Received: March, 2013
Fluorine Notes, 2013, 88, 3-4
Adducts of pyrrole with fluorinated olefines
Yu. Babkin, O.P. Yudina, S.E. Galan, S.V. Moiseew, A.F. Eleev
State Research Institute of Organic Chemistry & Technology
23, Shosse Entuziastov, 111024
Moscow, Russian Federation
e-mail: dir@gosniiokht.ru
Abstract:In the reaction methyl perfluoroacrylate with pyrrole produced methyl ether of 2,3,3-trifluoro-3-(1H-pyrryl-2’)-propionic acid, as the result additions in α-position of pyrrole. But the reaction pyrroles with terminal fluorolefins gives only compounds [2+4]-cycloadditions, as the mixing exo- and endo-stereoisomers. We did to isolation of stereo-isomers with a help flash-chromatography on spherical particles (size - 15μm) silica gels with chloroform (or dichloromethane) as eluent. In the reaction pyrroles with perfluoroallylbenzene has been tetracyclic compounds: perfluorobenz[1,2-e]-2,2,3-trifluoro-6-azatricyclo-[4.3.0.03,7]nonane-8.
Keywords: Pyrrole, methyltrifluoroacrylate, terminal fluoroelefins, [2+4]-cycloaddition, 5,5,6-trifluoro-6-exo-polyfluoroalkyl- and 5,5,6-trifluoro-6-endo-polyfluoroalkyl-7-azabicyclo-[2.2.1]hept-2-enes, flash-chromatography, NMR-spectroscopy 1H, 19F Рё 13C, HSQC and COSY-spectrum, MS-spectroscopy.
However [1] it was shown that, a reaction pyrrole and polyalkylpyrroles with acrylic acid and it derivatives give ambiguous results: addition acrylates in α-position of pyrroles [2]; double addition in α and α’ positions (by catalysis acids of Lewis) [3]; addition on the atom of nitrogen pyrroles (by basic catalysis) [4] and cycloadditions (catalysis by salts of Os2+) [5].
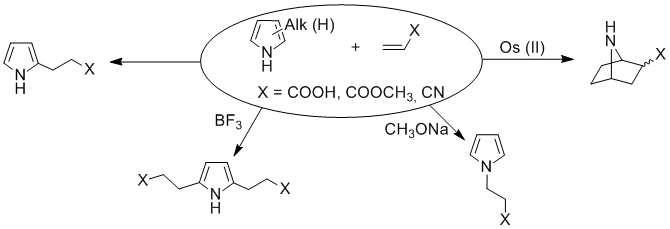
At the same time the research of reactionary ability of fluorinated acrylates, being representatives of functionally substituted for olefins, with an pyrrole which are know only in a few example [1]. The derivatives of pyrrole are presented by the considerable assortment of representatives of bioactive substances of the widest spectrum of action. It’s interestly to estimate possibility of receipt fluorinated adducts pyrroles as accessible precursors of new bioactive substances.
The reaction pyrrole with methyl perfluoroacrylates without solvents by 20В°C going to polymerization in the reaction mixture.
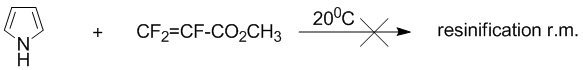
At the same time mixture of pyrrole (1M) and methyl ether of acrylic acidВ (1M) in a chloroform does not change in the interval of temperatures from 20 to 60В°C during a 7 days.
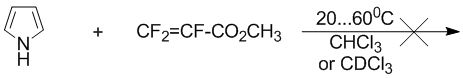
Heating of the same mixture of reagents in a dichloromethane by reflux during a 10 days with subsequent factious distillation of reactionary mass in vacuum allows to get methyl ether of 2,3,3-trifluoro-3-(1H pyrryl 2’)-propionic acid (1), being the product of additions of methyl perfluoroacrylate in α-position of pyrroles.

