Received: May , 2014
Fluorine Notes, 2014, 94, 3-4
SYNTHESIS AND ANTIBACTERIAL ACTIVITY OF SERIES FLUORINE-SUBSTITUTED ACRIDONEACETIC ACID DERIVATIVES
T.N.Kudryavtseva a, K.V.Bogatyrev a, L.G. Klimova b, E.A. Batuev c
aKursk State University, 305000 Kursk, Radishcheva, 33
e-mail: labOS.kgu@mail.ru
bKursk State Medical University, 305041 Kursk, K. Marksa, 3
cEngelhardt Institute of Molecular Biology, 119991 Moscow, Vavilova, 32
carboksyl@yandex.ru
Abstract: Some new derivatives of various fluoroacridoneacetic acids were obtained, their antimicrobial activity against a group of test strains of microorganisms was investigated, as well as the influence of the structure of the obtained compounds on their bactericidal action.
Keywords: Fluorine-substituted acridoneacetic acids, esters, amides, antimicrobial action
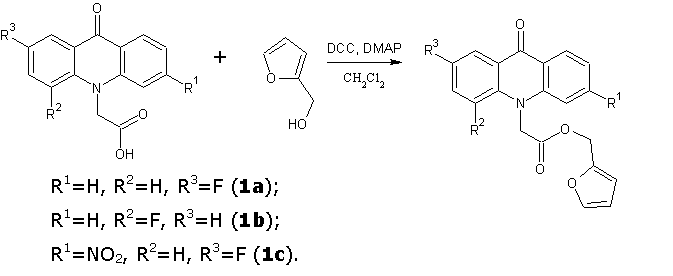
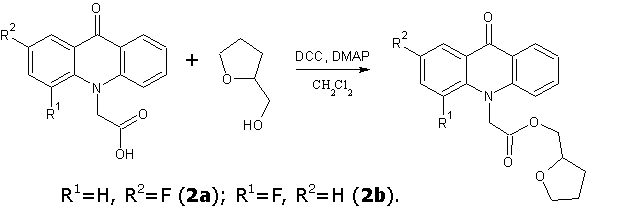
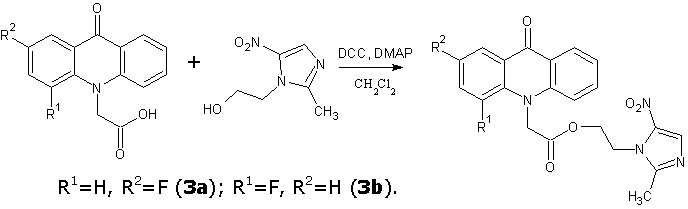
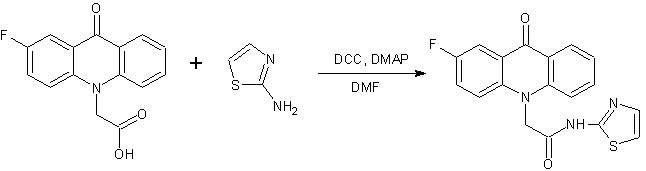
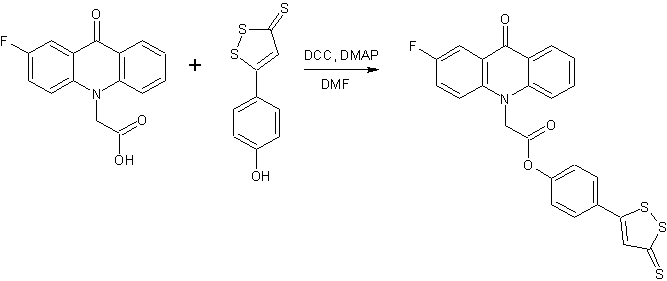
Continuing the search for biologically active compounds in the series of fluorine-substituted acridoneacetic acids (AAA) [1], we were tasked to synthesize a series of new products containing the condensed system of acridone coupled with additional pharmacophore heterocyclic moiety through an amide or ester bond. As these pharmacophores were taken 2-aminothiazole , 2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethanol , 2-(hydroxymethyl)furan, tetrahydro-2-furanmethanol , 4-methyl-5-(2-hydroxyethyl)thiazole and 5-(4-hydroxyphenyl)-3H-1,2-dithiole-3- thione.
