Received: May, 2013
Fluorine Notes, 2013, 89, 3-4
Stereoselective protonation adducts perfuoroallylbenzene with N-methylpyrrole
I.Yu. Babkin, O.P. Yudina, S.E. Galan, A.Yu. Lamanow, A.F. Eleev
State Research Institute of Organic Chemistry & Technology
23, Shosse Entuziastov, 111024
Moscow, Russian Federation
e-mail: dir@gosniiokht.ru
Abstract:The reaction of N-methylpyrrole with perftorallylbenzene leads to the formation of products [2 +4]-cycloaddition in a mixture of exo-and endo-isomers. The difference of reactivity of stereoisomers adduct allows to divide and allocate stereoisomers quantified in pure form as a chemical process, and by flash chromatography using silica gel as sorbent spherical high-density (particle size of 15 microns), and as the eluent - chloroform.
Keywords: N-Methylpyrrole, perfluoroallylbenzene, [2+4]-cycloaddition, 5,5,6-trifluoro-6 exo-polyfluoroalkyl- and 5,5,6-trifluoro-6-endo-polyfluoroalkyl-7-azabicyclo[2.2.1]hept-2-enes, flash-chromatography, NMR-spectroscopy 1H, 19F and 13C – spectrum, MS-spectroscopy.
Earlier [1-3], it was shown that pyrrole reacts with fluorinated olefins to form adducts of different structural type. Thus, the interaction of pyrrole with perfluoroallylbenzene is to form the product [2+4]-cycloaddition[4]. The division, formed in the reaction stereo-isomeric mixtures was performed using flash-chromatography.
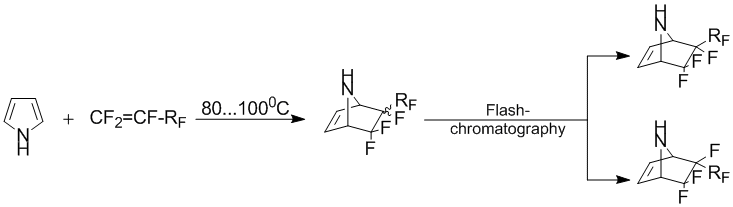
- RF = -CF2CF2H, -CF2CF2Cl, -CF2C6F5
Typically, the presence of the nitrogen atom is not substituted pyrrole cycle can promote the formation of various by-products [1, 4]. In this regard, it was estimated the change of reactivity pyrrole cycle introduction of a methyl group to the nitrogen atom. Thus, the exposure of a mixture of N-methylpyrrole and perfluoroallylbenzene with constant stirring and at a temperature of 80–130°C for 10 days leads only to the formation of the adduct [2 +4]-cycloaddition (1) as a mixture of stereoisomers relatively perfluorobenzylic group.
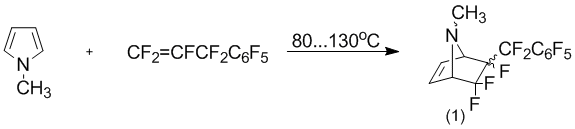
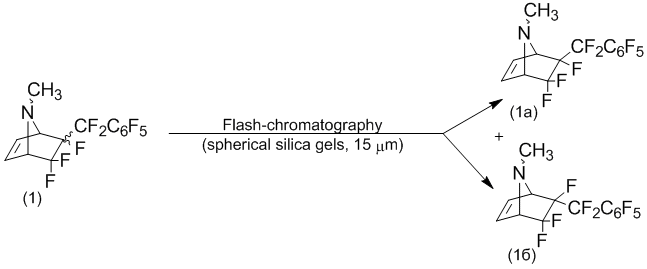
As shown previously [1], the stereo separation isomeric mixture of the compound (1) can be carried out by flash chromatography using as sorbent screened spherical silica of high density with particle size 15В Вµm.
