Received: December, 2014
DOI 10.17677/fn20714807.2015.02.01
Fluorine Notes, 2015, 99, 1-2
SYNTHESIS OF NEW ACRIDINES WITH FLUORINE-CONTAINING 1,2,4 AND 1,3,4- OXADIAZOLE FRAGMENT
A. Y. Lamanov, T.N. Kudryavtseva
Kursk State University, 305000 Kursk, Radishcheva, 33
e-mail: labOS.kgu@mail.ru
Abstract: New acridines with fluorine-containing 1,2,4- and 1,3,4-oxadiazole fragment was synthesized and reactivity of pentafluorophenyl derivatives with some nucleophiles was investigated.
Keywords: Acridine, fluorine-containing oxadiazoles
Currently, much attention is paid to the search for new biologically active substances of a number of acridine [1]. Such drugs based on acridine derivatives as mepacrine, acriflavine, amskarin were developed and produced by the pharmaceutical industry [2]. These drugs are used to treat malaria, infectious and cancer diseases. Like many agents used in chemotherapy, this drugs have some drawbacks, including toxic effects on various organs of the organism. Therefore, an important task facing chemists is to develop new and more effective drugs.
One of the directions of work, allow us to solve this problem, is a chemical modification of acridine. Introduction pharmacophoric groups in the molecule of acridine will allow to change character biological activity and the degree of the substance's action on the organism. The papers [3,4,5,6] demonstrates that such pharmacophore fragments may be 1,2,4 and 1,3,4-oxadiazoles. Furthermore, introducing fluorine atoms in molecules has a significant effect on the biological activity of the corresponding compounds. In particular, the introduction of the fluorine-containing alkyl substituents in the acridone molecule increases its antimalarial properties [7].
Thus, the aim of the work was to develop methods for the synthesis of some fluorinated acridine derivatives with 1,3,4 and 1,2,4-oxadiazole moiety.
The starting compounds for the synthesis of fluorinated derivatives of 1,3,4-oxadiazole was 2-(9-oxoacridine-10(9H)-yl)acetohydrazides [8]. Acridines 1a-2c, containing 1,3,4-oxadiazole fragment with fluorinated substituent, were obtained with yields of 60-80% by combining appropriate hydrazide with trifluoroacetic anhydride, perfluoropropionic anhydride or pentafluorobenzoyl chloride, followed by cyclization of the bis-acyl hydrazide in polyphosphoric acid (PPA).
Scheme 1
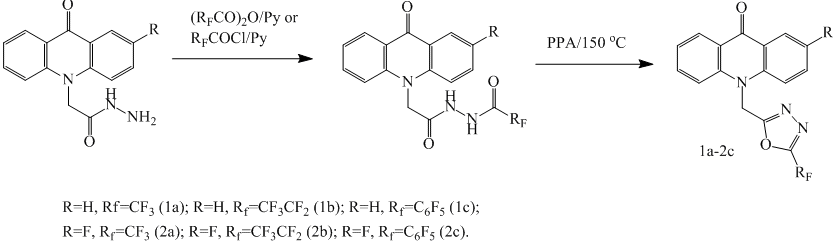
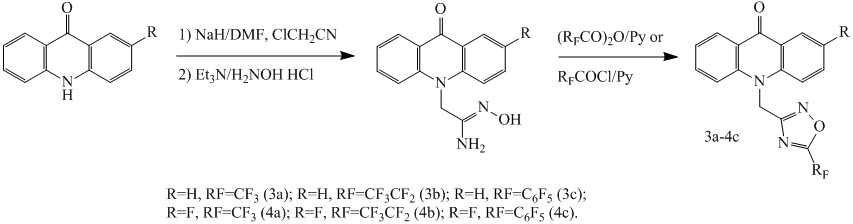
An efficient method for the preparation of fluorinated derivatives of 1,2,4-oxadiazole is using amidoximes and perfluorinated anhydrides or chlorides carboxylic acid as starting intermediates [9,10]. This method is suitable for the synthesis of 1,2,4-oxadiazol perfluorinated acridines, in our case it is practically does not give byproducts. The disadvantage of this way is the step of acridone’s alkylation with chloroacetonitrile in a system sodium hydride - N, N-dimethylformamide (DMF), that has a 36-45% conversion (HPLC), which reduces the yield of the desired compounds. Amidoxime was prepared by reaction of nitrile of acridone acetic acid with hydroxylamine in ethanol. Further reaction of amidoximes with trifluoroacetic anhydride, perfluoropropionic anhydride or pentafluorobenzoyl chloride by heating in toluene with pyridine leads to target acridone containing 1,2,4-oxadiazole moiety with perfluorinated substituent.
Scheme 2
It is known [11] that the fluorine atom in the 4-position substituent perfluorophenyl, related with 1,3,4-oxadiazole moiety is highly reactive and easily undergoes for nucleophilic substitution. Therefore it was of interest to investigate the reaction of compounds 1c, 2c with various nucleophiles. So in particular the corresponding compounds 5a-6e were obtained by reaction with amines in DMF (scheme 3), the results shown in Table 1.