The choice of heterocyclic pharmacophore groups was caused by the following reasons: some 2-aminothiazole derivatives have an antibacterial activity [2,3], the fragment of this compound also contains in structure of such known antimicrobial agents as sulfathiazole, nitazolum, carumonam, aztreonam, tenonitrozole, ceftriaxone [4].
Antimicrobial and antiprotozoal drug metronidazole (2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethanol) is widely used in clinical practice. This compound causes DNA damage due to formation of complexes or strand breaks and is effective against Trichomonas vaginalis, Entamoeba histolytica and obligate anaerobes, which are converted metronidazole into a reactive hydroxylamine metabolite, that lead to irreversible changes in the DNA structure [5,6].
The derivatives of 4-methyl-5-(2-hydroxyethyl)thiazole, which can be regarded as part of thiamine molecule (vitamin B1), have some useful pharmacological properties. Thus, the drug clomethiazole (5-(2-chloroethyl)-4-methylthiazole ethanedisulphonate) has sedative, hypnotic and anticonvulsant effects and fluorinated analogs of 4-methyl-5-(2-hydroxyethyl)thiazole show bacteriostatic activity [7,8].
The derivatives of 5-(4-hydroxyphenyl)-3H-1,2-dithiole-3-thione (ADT-OH), which is widely used as H2S-releasing compound in the organisms for the synthesis of pharmaceutical hybrids, were actively studied last years. It was found that 4-(3H-1,2-dithiole-3-thione-5-yl)phenyl esters of various carboxylic acids show anti-inflammatory and antitumor activity [9-11].
Fragments of 2-(hydroxymethyl)furan and tetrahydro-2-furanmethanol also contains in many biologically active substances[12,13].
One of the general procedure for synthesis of esters and amides is carbodiimide activation of carboxylic acid. In these reactions various carbodiimides are used, N,N-dicyclohexylcarbodiimide (DCC) is the most simple and cheap among them. This synthetic method was used to obtain the title compounds.
Tetrahydrofuran and furan-2-ylmethyl esters of various fluoroacridoneacetic acids were prepared in dichloromethane in the presence of DCC and a catalytic amount of N, N-dimethylaminopyridine (DMAP) at room temperature for 6 hours. The precipitate of N, N-dicyclohexylurea was filtered, the solvent was evaporated and the crude obtained products were purified by column chromatography (silica gel 60-Merck, eluent toluene: acetone: ethanol at a volume ratio 10:3:2).
Similarly 2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethyl and 2-(4-methyl-1,3-thiazol-5-yl)ethyl esters of 2- and 4-fluoroacridoneacetic acids were synthesized.
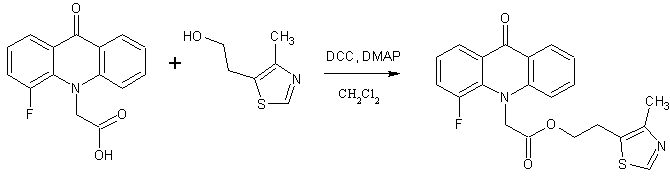
It should be noted that one of possible synthetic way for furan-2-ylmethyl and 2-(4-methyl-1,3-thiazol-5-yl)ethyl ester is transesterification of methyl, ethyl or butyl esters of the AAA by the corresponding alcohol with a five-membered heterocyclic moiety catalyzed NaOCH3. However, we found that a similar reaction with tetrahydro-2-furanmethanol leads to low yield of target ester.
Synthesis of 2-aminothiazole and ADT-OH derivatives was carried out more long time (8-10 hours). The final products have a poor solubility in dichloromethane, therefore the reaction was performed in DMF. The crude products were washed with hot CHCl3 solution to remove impurities of starting materials, N,N-dicyclohexylurea and N, N-dicyclohexylcarbamimidates of fluoroacridoneacetic acids.
The signals of protons corresponding acridone tricyclic system, as well as signals relating to the coupled pharmacophore heterocyclic moiety present in the 1H-NMR spectra of the obtained compounds.
In a previous paper [1] we studied the antimicrobial action of some 2-fluoroacridoneacetic acid derivatives, so it was of considerable interest to evaluate activity of substances with a fluorine atom in the 4-position of acridone ring. The antibacterial activity of compounds 2b, 3b and 4 (1% and 2% solution in DMSO) was investigated on a series of test strains of microorganisms, results are shown in Table 1.
