Received: August, 2014
DOI 10.17677/fn20714807.2015.01.02
Fluorine Notes, 2015, 98, 5-6
SYNTHESIS AND PROPERTIES OF HYDROPHOBIZATORS BASED ON ACRYLATES AND METHACRYLATES OF POLYFLUOROALCOXY-1-ALKANOLES
A. I. Rakhimov1,2, O. N. Kutyga1
1Volgograd State Technical University, 400131, Russia, Volgograd, Lenin prospect, 28
e-mail:
organic@vstu.ru
2Institute of Chemical problems of Ecology ANS RF, 400066, Volgograd PO box 127,
e-mail:
rakhimov@sprint-v.com.ru
Abstract: Polyfluoroalkyl ethers of acrylic, methacrylic acids and polymers based on them are obtained. Hydrophobic properties of polymers are studied.
Keywords: Polyfluoroalkyl α-chloroethers, polyfluoroalkoxy ethylacrylates, methacrylates, tissue hydrophobization.
Earlier [1-5] α-chloroethers containing different structural fragments including polyfluoroalkyl substituents have been synthesized. High reactivity of chlorine atom located at α-position to electron-seeking polyfluoroalkyl group is used in the reactions of nucleophilic substitution.
Based on polyfluorinated telomer alccohols α-chloroethers have been obtained, they have been transformed into corresponding monomers using the reaction with potassium salts of acrylic and methacrylic acids:
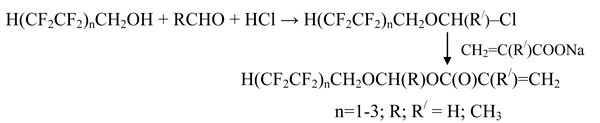
α-Chloroethers based on polyfluorinated alcohols are synthesized in chloroform with the yield of up to 71% [1]. These are easy boiling liquids purified from admixtures by vacuum distillation.
The reaction of potassium salt of acrylic or metacrylic acid is carried out in dry chladone-113 at zero temperature with its further increasing up to 40 °С. After separation of laid-down salt (potassium chloride) filtrate is washed through with 5% solution of potassium bicarbonate, water till reaching the neutral reaction, dried with magnesium sulphate [6]. After isolation of solvent under vacuum the residue is distilled in vacuum. The yield of monomers for metacrylates is about 86,2-91,4 %, and for acrylates it is somewhat lower -– 76,0-85,4 %. Physical and chemical properties of monomers are listed in Table 1.
Table 1. Polyfluoroalcoxy ethylacrylates and Metacrylates, H(CF2CF2)nCH2OCH(CH3)OC(О)C(R)=CH2
|
R |
n |
BP , °С/mm Hg |
n20D |
d20n |
|
Н |
1 |
57-58/3 |
1,3746 |
1,2752 |
|
СН3 |
1 |
57-58/5 |
1,3816 |
1,2343 |
|
Н |
2 |
79-80/5 |
1,3620 |
1,4236 |
|
СН3 |
2 |
71-78/4 |
1,3692 |
1,3712 |
|
Н |
3 |
78-79/4 |
1,3515 |
1,5491 |
|
СН3 |
3 |
82-83/4 |
1,3559 |
1,4798 |
Polymers based on polyfluoroalcoxy ethylacrylates and methacrylates are being obtained by radical polymerization in the block for corresponding monomers at t of 75-80 °С in 1-1,5 hour. Hydrophobizing properties of solutions in chladone 113 have been being studied using glass fibre cloth КТ-II-с 8/3-03-ТО. Glass fibre cloth was being put into the solution of fluoropolymer, and afterwards upon drying the surplus weight and water absorption in % have been determined (Table 2).
Table 2. Hydrophobic Properties of Glass Fibre Cloth Grade KT-II-c 8/3-03-TO Proccessed With Solutions Polyfluoroalcoxy ethylmethacrylates, H(CF2CF2)nCH2OCH(CH3)OC(О)C(СН3)–CH2–.
|
Hydrophobizing (Water-repellent) Compound |
Solution Concentration, % |
Sample Gain Weight, % |
Water Absorption |
|
n = 1 |
2 5 8 10 |
1 1,4 2,2 3,5 |
34,3 25,4 24,8 14,0 |
|
n = 2 |
2 5 8 10 |
1,2 2,4 3,2 4,2 |
24,3 15,7 14,8 14,0 |
|
n = 3 |
2 5 8 10 |
1,3 1,8 2,9 4,5 |
19,1 14,5 13,7 12,5 |
|
non-hydrophobized cloth |
- |
- |
66,1 |
As you can see from Table 2 the hydrophobization of cloth allows lowering the KT-II-c 8/3-03-TO glass fibre cloth’s water absorption more than twice. Hydrophobic properties of glass fibre cloth ЭЗ/I-100 (VII-76) have been studied using solutions of lower concentration (Table 3).