Scheme 3
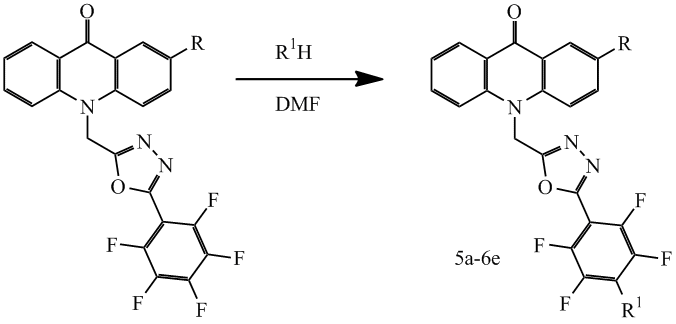
Table 1. Structure and yield compounds 5a-6e
Also the interaction with various alcohols was investigated for compounds 1c, 2c. Unlike the reaction with amines, the reaction with alcohols does not leak under the normal conditions. Using K2CO3 allowed to shift the equilibrium of the reaction to the right. But even in these conditions the interaction takes place considerably slower, and the reaction yield is slightly lower than in the reaction with amines (scheme 4), the results shown in Table 2.
Scheme 4
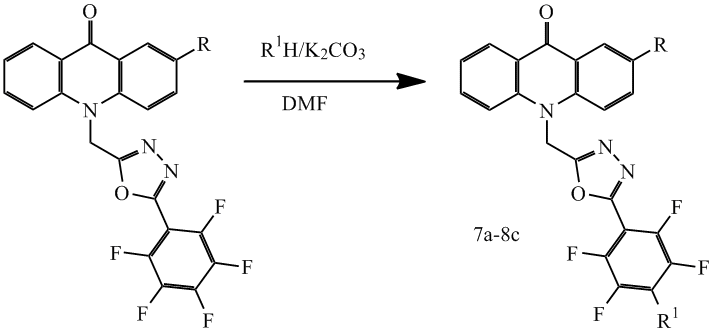
Table 2. Structure and yield compounds 7а-8с.
Thus obtained target fluorinated acridine derivatives including 1,3,4 and 1,2,4 oxadiazole moiety are of interest to study for their biological activity.
Experimental
Monitoring for the progress of the reaction and recording mass spectra of the synthesized compounds was performed using a Dionex Ultimate 3000 system with a mass spectrometric detector LCQ Fleet, (Thermo Scientific) mobile phase: ACN, H2O, 1% HCOOH. 1H NMR spectra were recorded on a Bruker AV-600, solvent DMSO-d6. Purification of the obtained compounds was performed using flash chromatography on a cartridge with the spherical silicagel, a particle size of 30 microns and a mass of 120 g (Interchim, PF 30SIHP / 120G). Eluent – from CH2Cl2 to CH2Cl2: MeOH - 10: 1. Used in the heterocyclic amines and alcohols - commercially available reagents.
General procedure for the synthesis of compounds 1a-2c
To acridone carboxylic acid hydrazide 1.87 mmol suspended in 5 ml of DMF was added 2 mmol of pyridine followed by adding 2 mmol of trifluoroacetic anhydride, or perfluoropropionic anhydride or pentafluorobenzoyl chloride and reaction was stirred for 5 hours. The hydrazide dissolves in the end of the reaction. The mixture was then poured into 50 ml of water, the precipitate was filtered, washed with water and dried. The obtained crude product was stirred with 8 g of 84% PPA and heated for 5 hours at 150 °C. The reaction mixture was cooled and poured into water. The precipitate was filtered, dried and purified by flash chromatography.
10-((5-(trifluoromethyl)-1,3,4-oxadiazol-2-yl)methyl)acridin-9(10H)-one (1a)
The crystalline substance of a pale yellow color. Yield: 70% m.p. 260-261 °C. Mass spectrum, m/z (Irel (%)): 346 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 6.17 (s, 2H, C(1a)H2); 7.4 (ddd, C(2)H, C(7)H, J = 7.93, J = 6.72, J = 1.03); 7.81-7.85 (m, 2H, C(4)H, C(5)H); 7.85-7.88 (m, 2H, C(3) H, C(6)H); 8.38 (dd, 2 H, C(1)H, C(8)H, J = 8.13, J = 1.49).
10-((5-(perfluoroethyl)-1,3,4-oxadiazol-2-yl)methyl)acridin-9(10H)-one (1b)
Yellow crystalline solid. Yield: 63% m.p. 177-178 ° C. Mass spectrum, m/z (Irel (%)): 414 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 6.20 (s, 2 H, C(1a)H2); 7.41 (ddd, C(2) , C(7)H, J = 7.9, J = 6.81, J = 0.97); 7.82-7.85 (m, 2H, C(4)H, C(5)H); 7.86-7.89 (m, 2H, C(3)H, C(6)H); 8.38 (dd, 2H, C(1) H, C(8)H, J = 8.01, J = 1.37).