Table 1. Antimicrobial activity of compounds 2b, 3b, 4.
|
Compound |
C, % |
E. coli (ATCC 25922) |
Ps. aeruginosa (ATCC 27853) |
Pr.vulgaris (ATCC 4636) |
S. aureus (ATCC 25923) |
B.subtilis (ATCC 6633) |
Candida albicans (NCTC2625) |
|
Growth inhibition areas, mm. |
|||||||
|
3b |
1 |
10.50В±0.40 |
8.50В±0.73 |
10.50В±0.61 |
8.00В±0.45 |
20.50В±0.92 |
11.00В±0.54 |
|
2 |
9.00В±0.79 |
7.00В±0.56 |
10.50В±0.67 |
8.00В±0.70 |
22.50В±0.85 |
11.00В±0.69 |
|
|
Metro-nidazole |
1 |
11.50В±0.39 |
20.00В±0.74 |
14.00В±0.42 |
22.00В±0.70 |
14.50В±0.37 |
20.00В±0.63 |
|
2 |
12.00В±0.35 |
21.00В±0.61 |
22.00В±0.73 |
25.00В±0.68 |
15.00В±0.40 |
25.00В±0.75 |
|
|
2b |
1 |
10.50В±0.54 |
10.50В±0.66 |
10.00В±0.60 |
9.00В±0.58 |
7.00В±0.38 |
12.00В±0.75 |
|
2 |
11.50В±0.71 |
11.50В±0.80 |
9.00В±0.47 |
9.00В±0.77 |
7.00В±0.42 |
11.00В±0.59 |
|
|
4 |
1 |
10.50В±0.84 |
8.00В±0.59 |
7.50В±0.63 |
8.00В±0.38 |
8.00В±0.40 |
10.50В±0.70 |
|
2 |
12.50В±0.62 |
8.50В±0.50 |
8.00В±0.45 |
8.50В±0.56 |
9.50В±0.81 |
12.00В±0.68 |
|
|
Rivanol |
1 |
12.75В±0.47 |
12.00В±1.14 |
12.50В±0.83 |
17.00В±1.02 |
14.05В±0.94 |
13.50В±0.56 |
|
2 |
14.50В±0.57 |
15.00В±0.93 |
15.00В±0.66 |
20.00В±0.97 |
15.00В±1.14 |
15.00В±0.96 |
|
It was of greatest interest to determine the antimicrobial activity of 4-fluoroacridoneacetic acid 2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethyl ester, because it was previously found that a similar ester of unsubstituted AAA inhibits the growth of microorganisms more effective than the starting drug metronidazole. [14]
However, the obtained product 3b generally showed lower activity than 2-(2-methyl-5-nitro-1H-imidazol-1-yl)-ethyl ester of AAA and only for values of growth inhibition areas of B.subtilis exceeded metronidazole. Introduction of fluorine in the 4-position of acridone ring also led to a reduction of the antibacterial action in case of the compounds 2b and 4. Table 2 shows the comparative activity of 2-(4-methyl-1,3-thiazol-5-yl)ethyl esters unsubstituted, 2- and 4-fluoroAAA.
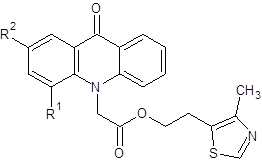
Table 2. Comparative antimicrobial activity of 2-(4-methyl-1,3-thiazol-5-yl)ethyl esters of various acridoneacetic acids.