However, it should be noted that the resulting stereo-isomers have significantly different reactivity in the reaction with gaseous hydrogen chloride in ether. Thus, it appears that exogenous perfluorobenzyl- stereoisomer (1a) does not react with hydrogen chloride, while the endo-isomer (1b) in quantitative yield into a hydrochloride. Moreover, the introduction of an excess of hydrogen chloride in ether solution of the compound (1), a mixture of stereoisomers, leads to the formation of HCl only (2) as a white crystalline precipitate. 6-exo-perfluorobenzyl-stereoisomers (1a) remains in solution without changes, so separation of endo-stereoisomer hydrochloride (2) of the exo-stereoisomer, formulation, there is no difficulty.
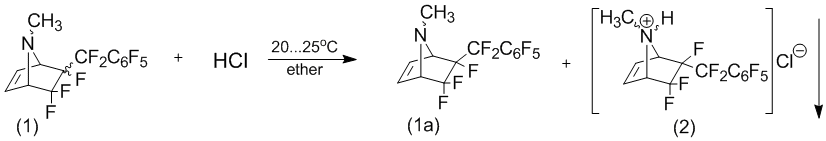
Hydrochloride treatment (2) sodium hydroxide was had 6-endo-perfluoro-N-methyl-5,5,6-trifluoro-7-azabicyclo[2.2.1]hept-2-ene in form base (1b).
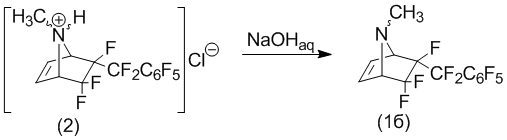
In turn, the interaction of the same structural type of compound, the 6-perfluorobenzyl-5,5,6-trifluoro-7-azabicyclo[2.2.1]hept-2-ene [1] with hydrogen chloride is not stereo-selective.
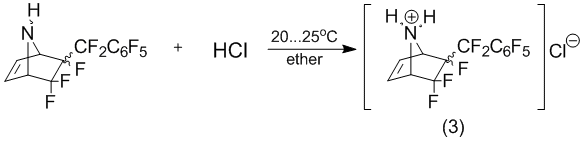
Moreover, the formation of the hydrochloride (3) is not stereo-selective even when added tenth equivalent of hydrogen chloride.
Thus, the introduction of a methyl group at the nitrogen atom of fluorine-containing 7-azabicyclo[2.2.1]hept-2-ene leads to a different reactivity of stereoisomers. For example, the possibility of separating a mixture of isomers (1a) and (1b) treatment with hydrogen chloride.
Experimental
The structure of the synthesized compounds were confirmed by the data of the MS, NMR 1H, 19F, 13C, HSQC Рё COSY-spectra.
NMR 1H, 19F, 13C, HSQC and COSY were recorded with a Agilent DD2 NMR System 600 spectrometer (in DMSO-d6 or CDCl3) by temperature 25В°C on standard methods 1D and 2D experimentation. Chemical shifts are given in parts per million (ppm) relative to the tetramethylsilane (1H, 13C) and trifluoroacetic acid (19F). The following abbreviations are used to describe peak patterns: s (singlet), d (doublet), dd (double doublet), tВ (triplet), m (multiplet), q (quadruplet), and br (broad). Coupling constants are given in hertz.
Mass (m/z, relative intensity) spectra were recorded with detector HP-5975C at a voltage of 70 eV.
N-Methyl-5,5,6-trifluoro-6-perfluorobenzyl-7-azabicyclo[2.2.1]hept-2-enes (1). In the Erlenmeyer flask (250 ml) was added 8,1 g (0,1 mol) N-methylpyrrole and 29,8 g (0,1 mol) perfluoroallylbenzene. The reactions mixture stirred under argons by 90–100°C for 10 day. Reactions mixture was cooled to room temperature. Starting compounds were evaporated under reduced pressure. A crude was dissolved in 50 ml chloroform and filtered by silica gel and washed with 300 ml chloroforms. Solvent was evaporated. The residue contained 26,4 г (69,6 %) N-methyl-5,5,6-trifluoro-6-perfluorobenzyl-7-azabicy-clo[2.2.1]hept-2-ene (1) as a mixture stereo-isomers.
Separation of the stereo-isomers N-methyl-5,5,6-trifluoro-6-perfluorobenzyl-7-azabicyclo[2.2.1]hept-2-ene (1) by flash-chromatography
The isolation of the stereo-isomeric mixture has been on cartridges with 300g silica gels as spherical particles, which has size 15В Ојm (Interchim, PFВ 15SIHP/300G) using dichloromethane as eluent.