By changing terminal perfluorolefins (if exchange carboxylic group on perfluoroalkyl group), between pyrroles and olefins has been only compounds (2-4), as products of [2+4]-cycloaddition. So, co-operation of pyrrole with such a perfluorolefins, as 4-hydroperfluorobut-1-ene, 4В chloroheptafluorobut-1-ene and perfluoroallylbenzene, has been only adducts (2-4). Thus, compounds (2-4) appear as mixture of the stereoisomeres relatively perfluoroakyl-groups.
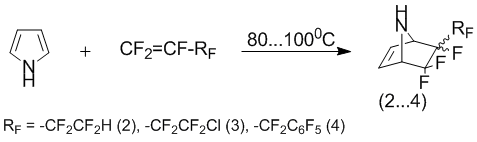
Attempt to highlight the individual adducts (2-4) as stereoisomers by fractional distillation and crystallization of the hydrochlorides has failed. Therefore, experiments were carried out on the selection of separation conditions stereoisomeric compounds (2-4) by flash chromatography. It was found that the use of sorbent spherical silica gel (particle size 15В Ојm) and eluent is chloroform (or dichloromethane), allows to separate quantitatively stereoisomeric mixture of compounds (2-4).
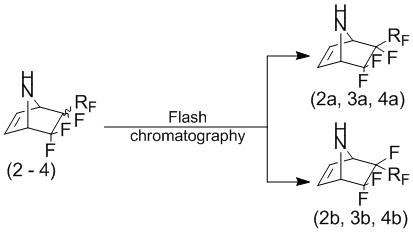
Interestingly, reacting pyrrole with 4-chloroheptafluorobut-1-ene and 4-hydroperfluorobut-1-ene at a higher temperature (130В°C) leads only to an increase in product yield and reduce resinification adducts (2, 3). The ratio of the stereoisomers (relative fluoroalkyl group) did not significantly change.
In the case of heating the mixture of pyrrole and perfluoroallylbenzene at 130°C it begins to apper a mixture of exo-perfluorobenzyl-stereoisomers (4a) and endo- perfluorobenzyl-stereoisomers (4b). After 20 hours, according to date GC-MS, begins conversion of compound (4a) to form product of elimination of one molecule of hydrogen fluoride (the mass of the molecular ion formed connections: 345, and the starting material - 365). Dedicated compounds in the individual form in the NMR 1H – spectra contains an identical compounds (4a), a set of signals, characterized by double bond proton bicyclic structure (6,55 ppm and 6,66 ppm) and “anchor” of protons (4,26 ppm and 4,48 ppm). COSY-spectrum also confirms the spin-spin interaction of the protons of the double bond and nodal protons. However, there is no a signal indicative of the nitrogen atom.
NMR spectra of 13C and HSQC retained the same set of signals characteristic of the carbon structure of the compound (4a).
Thus, we can assume that when heated 6-exo-perfluorobenzyl-5,5,6-trifluoro-7-azabicyclo[2.2.1]hept-2-ene (4a) to 110-130В°C it is intramolecular cyclization with elimination of fluoride hydrogen to form tetracyclic compound (5).
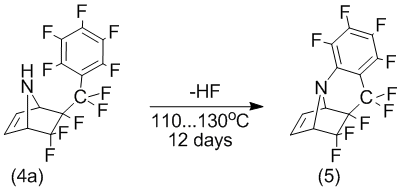
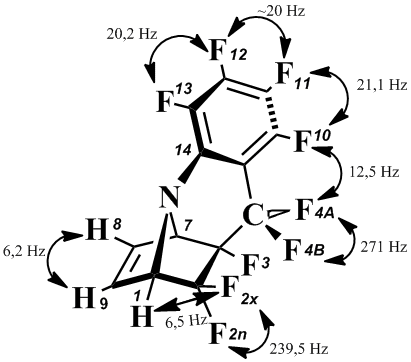
NMR 19F data also confirm the structure of the compound (5). Thus, the intensity of the signal corresponding to the fluorine atom in the ortho-position of the benzene ring is reduced by half, and the nature of the signals of the fluorine atoms in the meta-position is asymmetric:
However the formation of the adducts of fluorinated olefins adduct don’t occur directly to the nitrogen atom of the pyrrole.