|
Compound |
C, % |
E. coli (ATCC 25922) |
Ps. aeruginosa (ATCC 27853) |
Pr.vulgaris (ATCC 4636) |
S. aureus (ATCC 25923) |
B.subtilis (ATCC 6633) |
Candida albicans (NCTC2625) |
|
Growth inhibition areas, mm. |
|||||||
|
R1=H, R2=H |
1 |
9.00В±0.34 |
12.25В±1.04 |
9.00В±0.36 |
9.00В±0.46 |
10.00В±0.33 |
12.50В±0.47 |
|
2 |
9.00В±0.51 |
13.50В±0.56 |
10.00В±0.39 |
9.25В±0.52 |
10.50В±0.72 |
14.00В±0.32 |
|
|
R1=H, R2=F |
1 |
8.80В±0.71 |
10.05В±0.94 |
11.50В±0.32 |
14.00В±0.75 |
10.00В±0.37 |
13.50В±0.94 |
|
2 |
9.00В±0.75 |
13.50В±0.66 |
15.00В±1.09 |
14.50В±0.97 |
11.50В±0.32 |
15.00В±1.09 |
|
|
R1=F, R2=H |
1 |
10.50В±0.84 |
8.00В±0.59 |
7.50В±0.63 |
8.00В±0.38 |
8.00В±0.40 |
10.50В±0.70 |
|
2 |
12.50В±0.62 |
8.50В±0.50 |
8.00В±0.45 |
8.50В±0.56 |
9.50В±0.81 |
12.00В±0.68 |
|
It should be noted that 2-(4-methyl-1,3-thiazol-5-yl)ethyl ester of 2-fluoro AAA has a slightly higher antimicrobial activity than the unsubstituted analog, whereas the biological activity of a 4-fluoroAAA derivative generally decreased.
Compounds 2b and 4 on some indicators of activity are comparable to standard drug rivanol (closest structurally antibacterial drug – 2-ethoxy-6,9-diaminoacridine lactate).
Experimental
TLC was performed on plates «Sorbfil» PTLC-P-B-UV, eluent – toluene: acetone: ethanol in volume ratio of 10:3:2. IR spectra were obtained using a spectrometer FSM 1201 Monitoring, KBr tablets. Mass spectra were recorded on system ACQUITY UPLC H-Class with UV / mass detectors ACQUITY SQD Waters. 1H NMR spectra were recorded on spectrometer Bruker AV-600, solvent DMSO-d6.
The synthesis of various fluoroacridoneacetic acids and determination of antimicrobial activity were performed by the methods described previously [1]. Heterocyclic amines and alcohols used in this work – commercially available reagents.
General procedure for the synthesis of compounds 1-4
A mixture of the corresponding fluoroacridoneacetic acid (3 mmol), the alcohol, containing five-membered heterocyclic fragment, (3 mmol), 0.61 g (3 mmol) N,N-dicyclohexylcarbodiimide, 0.04 g (0.33 mmol) N,N-dimethylaminopyridine and 30 ml CH2Cl2 was stirred at room temperature for 5 - 6 hours. The precipitate N,N'-dicyclohexylurea was filtered off, the solvent was evaporated. Technical obtained product was purified by column chromatography on silica gel-60 Merck, eluent – toluene: acetone: ethanol at volume ratio 10:3:2.
General procedure for the synthesis of compounds 5,6
A mixture of the corresponding fluoroacridoneacetic acid (3 mmol), 2-aminothiazole or ADT-OH (3 mmol), 0.61 g (3 mmol) N,N-dicyclohexylcarbodiimide, 0.04 g (0.33 mmol) N,N-dimethylaminopyridine and 25 ml DMF was stirred at room temperature for 8-10 hours. The precipitate N,N'-dicyclohexylurea was filtered, the filtrate was poured into 50 ml of water. The formed precipitate was filtered off and washed with a hot solution of CHCl3 (3x20 mL).
Furan-2-ylmethyl(2-fluoro-9-oxoacridin-10(9H)-yl)acetate (1a)
Dark yellow crystalline solid. Yield: 83 %, m.p. 179-180 °C. Rf = 0.81. MS, m/z (Irel (%)): 352 [М+H]+ (100), 272 [C15H10FNO3 + H]+ (20), 226 [C14H10FNO – H]+ (84). IR (KBr) ν, сm-1: 3120–2856 (C—H); 1744 (C=Oest.); 1623 (C=Oacridone); 1603, 1493, 1470 (C-Cаr). 1H-NMR (DMSO-d6, δ, ppm, J/ Hz): 5.22 (s, 2 H, C(2a)H2); 5.52 (s, 2 H, C(1a)H2); 6.49 (t, 1 H, C(2b)H, J = 3.1, J =1.8); 6.56 (d, 1 H, C(1b)H, J = 3.1); 7.38 (t, 1 H, C(7)H, J = 7.4); 7.64 (d, 1 H, C(4)H, J = 8.8); 7.71 – 7.77 (m, 3 H, C(3)H, C(5)H, C(3b)H); 7.81(t, 1 H, C(6)H, J = 7.9); 7.99 (dd, 1 H, C(1)H, J = 8.7, J = 2.8); 8.34 (d, 1 H, C(8)H, J = 7.9).