25,0В g N-methyl-5,5,6-trifluoro-6-perfluorobenzyl-7-azabicy-clo[2.2.1]hept-2-ene (1) as the mixture of stereo-isomers was dissolved in 25В ml dichloromethane. An analysis fractions has made by GC-chromatography. The first fraction contained 11,2В g 6-endo-perfluorobenzyl-N-methyl-5,5,6-trifluoro-7-azabicyclo[2.2.1]hept-2-ene (1b), m.p.В 89,7В°C.; followed by 6-exo-perfluorobenzyl-N-methyl-5,5,6-trifluoro-7-azabicyclo[2.2.1]hept-2-ene (1a) 13,4В g, m.p.В 77,9В°C.
6-Endo-perfluorobenzyl-N-methyl-5,5,6-trifluoro-7-azabicyclo[2.2.1]hept-2-ene hydrochloride (2) and 6-exo-perfluorobenzyl-N-methyl-5,5,6-trifluoro-7-azabicyclo[2.2.1]hept-2-ene (1a).
20,0В g compounds (1) was dissolved in 200В ml anhydrous ether. By fast stirring to it was added dry hydrogen chloride before not obtained all precipitate. The precipitate was filtered off and washed to ether. Yield 9,8В g (quantitatively) 6-endo-perfluorobenzyl-N-methyl-5,5,6-trifluoro-7-azabicyclo[2.2.1]hept-2-ene hydrochloride (2), m.p.В 167,7В°C. Filtrate was evaporated and dried under reduced pressure by 1В mm for 4В h. Yield 9,3В g 6-exo-perfluorobenzyl-stereomers (1a).
6-Exo-perfluorobenzyl-N-methyl-5,5,6-trifluoro-7-azabicyclo[2.2.1]-hept-2-en (1a):
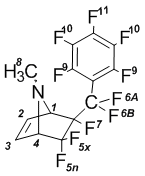
NMR 1H (CDCl3): 2,11В s (8-H, 3H); 3,87В dm (4-H, J5x-4 = 6В Hz, 1H); 4,32В m (1-H, 1H); 6,35В dm (2-H, J2-3 = 6В Hz, 1H); 6,39В dm (3-H, J3-2 = 6В Hz, 1H).
NMR 19F (CDCl3): –113,95 dm (5x-F, J5x-5n = 230 Hz, 1F);  105,57 d (5n-F, J5n-5x = 230 Hz, 1F);  97,85 dd (6A-F, J6A-6B = 283 Hz, 1F); –102,17 dt (6B-F, J6B-6A = 283 Hz, J = 35,2 Hz, 1F);  167,79 m (7-F, 1F);  161,42 t (9-F, J = 18,5 Hz, 2F); –136,48 m (10-F, 2F);  149,57 t (11-F, J = 21 Hz, 1F).
NMR 13C (CDCl3): 32,8 s (8-C); 70,0 d (1-C, J = 25,1 Hz); 72,1 dd (4-C, J’ = 22,4 Hz, J’’ = 26,5 Hz); 118,9 d (CF); 120,7 d (CF); 122,5 d (CF); 131,9 s (3-C); 132,7 s (2-C); 137,1 m (CF); 138,8 m (CF); 141,9 m (CF); 144,8 m (CF); 146,5 m (CF).
MS, m/z (%): 379 (M+, 1); 360 ([M-F]+, 4); 298 ([C9F10]+, 8); 279 ([C9F9]+, 7); 248 ([C8F8]+, 8); 217 ([C7F7]+, 26); 167 ([C6F5]+, 4); 81 (C5H7N+, 100).
6- Endo-perfluorobenzyl-N-methyl-5,5,6-trifluoro-7-azabicyclo[2.2.1]hept-2-en (1b)
A hydrochloride (2) (12,0 g) was added to 100 ml 2N solution sodium hydroxide. Reactions mixture was shaked for 10–15 min and extracted with dichloromethane (4 x 50 ml). The combined organic layers were dried under sodium sulfate. After drying, the solution was concentrated to give the crude 10,5 g (95,9%) 6-endo-perfluorobenzyl-N-methyl-5,5,6-trifluoro-7-azabicyclo[2.2.1]hept-2-ene (1b), m.p. 89,7°C.