Thus, it should be noted that the interaction of pyrrole with fluorinated olefins can both adducts accession in α-position of the pyrrole ring and [2+4]-cycloaddition and “domino”-reaction: adduct formation by reactions [2+4]-cycloaddition, followed by alkylation the resulting bicyclic secondary amine with perfluorobenzyl-group (for example, formation of compoud (5)).
Experimental
The structure of the synthesized compounds were confirmed by the data of the MS, NMR 1H, 19F, 13C, HSQC and COSY-spectra.
NMR 1H, 19F, 13C, HSQC and COSY were recorded with a Agilent DD2 NMR System 600 spectrometer (in DMSO-d6 or CDCl3) by temperature 25В°C on standard methods 1D and 2D experimentation. Chemical shifts are given in parts per million (ppm) relative to the tetramethylsilane (1H, 13C) and trifluoroacetic acid (19F). The following abbreviations are used to describe peak patterns: s (singlet), d (doublet), dd (double doublet), tВ (triplet), m (multiplet), q (quadruplet), and br (broad). Coupling constants are given in hertz.
Mass (m/z, relative intensity) spectra were recorded with detector HP-5975C at a voltage of 70 eV.
Methyl 2,3,3-trifluoro-3-(1H-pyrryl-2’)-propiolate (1)
A solution 6,7В g (0,1В mol) pyrrole and 14,0В g (0,1В mol) methyl ester perfluoroacrylic acids in 100В ml dichloromethane was refluxed for 10В days. The solvent was evaporated, and the residue was purified by vacuum distillation. B.p. В 43В°C/20В mm.
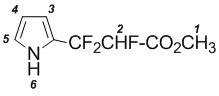
NMR 1H (CDCl3): 3,62В s (1-H, 3H); 6,11В m (4-H, 1H); 6,20В m (3-H, 1H); 6,74В m (5-H, 1H); 8,30В brВ s (6-H, 1H).
MS, m/e (%): 207 (M+,В 1); 187 ([M-HF]+; 100); 155 ([M-HF-OCH3]+ ;36); 129 ([M-HF-CO2CH3]+; 45).
5,5,6-Trifluoro-6-(2’-hydroperfluoroethyl)-7-azabicyclo-[2.2.1]hept-2-ens (2a и 2b)
A mixture of 4-hydroperfluorobut-1-ene (18,2 g; 0,10 mol) and pyrroles (30 g; 0,45 mol) was heated in steel autoclaves (150 ml) at 100°C for 10 days. The autoclave was cooled until 0°C. The reaction mixture has been distilled in vacuum. We received 6,4 g (0,026 mol; 26%) of colorless liquid with b.p. 59-62°C at 2 mm. The separation stereoisomeric mixture has been by flash chromatography on silica gel (cartridge 300g, spherical particles, size - 15μm, INTERCHIM) using dichloromethane as eluent. An analysis fractions has did by GC-chromatography. The first fraction contained 6 exo-(2’-hydroperfluoroethyl)-5,5,6-trifluoro-7-azabicyclo[2.2.1]hept-2-ene (2a) 1,8 g; followed by 6-endo-(2’-hydroperfuoroethyl)-5,5,6-trifluoro-7 azabicyclo[2.2.1]hept-2-ene (2b).
5,5,6-Trifluoro-6-exo-(2’ hydroperfluoroethyl)-7-azabicyclo-[2.2.1]hept-2-ene (2a)
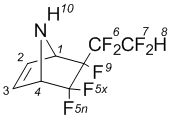
NMRВ 1H (CDCl3): 2,31В brВ s (10-H, 1H); 4,49В dВ m (1-H, 1H); 4,37В m (4-H, 1H); 6,70В dm (2-H, 1H); 6,74В dm (3-H, 1H); 6,87В t (8-H, 1H).