Furan-2-ylmethyl(4-fluoro-9-oxoacridin-10(9H)-yl)acetate (1b)
Brown crystalline solid. Yield: 85 %, m.p. 151-152 °C. Rf = 0.84 . MS, m/z (Irel (%)): 352 [М+H]+ (75), 272 [C15H10FNO3 + H]+ (31), 226 [C14H10FNO – H]+ (100). IR (KBr) ν, сm-1: 3108–2855 (C—H); 1744 (C=Oest.); 1638 (C=Oacridone); 1603, 1499, 1463 (C-Cаr). 1H-NMR (DMSO-d6, δ, ppm, J/ Hz): 5.27 (s, 2 H, C(2a)H2); 5.30 (s, 2 H, C(1a)H2); 6.50 (t, 1 H, C(2b)H, J = 3.0, J =1.8); 6.60 (d, 1 H, C(1b)H, J = 2.9); 7.31 – 7.35 (m, 1 H, C(2)H); 7.39 (t, 1 H, C(7)H, J = 7.4); 7.61 (d, 1 H, C(5)H, J = 8.8); 7.67 (dd, 1 H, C(3)H, J = 15.3, J = 7.8); 7.73 (s, 1 H, C(3b)H); 7.81 (t, 1 H, C(6)H, J = 7.8); 8.15 (d, 1 H, C(1)H, J = 8.1); 8.28 (d, 1 H, C(8)H, J = 8.1).
Furan-2-ylmethyl(2-fluoro-6-nitro-9-oxoacridin-10(9H)-yl)acetate (1c)
Dark orange crystalline solid. Yield: 74 %, m.p. 153-154 °C. Rf = 0.86. MS, m/z (Irel (%)): 397 [М+H]+ (64), 271 [C14H9FN2O3 – H]+ (100). IR (KBr) ν, сm-1: 3103–2857 (C—H); 1728 (C=Oest.); 1651 (C=Oacridone); 1611, 1483, 1462 (C-Cаr), 1534 (NO2). 1H-NMR (DMSO-d6, δ, ppm, J/ Hz): 5.24 (s, 2 H, C(2a)H2); 5.67 (s, 2 H, C(1a)H2); 6.47 (t, 1 H, C(2b)H, J = 3.3, J =1.5); 6.56 (d, 1 H, C(1b)H, J = 3.3); 7.69 (s, 1 H, C(3b)H); 7.78 – 7.82 (m, 2 H, C(3)H, C(4)H); 7.98 (d, 1 H, C(1)H, J = 8.4); 8.05 (d, 1 H, C(7)H, J = 8.8); 8.47 (s, 1 H, C(5)H); 8.51 (d, 1 H, C(8)H, J = 8.4).
Tetrahydrofuran-2-ylmethyl(2-fluoro-9-oxoacridin-10(9H)-yl)acetate (2a)
Pale yellow crystalline solid. Yield: 77 %, m.p. 147-148 °C. Rf = 0.79. MS, m/z (Irel (%)): 356 [М+H]+ (100), 272 [C15H10FNO3+H]+ (94), 226 [C14H10FNO – H]+ (35). IR (KBr) ν, сm-1: 3070–2855 (C—H); 1751 (C=Oest.); 1627 (C=Oacridone); 1603, 1493, 1466 (C-Cаr), 1074 (C-O-C in tetrahydrofuran fragment). 1H-NMR (DMSO-d6, δ, ppm, J/ Hz): 1.44 – 1.50 (m, 1 H, C(2b)H); 1.67 – 1.75 (m, 2 H, C(2b)H, C(3b)H); 1.82 – 1.88 (m, 1 H, C(3b)H); 3.55 – 3.60 (m, 2 H, C(4b)H2); 4.01 – 4.05 (m, 1 H, C(1b)H); 4.08 – 4.11 (m, 1 H, C(2a)H); 4.17 (dd, 1 H, C(2a)H, J=11.5, J=3.6); 5.49 (s, 2 H, C(1a)H2); 7.37 (t, 1 H, C(7)H, J=7.6); 7.66 (d, 1 H, C(4)H, J=8.6); 7.71 – 7.78 (m, 2 H, C(3)H, C(5)H); 7.82 (t, 1 H, C(6)H, J=7.6); 7.98 (dd, 1 H, C(1)H, J=8.6, J=2.6); 8.33 (d, 1 H, C(8)H, J=8.1).