10-((5-(perfluorophenyl)-1,3,4-oxadiazol-2-yl)methyl)acridin-9(10H)-one (1c)
The crystalline substance of a pale yellow color. Yield: 80% m.p. 222-223 ° C. Mass spectrum, m/z (Irel (%)): 444 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 6.25 (s, 2H, C(1a)H2); 7.40 (ddd, C(2)H, C(7)H, J = 7.9, J = 6.92, J = 0.86); 7.86 (ddd, C(4)H, C(5)H, J = 8.73, J = 6.95, J = 1.72); 7.91-7.95 (m, 2H, C(3)H, C(6)H); 8.38 (dd, 2H, C(1)H, C(8)H, J = 8.01, J = 1.6).
10-((5-(perfluorophenyl)-1,3,4-oxadiazol-2-yl)methyl)acridin-9(10H)-one (2a)
Yellow crystalline solid. Yield: 67% m.p. 263-264 ° C. Mass spectrum, m/z (Irel (%)): 364 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 6.18 (s, 2H, C(1a)H2); 7.41 (t, 1H, C(7)H, J = 7.1); 7.73-7.78 (m, 1H, C(3)H); 7.82-7.90 (m, 2H, C(4)H and C(5)H); 7.96 (dd, 1H, C(1)H, J = 9.44, J = 3.9); 8.00 (dd, 1H, C(6)H, J = 8.75, J = 3.15); 8.34-8.37 (m, 1H, C(8)H).
2-fluoro-10-((5-(perfluoroethyl)-1,3,4-oxadiazol-2-yl)methyl)acridin-9(10H)-one (2b)
Yellow crystalline solid. Yield: 60% m.p. 199-200 °C. Mass spectrum, m/z (Irel (%)): 414 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 6.21 (s, 2H, C(1a)H2); 7.40 (ddd, 1 H, C(7)H, J = 7.9, J = 6.87, J = 1.03); 7.75 (ddd, 1H, C(3)H, J = 9.44, J = 7.84, J = 3.20); 7.81-7.86 (m, 1H, C(5)H); 7.86-7.90 (m, 1H, C(4)H); 7.95-8.01 (m, 2H, C(1)H, C(6)H); 8.35 (dd, 1H, C(8)H, J = 8.01, J = 1.60).
2-fluoro-10-((5-(perfluorophenyl)-1,3,4-oxadiazol-2-yl)methyl)acridin-9(10H)-one (2с)
Yellow crystalline solid. Yield: 77% m.p. 228-229 ° C. Mass spectrum, m/z (Irel (%)): 462 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 6.27 (s, 2H, C(1a)H2); 7.41 (t, 1H, C(7)H, J = 7.27); 7.76-7.83 (m, 1H, C(3)H); 7.87 (m, C(5)H); 7.93 (d, 2H, C(4)H, J = 8.7); 7.97-8.07 (m, 2H, C(1)H, C(6)H); 8.36 (d, 1H, C(8) H, J = 7.55).
General procedure for the synthesis of compounds 3a-4c
To acridone (10 mmol) suspended in 20 ml of DMF was added 11 mmol of sodium hydride, stirred for 15 minutes and then 11 mmoles of chloroacetonitrile was added and stirred for 4 hours. Then mixture was poured into 150 ml of water, and the precipitate was filtered, washed with water and dried in vacuo. The resulting nitrile, 10 mmol of hydroxylamine hydrochloride and 10 mmol of triethylamine in 20 ml of ethanol was refluxed for 7 hours. After cooling, the precipitate was filtered, washed with ethanol and dried in vacuo.
To the amidoxime in 20 ml of toluene were added 10 mmol of pyridine, mixture was cooled to 0 ° C, then 10 mmol of trifluoroacetic anhydride or perfluoropropionic anhydride or pentafluorobenzoyl chloride was added and stirred for 3 hours. Reaction was stirred for 4 hours at room temperature and refluxed for 3 hours. The hot solution was filtered, the residue washed several times with hot toluene. The filtrates were combined, evaporated and the residue was purified by flash chromatography.
10-((5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl)methyl)acridin-9(10H)-one (3a)
Crystalline substance of light yellow color. Yield: 17% m.p. 190 -191 °C. Mass spectrum, m/z (Irel (%)): 346 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 6.12 (s, 2H, C(1a)H2); 7.37-7.42 (m, C(2)H, C(7)H, J = 7.95, J = 4.58, J = 3.26); 7.80-7.85 (m, 4H, C(3)H, C(4)H, C(5)H, C(6)H); 8.38 (dt, 2H, C(1)H, C(8)H, J = 7.78, J = 1.14).
