Received: July, 2011
Fluorine Notes, 2012, 80, 3-4
α-HALOGENALKYL ETHERS AND ACETALS BASED ON 1,1,5-TRIHYDROPERFLUORO PENTANE-1-OL
A. I. Rakhimov, O. N. Kutyga, A. V. Miroshnichenko, Nguyen Thi Bik Han
Volgograd State Technical University, Russia, 400131, Volgograd, Lenin Prosp., 28
e-mail: organic@vstu.ru
Abstract: α-Chloro- and α-bromo- ethers of 1,1,5-trihydroperfluoropentane-1-ol were synthesized by the reaction of H(CF2CF2)2CH2OH with ethanal, propanal, hydrogen chloride or hydrogen bromide. Unlike α-chloroethers α-bromoethers are unstable substances with high reactivity at low temperatures. α-Chloro- and α-bromo- ethers form acetals with alcohols and esters with carboxylic acid salts. Was made a comparison of possible application in organic synthesis of polyfluorinated α-chloro- and α-bromo- ethers.
Keywords: Polyfluorinated alcohol, α-chloro- and α-bromo- ethers of 1,1,5-trihydroperfluoropentane-1-ol, 1,1-di(1',1',5'-trihydroperfluoropentoxy)ethane, 1,1-di(1', 1',5'-trihydroperfluoropentoxy)propane.
The properties of polyfluorinated alcohols (PFA), synthesis and properties of α-chloro ethers, including those of α-chloro polyfluoroalkyl ethers were studied earlier [1-6].
Here the properties of α-chloro- and α-bromo- polyfluoroalkyl ethers and their possibilities of application in organic synthesis are compared by the example of 1,1,5-trihydroperfluoropentane-1-ol.
The solutions of 1,1,5-trihydroperfluoropentanol and acetic or propionic aldehyde in dry chloroform (at equimolar ratio of the reagents) were used in the synthesis of α-chloro-, and α-bromo- polyfluoroalkyl ethers.
To prepare α-chloro ether cooled chloroform solution of acetic aldehyde was added to 1,1,5-trihydroperfluoropentanol solution, and dry hydrogen chloride in 10-fold excess was passed through the resulting mixture during 2-2.5 hours. The reaction scheme was as follows:
CH3CHO + HCI → CH3CH(CI)OH
CH3CH(CI)OH+ H(CF2CF2)2CH2OH →CH3CH(CI)OCH2(CF2CF2)2 H+H2O
At the close of the reaction excess hydrogen chloride was blown off with inert gas flow (nitrogen or air). Water layer was separated, and that of organic was dried with the help of magnesium sulfate. Solvent being removed the residuum was distilled under vacuum. The yield of α-chloro ether was 69%.
The reaction mechanism most likely involves the interaction of polyfluorinated alcohol and aldehyde associates (I) with hydrogen chloride to give six-member transient state (II), transformed further to semiacetal (III), that reacts afterwards with hydrogen chloride resulting in α-chloropolyfluoroalkyl ether:
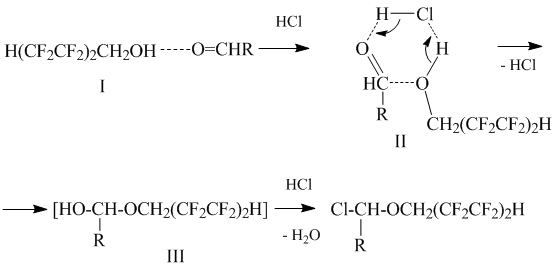
The formation of the transient semiacetal (III) was proved by the tracer method: reaction water molecules proved to contain isotopic oxygen inserted initially into original aldehyde molecules [6].
The yield of 1-chloro-1-(1',1',5'-trihydroperfluoropentoxy)ethane was 69%. It is a colourless easily distilled liquid with boiling temperature 52–53°C at 15 torr. The molecular structure of α-chloropolyfluoroalkyl ether was proved by its 1H NMR and IR spectra. In 1H NMR -spectra of the substance the presence of H(CF2CF2)2CH2-group is revealed by two specific signals of protons that belong to HCF2-group and to CF2CH2-group [7]. The proton of HCF2-group thanks both to near and far interactions with fluorine atoms manifests itself in the form of symmetric triplet of triplets, and δ = 5.72 ppm for the central signal of the central triplet. The spin-spin coupling constants are JH-F = 51.0 Hz and JHC-CF = 5.2 Hz. The spectrum of -CH2-group belongs to ABX2 type (X=F) with δÀ = 4.10 ppm, δB = 3.90 ppm. The signal of -CH-group is a quadruplet at about δ = 5.52–5.36 ppm. JH-H = 6.0 Hz. The protons of CH3-group are manifested by a doublet in strong field with δ = 1.70 ppm and δ = 1.60 ppm.