Tetrahydrofuran-2-ylmethyl(4-fluoro-9-oxoacridin-10(9H)-yl)acetate (2b)
Yellow crystalline solid. Yield: 82 %, m.p. 142-143 °C. Rf = 0.80. MS, m/z (Irel (%)): 356 [М+H]+ (100), 272 [C15H10FNO3+H]+ (81), 226 [C14H10FNO – H]+ (20). IR (KBr) ν, сm-1: 3071–2847 (C—H); 1750 (C=Oest.); 1640 (C=Oacridone); 1601, 1498, 1461 (C-Cаr), 1084 (C-O-C in tetrahydrofuran fragment). 1H-NMR (DMSO-d6, δ, ppm, J/ Hz): 1.53 – 1.59 (m, 1 H, C(2b)H); 1.73 – 1.78 (m, 2 H, C(2b)H, C(3b)H); 1.89 – 1.95 (m, 1 H, C(3b)H); 3.61 – 3.69 (m, 2 H, C(4b)H2); 4.06 – 4.09 (m, 1 H, C(1b)H); 4.14 – 4.17 (m, 1 H, C(2a)H); 4.24 (d, 1 H, C(2a)H, J=11.5); 5.28 (s, 2 H, C(1a)H2); 7.32 – 7.35 (m, 1 H, C(2)H); 7.39 (t, 1 H, C(7)H, J=7.4); 7.62 (d, 1 H, C(5)H, J=8.6); 7.69 (dd, 1 H, C(3)H, J=15.1, J=7.8); 7.82 (t, 1 H, C(6)H, J=7.5); 8.15 (d, 1 H, C(1)H, J=7.7); 8.29 (d, 1 H, C(8)H, J=7.9).
2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethyl(2-fluoro-9-oxoacridin-10(9H)-yl)acetate (3a)
Light yellow crystalline solid. Yield: 62 %, m.p. 224-225 °C. Rf = 0.68. MS, m/z (Irel (%)): 425 [М+H]+ (100), 298 [C17H14FNO3 – H]+ (65), 272 [C15H10FNO3 + H]+ (67), 226 [C14H10FNO – H]+ (29), 212 [C13H8FNO – H]+ (8). IR (KBr) ν, сm-1: 3129 – 2847 (C—H); 1742 (C=Oest.); 1619 (C=Oacridone); 1601, 1493, 1462 (C=C, C=N); 1526 (NO2). 1H-NMR (DMSO-d6, δ, ppm, J/ Hz): 2.28 (s, 3 H, CH3); 4.53 (t, 2 H, C(3a)H2, J=5.1); 4.58 (t, 2 H, C(2a)H2, J=5.0); 5.39 (s, 2 H, C(1a)H2); 7.38 (t, 1 H, C(7)H, J=7.5); 7.50 (d, 1 H, C(4)H, J=8.7); 7.58 – 7.64 (m, 2 H, C(3)H, C(5)H); 7.68 (t, 1 H, C(6)H, J=7.8); 7.79 (dd, 1 H, C(1)H, J=8.8, J=2.7); 7.98 (s, 1 H, C(1b)H); 8.33 (d, 1 H, C(8)H, J=8.0).
2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethyl(4-fluoro-9-oxoacridin-10(9H)-yl)acetate (3b)
Pale brown crystalline solid. Yield: 65 %, m.p. 207-208 °C. Rf = 0.71. MS, m/z (Irel (%)): 425 [М+H]+ (95), 298 [C17H14FNO3 – H]+ (100), 272 [C15H10FNO3 + H]+ (28), 254 [C15H10FNO2 – H]+ (12), 226 [C14H10FNO – H]+ (73), 212 [C13H8FNO – H]+ (13). IR (KBr) ν, сm-1: 3129 – 2856 (C—H); 1738 (C=Oest.); 1636 (C=Oacridone); 1610, 1601, 1497, 1462 (C=C, C=N); 1519 (NO2). 1H-NMR (DMSO-d6, δ, ppm, J/ Hz): 2.26 (s, 3 H, CH3); 4.58 (t, 2 H, C(3a)H2, J=5); 4.62 (t, 2 H, C(2a)H2, J=4.8); 5.18 (s, 2 H, C(1a)H2); 7.32 – 7.35 (m, 1 H, C(2)H); 7.39 (t, 1 H, C(7)H, J=7.4); 7.49 (d, 1 H, C(5)H, J=8.8); 7.63 (dd, 1 H, C(3)H, J=15.2, J=7.8); 7.78 (t, 1 H, C(6)H, J=7.8); 7.95 (s, 1 H, C(1b)H); 8.14 (d, 1 H, C(1)H, J=7.8); 8.28 (d, 1 H, C(8)H, J=7.8).