10-((5-(perfluoroethyl)-1,2,4-oxadiazol-3-yl)methyl)acridin-9(10H)-one (3b)
Yellow crystalline substance. Yield: 18% m.p. 138-139 °C. Mass spectrum, m/z (Irel (%)): 396 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 6.14 (s, 2H, C(1a)H2); 7.40 (ddd, C(2)H, C(7)H, J = 7.95, J = 4.58, J = 3.26); 7.82-7.84 (m, 4H, C(3)H, C(4)H, C(5)H, C(6)H); 8.37-8.39 (m, 2H, C(1)H, C(8)H).
10-((5-(perfluorophenyl)-1,2,4-oxadiazol-3-yl)methyl)acridin-9(10H)-one (3c)
The crystalline substance of a pale yellow color. Yield: 19% m.p. 160-161 °C. Mass spectrum, m/z (Irel (%)): 444 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 6.15 (s, 2H, C(1a)H2); 7.39 (ddd, 2H, C(2)H, C(7)H, J = 7.90, J = 6.75, J = 1.03); 7.82-7.86 (m, 2H, C(4)H, C(5)H); 7.86-7.90 (m, 2H, C(3)H, C(6)H); 8.38 (dd, 2H, C(1)H, C(8)H, J = 7.95, J = 1.66).
2-fluoro-10-((5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl)methyl)acridin-9(10H)-one (4a)
The crystalline substance of a pale yellow color. Yield: 20% m.p. 192-193 ° C. Mass spectrum, m/z (Irel (%)): 364 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 6.14 (s, 2H, C(1a)H2); 7.41 (ddd, 1H, C(7)H J = 7.93, J = 4.95, J = 2.92); 7.76 (ddd, 1 H, C(5)H J = 9.44, J = 7.84, J = 3.2); 7.83-7.87 (m, 2H, C(3)H, C(4)H); 7.92-7.95 (m, 1H, C(1)H); 8.01 (dd, C(6)H J = 8.81, J = 3.2); 8.35-8.37 (m, 1H, C(8)H).
2-fluoro-10-((5-(perfluoroethyl)-1,2,4-oxadiazol-3-yl)methyl)acridin-9(10H)-one (4b)
Yellow crystalline substance. Yield: 77% m.p. 141-142 °C. Mass spectrum, m/z (Irel (%)): 414 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 6.16 (s, 2H, C(1a)H2); 7.40 (ddd, 1H, C(7)H, J = 7.95, J = 5.09, J = 2.75); 7.76 (ddd, 1H, C(5)H, J = 9.41, J = 7.81, J = 3.26); 7.82-7.87 (m, 2H, C(3)H, C(4)H); 7.93 (dd, 1H, C(1)H, J = 9.5, J = 4,01); 8.01 (dd, 1H, C(6)H J = 8.75, J = 3.15); 8.34-8.37 (m, 1H, C(8)H).
2-fluoro-10-((5-(perfluorophenyl)-1,2,4-oxadiazol-3-yl)methyl)acridin-9(10H)-one (4с)
The crystalline substance of a pale yellow color. Yield: 17% m.p. 166-167 ° C. Mass spectrum, m/z (Irel (%)): 462 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 6.16 (s, 2H, C(1a)H2); 7.33 (dt, 1H, C(7)H, J = 7.4, J = 0.9); 7.48 (ddd, 1H, C(3)H, J = 9.3, J = 7.4, J = 3.2); 7.71-7.78 (m, 3H, C(5)H, C(4)H, C(6)H; 8.14 (dd, 1H, C(1), J = 8.6, J = 3.1) 8.45 (d, 1H, C(8)H, J = 8.01, J = 1.5).
General procedure for the synthesis of compounds 5a-6e
To a 0.226 mmol 1c or 2c component in 1 ml of DMF was added 0.23 mmol of the amine and stirred 3-8 hours at room temperature. The solution was then poured into 20 ml of water, the precipitate was filtered, dried and purified by flash chromatography.
10-((5-(2,3,5,6-tetrafluoro-4-(4-methylpiperazin-1-yl)phenyl)-1,3,4-oxadiazol-2-yl)methyl)acridin-9(10H)-one (5a)
Yellow crystalline solid. Yield: 91% m.p. 222-223 ° C. Mass spectrum, m/z (Irel (%)): 524 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 2.35 (br. s. 3H, C(5a)H3); 2.55-2.7 (m, 4H, C(2a)H2, C(2b)H2); 3.2-3.5 (m, 4H, C(3a)H2, C(3b) H2); 6.06 (s, 2H, C(1a)H2); 7.32 (t, 2H, C(2)H, C(7)H, J = 7.34); 7.77 (ddd, 2H, C(4)H, C(5)H, J = 8.67, J = 7.01, J = 1.6); 7.84 (d, 2H, C(3)H, C(6)H, J = 8.7); 8.38 (dd, 2H, C(1)H, C(8)H, J = 7.9, J = 1.6).