NMRВ 19F (CDCl3): -110,9В dВ qВ d (5x-F, J5x-4=5,9 Hz; J5xВ 5n=228,9; 1F); В 104,71В d (5n-F, J5x-5n=228,9; 1F); В 123,9В ddt (6A-F; J6-7=9,4В Hz; J6AВ 6B=277,7В Hz; 1F); В 124,2В dm (6B-F; J6AВ 6B=277,7В Hz; 1F); В 135,1В dd (7A-F; J7-6=9,4В Hz; J7AВ 7B=299,3В Hz; 1F); В 137,3В ddm (7B-F; J7-6=9,4В Hz; J7AВ 7B=299,3В Hz; 1F); В 169,6В m (9-F, 1F).
NMR13C (CDCl3): 62,3 (1-C; 1C); 64,3 (4-C; 1C); 107,6 (C-F, 1C); 108,6 (C-F; 1C); 109,1 (C-F; 1C); 110,6 (C-F; 1C); 138,1 (3-C; 1C); 139,1 (2-C; 1C).
MS, m/e (%): 249 (M+,В 1); 230 ([M-F]+,В 2); 210 ([M-2HF]+; 2); 198 ([M-CF2H]+; 6); 148 ([M-C2F4H]+; 5); 67 ([C4H5N]+; 100).
5,5,6-Trifluoro-6-endo-(2’- hydroperfluoroethyl)-7-azabicyclo-[2.2.1]hept-2-ene (2b)
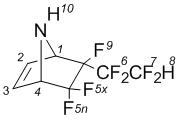
NMRВ 1H (CDCl3): 2,81В brВ s (10-H, 1H); 4,95В dm (1-H, 1H); 5,01В m (4-H, 1H); 6,67В dm (2-H, 1H); 6,74В dm (3-H, 1H); 6,59В t (8-H, 1H).
NMR 19F (CDCl3): –108,7 dqd (5x-F, J5x-5n=235,9 Hz, 1F);  106,60 dq (5n-F, J5x-5n=235,9 Hz, 1F); –122,0 d (6A-F, J6A-6B=289Hz, J6 7=8,1 Hz, 1F); –124,2 dm (6B-F, J6A-6B=289 Hz, J6-7=8,1 Hz, 1F);  137,52 ddm (7A-F, J6-7=8,1 Hz, J7A-7B=305 Hz; J7-8=51 Hz, 1F);  139,3 ddm (7B-F, J6-7=8,1 Hz, J7A-7B=305 Hz; J7-8=51 Hz, 1F); –170,5 m. (9 F, 1F).
NMRВ 13C (CDCl3): 62,8 (1-C; 1C); 64,3 (4-C; 1C); 107,6 (C-F, 1C); 108,7 (C-F; 1C); 109,1 (C-F; 1C); 110,6 (C-F; 1C); 133,5 (3-C; 1C); 134,0 (2-C; 1C).
MS, m/e (%): 230 ([M-F]+,В 1); 210 ([M-2HF]+; 1); 198 ([M-CF2H]+; 2); 148 ([MВ C2F4H]+, 5); 98 ([C5H5FN]+, 9); 67 ([C4H5N]+; 100).