2-(4-methyl-1,3-thiazol-5-yl)ethyl(4-fluoro-9-oxoacridin-10(9H)-yl)acetate (4)
Yellow crystalline solid. Yield: 80 %, m.p. 146-147 °C. Rf = 0.76. MS, m/z (Irel (%)): 397 [М+H]+ (100), 272 [C15H10FNO3+H]+ (88), 226 [C14H10FNO – H]+ (30), 213 [C13H8FNO]+ (7). IR (KBr) ν, сm-1: 3073–2855 (C—H); 1753 (C=Oest.); 1647 (C=Oacridone); 1602, 1501, 1462 (C-Cаr); 1412 (thiazole ring). 1H-NMR (DMSO-d6, δ, ppm, J/ Hz): 2.26 (s, 3 H, CH3); 3.15 (t, 2 H, C(3a)H2, J=6.3); 4.39 (t, 2 H, C(2a)H2, J=6.3); 5.23 (s, 2 H, C(1a)H2); 7.32 – 7.36 (m, 1 H, C(2)H); 7.39 (t, 1 H, C(7)H, J=7.4); 7.51 (d, 1 H, C(5)H, J=8.8); 7.66 (dd, 1 H, C(3)H, J=15.0, J=7.9); 7.80 (t, 1 H, C(6)H, J=7.9); 8.15 (d, 1 H, C(1)H, J=8.1); 8.29 (d, 1 H, C(8)H, J=7.9); 8.82 (s, 1 H, C(1b)H).
2-(2-fluoro-9-oxoacridin-10(9H)-yl)-N-(1,3-thiazol-2-yl)acetamide (5)
Pale yellow crystalline solid. Yield: 89 %, m.p. 299-300 °C. Rf = 0.81. MS, m/z (Irel (%)): 354 [М+H]+ (100), 254 [C15H10FNO2 – H]+ (100). IR (KBr) ν, сm-1: 3329 (N—H); 3164 – 2851 (C—H); 1683 (C=Oamide); 1620 (C=Oacridone); 1603, 1564, 1487, 1462 (C-Cаr). 1H-NMR (DMSO-d6, δ, ppm, J/ Hz): 5.54 (s, 2 H, C(1a)H2); 7.26 (d, 1 H, C(2b)H, J=3.5); 7.37 (t, 1 H, C(7)H, J=7.1); 7.53 (d, 1 H, C(1b)H, J=3.5); 7.67 (d, 1 H, C(4)H, J=8.4); 7.74 (d, 1 H, C(5)H, J=8.6); 7.78 – 7.85 (m, 2 H, C(6)H, C(3)H); 8.00 (dd, 1 H, C(1)H, J=7.6, J=2.8); 8.35 (d, 1 H, C(8)H, J=7.5); 12.76 (s, 1 H, NH).
4-(3-thioxo-3H-1,2-dithiol-5-yl)phenyl(2-fluoro-9-oxoacridin-10(9H)-yl)acetate (6)
Dark orange crystalline solid. Yield: 71 %, m.p. 216-217 °C. Rf = 0,84. MS, m/z (Irel (%)): 480 [М+H]+ (100), 226 [C14H10FNO – H]+ (10). IR (KBr) ν, сm-1: 3061–2855 (C—H); 1753 (C=Oest.); 1642 (C=Oacridone); 1603, 1485, 1466 (C-Cаr). 1H-NMR (DMSO-d6, δ, ppm, J/ Hz): 5.79 (s, 2 H, CH2); 7.41 (t, 1 H, C(7)H, J = 7.2); 7.47 (d, 2 H, C(1а)H, C(2а)H, J = 8.3); 7.76 – 7.80 (m, 1 H, C(3)H); 7.83 (s, 1 H, C(5а)H); 7.87 – 7.90 (m, 2 H, C(4)H, C(5)H); 7.98-8.03 (m, 4 H, C(1)H, C(6)H, C(3а)H, C(4а)H); 8.36 (d, 1 H, C(8)H, J = 8.0).