10-((5-(2,3,5,6-tetrafluoro-4-(pyridin-2-ylmethylamino)phenyl)-1,3,4-oxadiazol-2-yl)methyl)acridin-9(10H)-one (5b)
Yellow crystalline solid. Yield: 81% m.p. 218-219 ° C. Mass spectrum, m/z (Irel (%)): 532 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 4.69 (d, 2H, C(2a)H2, J = 6.5); 6.18 (s, 2H, C(1a)H2); 7.25-7.29 (m, 1H, C(4a)H); 7.8 (d, 1H, C(6a)H, J = 7.8); 7.39 (dt, 2H, C(2)H, C(7)H J = 7.4, J = 0.8); 7.43-7.47 (m, NH); 7.77 (dt, 1H, C(5a)H, J = 7.7, J = 1.8); 7.85 (ddd, C(4)H, C(5)H, J = 8.7, J = 6.9, J = 1.8); 7.9-7.93 (m, 2H, C(3)H, C(6)H); 8.38 (dd, 2H, C(1)H, C(8)H, J = 8, J = 1.7); 8.5-8.52 (m, 1H, C(3a)H).
10-((5-(4-(cyclopropylamino)-2,3,5,6-tetrafluorophenyl)-1,3,4-oxadiazol-2-yl)methyl)acridin-9(10H)-one (5c)
The crystalline substance of a pale yellow color. Yield: 70% m.p. 219-220 ° C. Mass spectrum, m/z (Irel (%)): 481 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 0.61-0.73 (m, 4H, C(3a)H2, C(3b)H2); 2.85-2.91 (m, 1H, C(2a)H2); 6.19 (s, 2H, C(1a)H2); 7.15-7.17 (m, NH); 7.40 (td, 2H, C(2) H, C(7)H, J = 7.44, J = 0.8); 7.85 (ddd, 2H C(3)H, C(6)H, J = 8.73, J = 6.95, J = 1.72); 7.93 (d, 2H, C(4)H, C(5)H, J = 8.81); 8.38 (dd, 2H, C(1)H, C(8)H, J = 8.01, J = 1.72).
10-((5-(2,3,5,6-tetrafluoro-4-(3-hydroxypropylamino)phenyl)-1,3,4-oxadiazol-2-yl)methyl)acridin-9(10H)-one (5d)
The crystalline substance of a pale yellow color. Yield: 62% m.p. 227-228 ° C. Mass spectrum, m/z (Irel (%)): 499 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 1.68-1.74 (m, 2H, C(3a)H2); 3.42-3.50 (m, 4H, C(2a)H2, C(4a)H2); 4.56 (t, OH, J = 5.04); 6.18 (s, 2H, C 1a)H2); 6.78-6.85 (m, NH); 7.37-7.42 (m, 2H, C(2)H, C(7)H); 7.85 (ddd, 2H C(3)H, C(6)H, J = 8.7, J = 6.98, J = 1.72); 7.92 (m, 2H, C(4)H, C(5)H); 8.37 (dd, 2H, C(1)H, C(8)H, J = 7.95, J = 1.66).
ethyl 2-(2,3,5,6-tetrafluoro-4-(5-((9-oxoacridin-10(9H)-yl)methyl)-1,3,4-oxadiazol-2-yl)phenylamino)acetate (5e)
The crystalline substance of a pale yellow color. Yield: 48% m.p. 217-218 ° C. Mass spectrum, m/z (Irel (%)): 527 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 1.17-1.20 (m, 3H, C(4a)H3); 4.12-4.16 (m, 4H, C(3a)H2, C(2a)H2); 6.18 (s, 2H, C(1a)H2); 7.24-7.29 (m, NH); 7.38-7.41 (m, 2H, C(2)H, C(7)H); 7.83-7.87 (m, 2H, C(3)H, C(6)H); 7.91-7.94 (m, 2H, C(4)H, C(5)H); 8.36-8.39 (m, 2H, C(1)H, C(8)H).
2-fluoro-10-((5-(2,3,5,6-tetrafluoro-4-(4-methylpiperazin-1-yl)phenyl)-1,3,4-oxadiazol-2-yl)methyl)acridin-9(10H)-one (6a)
Yellow crystalline solid. Yield: 89% m.p. 220-221 °C. Mass spectrum, m/z (Irel (%)): 542 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 2.27 (br. s. 3H, C(5a)H3); 2.43-2.51 (m, 4H, C(2a)H2, C(2b)H2); 3.29-3.39 (m, 4H, C(3a)H2, C(3b)H2); 6.02 (s, 2H, C(1a)H2); 7.31 (t, 1H, C(7)H, J = 7.4); 7.51-7.57 (m, 1H, C(3)H); 7.76 (t, 1H, C(5)H, J = 7.67); 7.79-7.84 (m, 1H, C(4)H); 7.87-7.93 (m, 1H, C(6)H); 7.87-7.92 (m, 1H, C(1)H); 8.36 (d, 1H, C(8)H, J = 8.01).