The resulting α- chloro ether was used in the reaction with 1,1,5- trihydroperfluoropentanol. At the same time obtained acetal – 1,1-di(1',1',5'-trihydroperfluoropentoxy)ethane. The process was conducted in dry hexane at 30°C during 3 hours, hydrogen chloride as released was removed by dry nitrogen flow. The reaction scheme was as follows:
CH3CH(CI)OCH2(CF2CF2)2H + H(CF2CF2)2CH2OH → HCI + CH3CH[OCH2(CF2CF2)2H]2
The yield of acetal was 51%. The said acetal was a colourless high-boiling liquid (boiling temperature 91–92°C at 2 torrs). The acetal structure was proved by its 1H NMR and IR spectra. In the 1H NMR spectra HCF2CF2-group appears as a symmetrical triplet of triplets with similar values of the constants corresponding to HCF2CF2-group in the α- chloropolyfluoroalkyl ether, which are δHCF2 = 5.74 ppm and JH-C = 53 Hz, JHC-CF = 4.4 Hz. The protons of CH2-O-group give a triplet centred at 3.65 ppm and JHC-CF = 14 Hz. The protons of alkyl groups are easily identified by the position of their signal and the character of their spin-spin coupling.
α-Bromoether of 1,1,5-trihydroperfluoropentanol was obtained by the example of propionic aldehyde. The reaction was carried out in a flow of dry compressed air supply of hydrogen bromide to a cooled to a temperature of -12 ÷ -14 °C chloroform solution of 1,1,5- trihydroperfluoropentanol and propionic aldehyde, containing anhydrous sodium sulfate as a desiccant. The reaction is similar to obtaining of α-chloro ether as shown above:
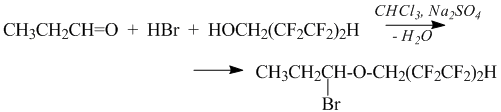
The participation of hydrogen bromide in this process is controlled conveniently by the change in colour: being saturated with hydrogen bromide the reaction mass becomes yellowish, but this colour disappears after smooth warming of the reaction mass to 25°C and keeping it at this temperature for 1-2 hours. However, longer exposition and higher process temperature result in dark colour of the reaction mass due to decomposition of α-bromoether.
High reactivity of α-bromoether at low temperature (-12÷-14°C) was used to substitute bromine by polyfluoroalkoxy–substituent in the synthesis of acetal at the excess presence of polyfluorinated alcohol. The nucleophilic substitution follows the scheme:
CH3CH2CH(Br)OCH2(CF2CF2)2H + H(CF2CF2)2CH2OH → HBr + CH3CH2CH[OCH2(CF2CF2)2H]2
The finish stage of the reaction was carried out at temperature not exceeding 20-25°C. The yield of 1,1-di(1,1,5-trihydroperfluoropentoxy)-propane was 58.3%. The resulting acetal is a transparent colourless liquid (boiling temperature 76°C at 1 torr).
The IR-spectrum of acetal has an intensive band at 1318cm-1 belongs to C-O-C-O-C bond system. A band at 1126 cm-1 belongs to HCF2-group. Strong bands at 3010, 2980, 2920 cm-1 are associated with methyl-, methylene-, and methine-groups correspondingly.
The 1H NMR -spectrum of acetal is characterized by the triplet of triplets with 5.77 ppm, that corresponds to the proton of HCF2CF2-group. Splitting of the triplet is due to spin-spin coupling between the proton and fluorines that belong to group HCF2CF2 : JH-C = 53 Hz and JHC-CF = 4.4 Hz. An intensive triplet at 3.9 ppm is associated with the protons of methylene group in –O-CH2-CF2, and its multiplicity is due to the interaction between protons and fluorines with JHC-CF = 14 Hz. CH3CH2CH-group manifests itself as a triplet at 4.6 ppm, that corresponds to the proton in CH-group. A multiplet at 1.6 ppm and a triplet at 0.9 ppm belong to the protons of the methylene group and methyl-group correspondingly.