Table 3. Hydrophobic Properties of Glass Fibre Cloth ЭЗ/I-100 (VII-76) Processed With Solutions Of Polymfluoroalkoxy ethylmethacrylates, H(CF2CF2)nCH2OCH(CH3)OC(О)C(СН3)–CH2–.
|
Hydrophobizing (Water-repellent) Compound |
Solution Concentration, % |
Sample Gain Weight, , % |
Water Absorption |
Capillarity, % |
|
n = 1 |
0,5 1 2 3 |
0,3 0,5 0,8 1,3 |
27,4 26,6 25,8 25,2 |
80 75 70 65 |
|
n = 2 |
0,5 1 2 3 |
0,4 0,7 1,2 1,5 |
26,4 25,7 24,3 24,1 |
72 68 65 64 |
|
n = 3 |
0,5 1 2 3 |
0,5 0,7 1,3 1,5 |
25,2 25,4 24,0 23,2 |
70 67 67 65 |
|
unhydrophobized cloth |
|
|
28,9 |
90 |
Tensile strength of glass fibre cloth elemental threads (Table 4) processed with hydrophobizators is very important..
Table 4. Tensile strength of Glass Fibre Cloth Elemental Threads Processed With 0,5 % Solution of Polyfluoroalkoxy ethylmethacrylate At T 105 °С, Time Period – 20 min.
|
Serial # |
Hydrophobized Cloth, Tensile Strength, kg · sec |
Serial # |
Hydrophobized Cloth, Tensile Strength, kg · sec |
|
1 |
0,95 |
1 |
0,75 |
|
2 |
0,99 |
2 |
0,85 |
|
3 |
0,95 |
3 |
0,88 |
| |
ср. 0,96 |
|
ср. 0,82 |
As you can see from Table 4 the elemental threads of glass fibre cloth processed with the solution of polyfluoroalkoxy ethylmethacrylate are of somewhat bigger tensile strength than unhydrophobized cloth.
Thus, polymers based on polyfluoroalkoxy ethylacrylates and methacrylates can be recommended as hydrophobizators.
References
- A. I. Rakhimov, O. N. Kutyga, and A. A. Bakshaeva , Synthesis of α-Chloropolyfluoroalkyl Ethers and Acetals Based on Them, Russian Journal of General Chemistry (Zhurnal Obshchei Khimii) , 2011, vol. 81, No. 3, p. 559-562.
- A. I. Rakhimov, O. N. Kutyga, and A. A. Bakshaeva, Synthesis of Di(1,1,3-trihydroperfluoropropoxy)methane and Di(1,1,3-trihydroperfluoropropoxy)ethane, Russian Journal of General Chemistry (Zhurnal Obshchei Khimii), 2009, Vol. 79, No 11, p. 2452-
- A. I. Rakhimov, Chemistry and Technology of fluoroorganic compounds, Moscow, Khimia, 1986, 389 p.
- A. I. Rakhimov and E. Tikhonova, Russian Journal of General Chemistry (Zhurnal Obshchei Khimii), 1977, Vol. 8, pp. 318-324.
- A. I. Rakhimov and O. S. Bogdanova, Synthesis of Acrylates and Methacrylates Based on tert-Butylperoxy-α-Chloroalkoxyethanes, Russian Journal of General Chemistry (Zhurnal Obshchei Khimii) , 2011, vol. 81, No. 11, p. 2379
- A. I. Rakhimov, O. N. Kutyga, A. V. Miroshnichenko, Nguyen Thi Bik Han, α-HALOGENALKYL ETHERS AND ACETALS BASED ON, Fluorine notes, 2012, Iss. 1(80), /public/2012/1_2012/letters/index.html.
Recommended for publication by Prof. A. Rakhimov
Fluorine Notes, 2015, 98, 5-6