2-fluoro-10-((5-(2,3,5,6-tetrafluoro-4-(pyridin-2-ylmethylamino)phenyl)-1,3,4-oxadiazol-2-yl)methyl)acridin-9(10H)-one (6b)
Yellow crystalline solid. Yield: 78% m.p. 222-223 °C. Mass spectrum, m/z (Irel (%)): 550 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 4.64-4.71 (m, 2H, C(2a)H2); 6.19 (s, 2H, C(1a)H2); 7.27 (ddd, 1H, C(4a)H, J = 7.47, J = 7.84, J = 1.09); 7.36 (d, 1H, C(6a)H, J = 7.9); 7.38-7.42 (m, 1H, C(7)H); 7.46 (t, NH, J = 6.52); 7.75-7.80 (m, 2H, C(3)H, C(5a)H); 7.84-7.87 (m, 1H, C(5)H); 7.90-7.93 (m, 1H, C(4)H); 7.99-8.03 (m, 2H, C(1)H, C(6)H); 8.34-8.36 (m, 1H, C(8)H); 8.51 (ddd, 1H, C(3a)H, J = 4.84, J = 1.75, J = 0.97).
10-((5-(4-(cyclopropylamino)-2,3,5,6-tetrafluorophenyl)-1,3,4-oxadiazol-2-yl)methyl)-2-fluoroacridin-9(10H)-one (6c)
Yellow crystalline solid. Yield: 75% m.p. 233-234 °C. Mass spectrum, m/z (Irel (%)): 499 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 0.61-0.73 (m, 4H, C(3a)H2, C(3b)H2); 2.85-2.91 (m, 1H, C(2a)H2); 6.21 (s, 2H, C(1a)H2); 7.17 (d, NH, J = 2.06); 7.41 (ddd, 1 H, C (7) H, J = 7.93, J = 6.95, J = 0.8); 7.79 (ddd, 1 H, C(3)H, J = 9.44, J = 7.78, J = 3.26); 7.87 (ddd, 1H, C(5)H, J = 8.75, J = 6.92, J = 1.72); 7.91-7.94 (m, 1H, C(4)H); 8.0-8.04 (m, 2H, C(1)H, C(6)H); 8.36 (dd, 1H, C(8)H, J = 8, J = 1.7).
2-fluoro-10-((5-(2,3,5,6-tetrafluoro-4-(3-hydroxypropylamino)phenyl)-1,3,4-oxadiazol-2-yl)methyl)acridin-9(10H)-one (6d)
The crystalline substance of a pale yellow color. Yield: 70% m.p. 230-231 °C. Mass spectrum, m/z (Irel (%)): 517 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 1.71 (quintet, 2H, C(3a)H2, J = 6.58); 3.42-3.50 (m, 4H, C(2a)H2, (4a)H2); 4.30-4.75 (m, OH); 6.19 (s, 2H, C(1a)H2); 6.78-6.85 (m, NH); 7.40 (td, 1H, C(7)H, J = 7.44, J = 0.8); 7.78 (ddd, 1H, C(3)H, J = 9.41, J = 7.81, J = 3.26); 7.86 (ddd, 1H, C(5)H, J = 8.75, J = 6.92, J = 1.72); 7.89-7.95 (m, 1H, C(4)H); 8.01 (td, 2H, C(1)H, C(6)H, J = 8.98, J = 3.66); 8.35 (dd, 1H, C(8)H, J = 8.01, J = 1.6).
ethyl 2-(2,3,5,6-tetrafluoro-4-(5-((2-fluoro-9-oxoacridin-10(9H)-yl)methyl)-1,3,4-oxadiazol-2-yl)phenylamino)acetate (6e)
The crystalline substance of a pale yellow color. Yield: 56% m.p. 223-224 °C. Mass spectrum, m/z (Irel (%)): 545 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 1.19 (t, 3H, C(4a)H3, J = 7.1); 4.11-4.18 (m, 4H, C(3a)H2, C(2a)H2); 6.20 (s, 2 H, C(1a)H2); 7.27 (t, NH, J = 6.58); 7.41 (td, 1H, C(7) H, J = 7.4, J = 0.9); 7.76-7.81 (m, 1H, C(3)H); 7.84-7.89 (m, 1H, C(5)H); 7.91-7.94 (m, 1H, C(4)H); 7.99-8.05 (m, 2H, C(1)H, C(6)H); 8.36 (dd, 1H, C(8)H, J = 8.01, J = 1.6).
General procedure for the synthesis of compounds 7a-8c
To a 0.226 mmol 1c or 2c component in 1 ml of DMF was added 0.23 mmole alcohol and 0.23 mmol K2CO3 and reaction was stirred for 24 hours at room temperature. The solution was then poured into 20 ml of water, the precipitate was filtered, dried and purified by flash chromatography.