The replacement of halogen in α-chloro- and α-bromo- ethers of 1,1,5-trihydroperfluoropentane-1-ol by an ester-group occurs at mild conditions.
α-Bromoether reacts with sodium acetate at the conditions of its formation (the step of ether release is then excluded) at temperature -8÷-11°C. The yield of 1-(1',1',5'-trihydroperfluoropentoxy)-1-acetoxy-propane is 65% (transparent colourless liquid with boiling temperature 64 °C at 1 torr). The reaction follows the scheme:
CH3CH2CH(Br)OCH2(CF2CF2)2H + CH3COONa → NaCI + CH3CH2CH(OC(O)CH3)OCH2(CF2CF2)2H
In the IR-spectrum of 1-(1',1',5'-trihydroperfluoropentoxy)-1-acetoxypropane there is a narrow absorption band at 1804 cm-1, that is typical for stretching vibrations νC=O of an ester group with an electron withdrawing polyfluoroalkyl substituent. CF2 –group absorbs at 1138 cm-1. The absorbance at 3010, 2974, 2920 cm-1 is due to C-H stretching vibrations.
In the 1H NMR -spectrum at 5.82 ppm there is a triplet of triplets typical for earlier discussed HCF2CF2 –group. There is also a similar intensive triplet at 3.9 ppm, that belongs to the protons of methylene group in -O-CH2-CF2.
At 4.76 ppm there is a triplet that corresponds to the proton of the methine group, while the triplet at 0.90 ppm and the singlet at 1.50 ppm is indication that there are protons belonging to methylene and methyl groups.
It has been established that α-chloro ethers (unlike α-bromoethers) react with salts of carbonic acids at more hard conditions. By the example of the reaction between 1-chloro-1-(1',1',5'-trihydroperfluoropentoxy)ethane and sodium acrylate it was shown that the substitution of chlorine by an acrylate-group occurs at 40 °C resulting in the formation of 1-(1',1',5'-trihydroperfluoropentoxy)-1-acryloxy- ethane:
CH3CH(CI)OCH2(CF2CF2)2H + CH2=CHCOONa → NaCI + CH3CH(OC(O)CH=CH2)OCH2(CF2CF2)2H
The monomer thus produced with the yield 80% is a colourless liquid with boiling temperature 79-80°C at pressure 5 torr. This monomer can be used for modifying the polymer compositions to increase wear, light and thermal stability of the obtained materials and to make them hydrophobic.
Experimental
The chemical structure of the produced substances was proved by their IR- and 1H NMR -spectra. IR- spectra of liquid compounds were taken at "Spekord–M82" instrument in thin film and in chloroform solution. 1H NMR–spectra were recorded at Mercury-300 (Varian), operating frequency 300 MHz, internal standard tetramethylsilane, solvent – carbon tetrachloride.
Synthesis of 1-chloro-1-(1',1',5'-trihydroperfluoropentoxy)ethane. 0.20 Moles of acetic aldehyde in 20 ml of chloroform were added to the solution of 0.20 moles of 1,1,5-trihydroperfluoropentanol in 50 ml of dry chloroform, cooled to -5°C. 1.5–2 moles of dry hydrogen chloride were passed through the reaction mixture during 2–2.5 hours, at -5 ÷ +5 °C. As the reaction finished the surplus hydrogen chloride was blown off by dry inert gas flow (nitrogen). The water layer was then separated, and the organic layer was dried with magnesium sulfate. The solvent being removed the residuum was rectified in vacuum. The yield of α-chloro ether was 69% (boiling temperature 52–53°C at 15 torr, nD20=1.3460, d420 =1.4925). Composition as determined, %: C 28.49; H 2.35; Cl 11.98; F 48.00. C7H8OF4Cl. Composition as calculated, %: C28.52; H 2.38; Cl 12.0. F 51.61.