6-Endo-perfluorobenzyl-N-methyl-5,5,6-trifluoro-7-azabicyclo[2.2.1]-hept-2-en (1b):
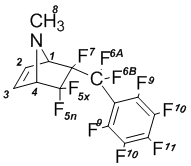
NMR 1H (CDCl3): 2,25В s (8-H, 3H); 3,84В dm (4-H, J5x-4 = 5В Hz, 1H); 3,96В dm (1-H, J1-7 = 4,5В Hz, 1H); 6,39В dm (2-H, J2-3 = 6В Hz, 1H); 6,41В dm (3-H, J3-2 = 6В Hz, 1H).
NMR 19F (CDCl3): –109,8 dm (5x-F, J5x-5n = 228 Hz, 1F); –106,7 d (5n-F, J5n-5x = 228 Hz, 1F);  101,6 ddq (6A-F, J6A-6B = 290 Hz, J = 10,4 Hz, J = 21,0 Hz, 1F); –96,1 dt (6B-F, J6B-6A = 290 Hz, J = 25,2 Hz, 1F); –165,1 m (7-F, 1F);  160,0 t (9-F, 2F); –137,4 m (10-F, 2F);  148,3 t (11-F, J = 20,5 Hz, 1F).
NMR 13C (CDCl3): 33,3 s (8-C); 71,7 dd (1-C, J’ = 22 Hz, J’’ = 27 Hz); 72,5 dd (4-C, J’ = 3 Hz, J’’ = 19 Hz); 121,4 (CF); 123,1 (CF); 131,5 (3-C); 131,8 (2-C); 137,3 d (CF); 139,0 (CF); 142,3 (CF); 144,6 (CF); 146,3 (CF).
MS, m/z (%): 379 (M+,В 1); 360 ([M-F]+,В 2); 298 ([C9F10]+;7); 279 ([C9F9]+; 3); 248В ([C8F8]+; 5); 217В ([C7F7]+; 23); 67 ([C5H7N]+; 100).
5,5,6-Trifluoro-6-perfluorobenzyl-7-azabicyclo[2.2.1]hept-2-ene hydrochloride (3)
5,0В g 5,5,6-trifluoro-6-perfluorobenzyl-7-azabicyclo[2.2.1]hept-2-ene (3) were dissolved in 50В ml anhydrous ether. By fast stirring to it was added dry hydrogen chloride before not obtained all precipitate. The precipitate was filtered off and washed to ether. Yield 5,5В g (quantitatively) as a mixture the stereo isomers of 6-endo-perfluorobenzyl-5,5,6-trifluoro-7-azabicyclo[2.2.1]hept-2-ene hydrochlo-ride (3).
References
- Kobayashi Y., Honda M., Hanzawa Y., Kumsdaki I., J. Chem. Soc., Perkin. Trans. 1, 1970, p. 1743.
- Kobayashi Y., Kumsdaki I., Ohsawa A., Sekine Y., Ando A. Studies on Organic Fluorine Compounds. Part 23. Diels-Alder Reactions 1,2,3,4-Tenrakis(trifluoromethyl)-5-thiabicyclo[2.1.0]pent-2-ene. – J. Chem. Soc., Perkin. Trans. 1, 1977, pp. 2355-2357.
- Barlow M.G., Haszeldine R.N., Hubbard R. Valence-bond Isomer Chemistry. Part II. Diels-Alder Reactions of Hexafluorobicyclo[2.2.0]hexa-2,5-diene. Pyrrole as a diene. J. Chem. Soc. (C), 1971, Vil. 1, pp.90-95
- Babkin I.Yu., Yudina O.P., Galan S.E., Moiseew S.V., Eleev A.F. Adducts of pyrrole with fluorinated olefines. Fluorine Notes, 2013, vol. 3(88), URL: /public/2013/3_2013/letters/letter2.html
Recommended for publication by Prof. A. F. Eleev
Fluorine Notes, 2013, 89, 3-4