10-((5-(2,3,5,6-tetrafluoro-4-(2-(piperidin-1-yl)ethoxy)phenyl)-1,3,4-oxadiazol-2-yl)methyl)acridin-9(10H)-one (7a)
The crystalline substance of a pale yellow color. Yield: 45% m.p. 270-271 ° C. Mass spectrum, m/z (Irel (%)): 553 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 1.26-1.42 (m, 6H, C(5a)H2, C(5b)H2, C(5c)H2); 2.29-2.48 (m, 4H, C(4a)H2, C(4b)H2); 2.62-2.77 (m, 2H, C(3a)H2); 4.42-4.51 (m, 2H, C(2a)H2); 6.23 (s, 2H, C(1a)H2); 7.41 (t, 2H, C(2)H, C(7)H, J = 7.72); 7.84-7.88 (m, 2H, C(3)H, C(6)H); 7.92-7.95 (m, 2H, C(4)H, C(5)H); 8.37 (dd, 2H, C(1)H, C(8)H, J = 7.95, J = 1.66).
10-((5-(2,3,5,6-tetrafluoro-4-(2-morpholinoethoxy)phenyl)-1,3,4-oxadiazol-2-yl)methyl)acridin-9(10H)-one (7b)
Crystalline solid of light yellow color. Yield: 44% m.p. 232-233 °C. Mass spectrum, m/z (Irel (%)): 555 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 2.39-2.47 (m, 4H, C(4a)H2, C(4b)H2); 2.7-2.76 (m, 2H, C(3a)H2); 3.43-3.5 (m, 4H, C(5a)H2, C(5b)H2); 4.48 (t, 2H, C(2a)H2, J = 5.15); 6.23 (s, 2H, C(1a)H2); 7.41 (t, 2H, C(2)H, C(7)H, J = 7.38); 7.86 (ddd, 2H, C(5)H, J = 8.7, J = 6.98, J = 1.72); 7.9-7.96 (m, 2H, C(3)H, C(6)H); 8.3 (dd, 2H, C(1)H, C(8)H, J = 7.9, J = 1.6).
10-((5-(2,3,5,6-tetrafluoro-4-(2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethoxy)phenyl)-1,3,4-oxadiazol-2-yl)methyl)acridin-9(10H)-one (7c)
White crystalline solid. Yield: 49% m.p. 185-186 °C. Mass spectrum, m/z (Irel (%)): 595 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 2.52 (m, 3H, CH3); 4.74-4.80 (m, 4H, C(3a)H2, C(2a)H2); 6.22 (s, 2H, C(1a)H2); 7.39 (ddd, 2H, C (2) H, C (7) H, J = 7.92, J = 6.95, J = 0.8); 7.84 (ddd, 2H, C(5)H, J = 8.75, J = 6.92, J = 1.72); 7.90-7.93 (m, 2 H, C (3) H, C (6) H); 8.07 (s, 1H, C(1b)H); 8.37 (dd, 2H, C(1)H, C(8)H, J = 8.01, J = 1.6).
2-fluoro-10-((5-(2,3,5,6-tetrafluoro-4-(2-(piperidin-1-yl)ethoxy)phenyl)-1,3,4-oxadiazol-2-yl)methyl)acridin-9(10H)-one (8a)
Crystalline solid of light yellow color. Yield: 43% m.p. 270-271 °C. Mass spectrum, m/z (Irel (%)): 571 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 1.25-1.39 (m, 6H, C(5a)H2, C(5b)H2, C(5c)H2); 2.27-2.43 (m, 4H, C(4a)H2 C(4b)H2); 2.60-2.72 (m, 2H, C(3a)H2); 4.44 (t, 2H, C(2a)H2, J = 4.86); 6.24 (s, 2H, C(1a)H2); 7.38-7.43 (m, 1H, C(7)H); 7.75-7.80 (m, 1H, C(3)H); 7.86 (ddd, 1H, C(5)H, J = 8.73, J = 6.95, J = 1.72); 7.93 (d, 1H, C(4)H, J = 8.81); 7.98-8.05 (m, 2H, C(1)H, C(6)H); 8.33-8.36 (m, 1H, C(8)H).
10-((5-(2,3,5,6-tetrafluoro-4-(2-morpholinoethoxy)phenyl)-1,3,4-oxadiazol-2-yl)methyl)acridin-9(10H)-one (8b)
Crystalline solid of light yellow color. Yield: 40% m.p. 240-241 ° C. Mass spectrum, m/z (Irel (%)): 573 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 2.37-2.47 (m, 4H, C(4a)H2, C(4b)H2); 2.68-2.76 (m, 2H, C(3a)H2); 3.43-3.50 (m, 4H, C(5a)H2, C(5b)H2); 4.48 (t, 2H, C(2a)H2, J = 5.21); 6.25 (s, 2H, C(1a)H2); 7.40-7.43 (m, 1H, C(7)H); 7.79 (ddd, 1H, C(3)H, J = 9.38, J = 7.78, J = 3.2); 7.85-7.89 (m, 1H, C(5)H); 7.92-7.95 (m, 1H, C(4)H); 7.99-8.05 (m, 2H, C(1)H, C(6)H); 8.35-8.37 (m, 1H, C(8)H).