Synthesis of 1,1-di(1',1',5'-trihydroperfluoropentoxy)ethane. 0.05 Moles of 1,1,5-trihydroperfluoropentanol in 20 ml of dry hexane were added dropwise to 0.05 moles of 1-chloro-1-(1',1',5'-trihydroperfluoropentoxy)ethane dissolved in 50ml of dry hexane cooled to 0°C. To remove the released hydrogen chloride dry nitrogen flow was passed through the reaction mixture. The reactor temperature was gradually raised to 30 °C, and the reaction mixture was being stirred during 3 hours. The solvent being removed the residuum was rectified in vacuum. The yield was 51%. The boiling temperature was 91–92°C at 2 torr. nD20=1.3331, d420=1.6011. Composition as determined, %: C 29.32; H 2.01; F 60.71. C12H10O2F16. Composition as calculated, %: C 29.32; H 2.04; F 62.04.
Synthesis of 1-(1',1',5'-trihydroperfluoropentoxy)-1-acetoxy-propane. The reactor was charged with 0.106 moles of sodium sulfate, 90 ml of chloroform, and 0.106 moles of 1,1,5-trihydroperfluoropentanol mixed with 0.106 moles of propionic aldehyde. The reaction mixture was cooled to –12°C. After that 0.106 moles of HBr were added in air flow (the amount was determined by weigh). By the finish of hydrogen bromide delivery the reaction mass colour changed to light yellow. The reaction mixture was kept at temperature –12 ÷ –14 °C during 3 hours. 0.106 Moles of sodium acetate being added the reaction mixture became colourless. The mixture then was stirred for 2 hours at temperature –11 ÷ –8 °C. This being done the reaction mass was filtered from sodium sulfate and sodium bromide, and chloroform was removed. The residuum was rectified under vacuum. The yield was 65%. 1-(1',1',5'-Trihydroperfluoropentoxy)-1-acetoxy-propane is a transparent colourless liquid with boiling temperature 64 °C at 1 torr, nD20 = 1.3745. Composition as determined, %: F 45.19. C10H12O3F8. Composition as calculated, %: F 45.78.
Synthesis of 1-(1',1',5'-trihydroperfluoropentoxy)-1-acryloxyethane. 0.05 Moles of 1-chloro-1-(1',1',5'-trihydroperfluoropentoxy)ethane were added to 40 ml of dry freon-113, and 0.06 moles of sodium acrylate were added under stirring, then temperature was increased to 40°C. After 6 hours of continuous stirring of the reaction mixture the precipitation was removed and the filtrate was washed with 5% solution of sodium bicarbonate, then with water (till neutral reaction), and dried with magnesium sulfate. The solvent being removed the residuum was rectified under vacuum. The yield of acrylate was 80%. Its boiling temperature 79–80 °C at 5 torr, nD20= 1.3620, d420= 1.4236. Composition as determined, %: C 36.29; H 3.03; F 44.24. C10H10O3F8. Composition as calculated, %: C 36.36; H 3.03; F 46.06.
References
- Rakhimov, A.I. Zh.Org.Chem. / A. I. Rakhimov, O. N Kutyga, A. A. Bakshaeva - 2009. vol. 79., Iss. 11. - p. 1026.
- Lovelace, L. Aliphatic fluorinated compounds / L. Lovelace, D. Roach, W. Postelnek - Moscow: I. L. 1961. 345 p.
- Krolevets, A. A. Chemistry of aliphatic organofluorine alcohols / Results of science and technology. A series of organic chemistry. Moscow. 1985. vol. 6. p. 144-154.
- Storozhakova, N. A. Chemistry and Technology of Elementorganic monomers and polymers. / N. A. Storozhakova, V. F. Zheltobryuhov – Volgograd. 1999. p. 105-111.
- Rakhimov, A. I. News of the Volgograd State Technical University. Chemistry and Technology of Elementorganic monomers and polymers / A. I. Rakhimov, A. V. Nalesnaya, O. V. Vostrikova, N. A. Storozhakova - Volgograd. 2004. vol. 1., Iss. 2. - p. 39-41.
- Pokonova, J. V. Halogen ethers. Methods of preparation, properties and application - Moscow: Khimiya, 1966. 339 p.
- Ionin, B. I. NMR spectroscopy in organic chemistry / B. I. Ionin, B. A. Ershov, A. I. Koltsov - Leningrad: Khimiya, 1983. 269 p.
Recommended for publication by Prof. Alexander I. Rakhimov
Fluorine Notes, 2012, 80, 3-4