This work was financially supported by the Ministry of Education and Science of the Russian Federation.
References
- Kudryavtseva T.N., Bogatyrev K.V., Sysoev P.I., Yar Zar Htun, Klimova L.G. Synthesis and study of antibacterial activity of some fluorosubstituted acridones derivatives // Fluorine notes: online journal.В 2013.В NВ 2В (87),В URL: /public/2013/2_2013/letters/rusindex.htmlВ (UsageВ date 11.04.2014).
- US Pat. 5856347 (1999).
- Vukovic N., Sukdolak S., Solujic S., Milosevic T. Synthesis and antimicrobial evaluation of some novel 2-aminothiazole derivatives of 4-hydroxy-chromene-2-one // Archiv der Pharmazie. 2008. N 8. P. 491 – 496.
- Kleemann A., Engel J. Pharmaceutical Substances. В Stuttgart - New York: Thieme, 2001. 2409 СЃ.
- US Pat. 2013/0259947 A1 (2013).
- Beena, Kumar N., Rohilla R.K., Roy N., Rawat D.S. Synthesis and antibacterial activity evaluation of metronidazole–triazole conjugates // Bioorg. Med. Chem. Let. 2009. N 5. P. 1396–1398.
- Antonov E. A., Zhirov A. A., Kirsanov A. T., Krivolapov Yu. A., Sorokin A. A., Markovich Yu. D., Panfilov A. V. Synthesis of 5-(2-chloroethyl)-4-methylthiazole using the reaction of 5-(2-hydroxyethyl)-4-methylthiazole with phosphorus pentachloride // Pharm. Chem. J. 1999. N 33. P. 658-660.
- Lowe G., Potter B.V. L. Bacteriostatic activity of fluoro-analogues of 5-(2-hydroxyethyl)-4-methylthiazole, a metabolic intermediate in the biosynthesis of thiamine // J. Chem. Soc., Perkin Trans. 1980.N 1. P. 2026-2028.
- Li L., Rossoni G., Sparatore A., Lee L.C., Soldato P. D., Moore P. K. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative // Free Radical Biology & Medicine. 2007. N 5. P. 706–719.
- Kodela R., Chattopadhyay M., Kashfi K. NOSH-Aspirin: A novel nitric oxideв€’hydrogen sulfide-releasing hybrid: a new class of anti-inflammatory pharmaceuticals // Med. Chem. Lett. 2012. N 3. P. 257в€’262.
- Perrino E., Cappelletti G., Tazzari V., Giavini E., Soldato P.D., Sparatore A. New sulfurated derivatives of valproic acid with enhanced histone deacetylase inhibitory activity // Bioorg. Med. Chem. Let. 2008. N 6. P. 1893–1897.
- Milazzo I., Blandino G., Caccamo F., Musumeci R., Nicoletti G., Speciale A. Faropenem, a new oral penem: antibacterial activity against selected anaerobic and fastidious periodontal isolates // Journal of Antimicrobial Chemotherapy. 2003. N 3. P. 721–725.
- Hao J., Chen B., Yao Y., Hossain M., Nagatomo T., Yao H., Kong L., Sun H. Practical access to four stereoisomers of naftidrofuryl and their binding affinity towards 5-hydroxytryptamine 2A receptor // Bioorg. Med. Chem. Let. 2012. N 10. P. 3441–3444.
- Bogatyrev K.V., Kudryavtseva T.N., Bushina L.G. Klimova L.G. Synthesis and antimicrobial activity of some new acridonecarboxylic acids derivatives // Scientific Notes: The online academic journal of Kursk State University. 2013. N 3. Vol 2. URL: http://scientific-notes.ru/pdf/032-018.pdf (date of access 11.04.2014).
Recommended for publication by Prof. A.F. Eleev
Fluorine Notes, 2014, 94, 3-4