2-fluoro-10-((5-(2,3,5,6-tetrafluoro-4-(2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethoxy)phenyl)-1,3,4-oxadiazol-2-yl)methyl)acridin-9(10H)-one (8c)
Crystalline solid of light yellow color. Yield: 52% m.p. 190-191 ° C. Mass spectrum, m/z (Irel (%)): 613 [M + H]+ (100). 1H NMR (DMSO-d6, δ, ppm, J/Hz): 2.52 (s, 3H, CH3); 4.74-4.81 (m, 4H, C(3a)H2, C(2a)H2); 6.22 (s, 2H, C(1a) H2); 7.39 (td, 1H, C(7)H, J = 7.44, J = 0.8); 7.77 (ddd, 1H, C(3)H, J = 9.5, J = 7.78, J = 3.2); 7.85 (ddd, 1H, C(5)H, J = 8.7, J = 6.92, J = 1.77); 7.9-7.93 (m, 1H, C(4)H); 7.97-8.03 (m, 2H, C(1)H, C(6)H); 8.06 (s, 1H, C(1b)H); 8.34 (dd, 1H, C(8)H, J = 8.01, J = 1.6).
Work was financially supported by the Ministry of Education and Science (Research project number 1399 in the framework of the state task 2014/349 of the Ministry of Education and Science of the Russian Federation.
References
- T.N. Kudryavtseva, K.V. Bogatyrev, L.G. Klimova, E.A. Batuev. Synthesis and antibacterial activity of series fluorine-substituted acridoneacetic acid derivatives // Fluorine notes: online journal. 2014. N 3 (94), URL: /public/2014/3_2014/letters/index.html
- Kleemann A., Engel J. Pharmaceutical Substances. Stuttgart - New York: Thieme, 2001. 2409 с.
- Trilok Chandra, Neha Garg, Suman Lata, K.K. Saxena, Ashok Kumar. Synthesis of substituted acridinyl pyrazoline derivatives and their evaluation for anti-inflammatory activity // Eur. J. Med. Chem. 2010. V. 45, N. 5, P. 1772–1776
- Anees Pangal, Javed A. Shaikh. Various pharmacological aspects of 2, 5-disubstituted 1,3,4-oxadiazole derivatives: a Review // Res. J. Chem. Sci. 2013, V. 3(12), P. 79-89.
- Andrea Pace and Paola Pierro. The new era of 1,2,4-oxadiazoles // Org. Biomol. Chem. 2009, 7, 4337–4348.
- Jonas Boström, Anders Hogner, Antonio Llinàs, Eric Wellner, and Alleyn T. Plowright. Oxadiazoles in Medicinal Chemistry // J. Med. Chem. 2012, V. 55, N. 5, P. 1817−1830.
- Rolf W. Winter, Jane X. Kelly, Martin J. Smilkstein, Rozalia Dodean, Grover C.Bagby, R. Keaney Rathbun, Joshua I. Levin, David Hinrichs, Michael K. Riscoe. Evaluation and lead optimization of anti-malarial acridones // Experimental Parasitology. 2006, V. 114, N 1, P. 47–56.
- Yu. D Markovich, P.I Sysoev, T. N Kudryavtseva, N. N Sergeyev, L. G Klimov. Synthesis and study of biological activity arilidengidrazidov akridonuksusnoy acid // Scientific notes. Electronic scientific journal Kursk State University. 2013.N 3 (27). Volume 2. URL http://scientific-notes.ru/pdf/032-003.pdf.
- J.P. Critchley, J.S. Pippett. The synthesis and stability of some perfluoroalkyl- and perfluoroalkylene-1,2,4- and 1,3,4-oxadiazoles // Journal of Fluorine Chemistry. 1972, V. 2, N. 2, P. 137–156.
- Silvestre Buscemi, Andrea Pace, Antonio Palumbo Piccionello, Nicolò Vivona. Synthesis of fluorinated first generation starburst molecules containing a triethanolamine core and 1,2,4-oxadiazoles // Journal of Fluorine Chemistry. 2006. V. 127, N. 12, P. 1601–1605.
- Jianfu Ding and Michael Day. Novel Highly Fluorinated Poly(arylene ether-1,3,4-oxadiazole)s, Their Preparation, and Sensory Properties to Fluoride Anion // Macromolecules. 2006, V. 39, N. 18, P. 6054–6062.
Recommended for publication by Prof. A. F. Eleev
Fluorine Notes, 2015, 99, 1-2