5,5,6-Trifluoro-6-(2’-chloroperfluoroethyl)-7-azabicyclo-[2.2.1]hept-2-enes (3a and 3b)
A mixture of 4-chloroheptafluorobut-1-ene (30,0 g; 0,14 mol) and pyrroles (29,1 g; 0,43 mol) was heated in steel autoclaves (150 ml) at 100°C for 10 days. The autoclave was cooled until 0°C. The reaction mixture has been distilled in vacuum. Has been received 7,1 g 5,5,6-trifluoro-6-(2’-chloroperfluoroethyl)-7-azabicyclo[2.2.1]hept-2-ene of colorless liquid with b.p. 72-74°C at 1,5 mm. The separation stereoisomeric mixture has been by flash chromatography on silica gel (cartridge 300g, spherical particles, size - 15μm, INTERCHIM) using dichloromethane as eluent. An analysis fractions has did by GC-chromatography. The first fraction contained 5,5,6-trifluoro-6-exo-(2’-hydroperfluoroethyl)-7-azabicyclo[2.2.1]hept-2-ene (3a) 4,92 g; followed by 6-endo-(2’-hydroperfuoroethyl)-5,5,6-trifluoro-7-azabicyclo[2.2.1]-hept-2-ene (3b) 9,27 g.
5,5,6-Trifluoro-exo-6-(2'-chloroperfluoroethyl)-7-azabicyclo-[2.2.1]hept-2-ene (3a)
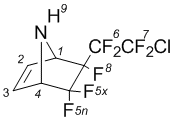
NMRВ 1H (CDCl3): 2,3В brВ s (9-H, 1H); 4,2В dm (4-H, 1H); 4,6В m (1-H, 1H); 6,6В dm (2-H; J2-3=5,5В Hz; 1H); 6,6В dm (3-H; J3-2=5,5В Hz; 1H).
NMRВ 19F (CDCl3): В 110,2В dm (5x-F, J5x-4=6В Hz; J5x-5n=237В Hz; 1F); В 105,9В d (5n-F, J5x-5n=237В Hz; 1F); В 112,2В dm (6A-F; J6AВ 6B=289В Hz; 1F); В 114,4В ddm (6B-F; J6AВ 6B=289В Hz; 1F); В 64,8В dm (7A-F; J7AВ 7B=178В Hz; 1F); В 65,6В dt (7B-F; J7B-6B=21В Hz; J7AВ 7B=178В Hz; 1F); В 164,41В m (8-F, 1F).
NMRВ 13C (CDCl3): 65,8 (4-C; 1C); 66,6 (1-C; 1C); 125,95 (C-F, 1C); 123,8 (C-F; 1C); 114,4 (C-F; 1C); 111,8 (C-F; 1C); 135,2 (2-C; 1C); 136,7 (3-C; 1C).
MS, m/e (%): 283 (M+,В 1); 264 ([M-F]+,В 1); 248 ([M-Cl]+; 1); 198 ([M-CF2Cl]+; 23); 148 ([M-C2F4Cl]+; 9); 67 ([C4H5N]+; 100)
5,5,6-Trifluoro-endo-6-(2'-chloroperfluoroethyl)-7-azabicyclo-[2.2.1]hept-2-ene (3b)
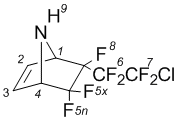
NMRВ 1H (CDCl3): 2,83 brВ s (9-H, 1H); 4,09В dm (4-H, 1H); 4,32В m (1-H, 1H); 6,42В dm (2-H; J2-3=4,8В Hz; 1H); 6,52В dm (3-H; J3-2=4,8В Hz; 1H).
NMR 19F (CDCl3): –109,7 dd (5x-F, J5x-5n=232 Hz, 1F);  105,6 dq (5n-F, J5x-5n=232 Hz, 1F); –111,8 dd (6A-F, J6A-6B=288 Hz, J6A-7A=17 Hz, 1F); –114,99 ddm (6B-F, J6A-6B=288 Hz, J6B-7B=23 Hz, 1F);  66,7 dm (7A F, J7A 7B=179 Hz; 1F);  67,4 ddm (7B-F, J6B-7B=23 Hz, J7A-7B=179 Hz);  168,9 m (8 F, 1F).
NMRВ 13C (CDCl3): 64,2 (4-C; 1C); 66,1 (1-C; 1C); 111,6 (C-F, 1C); 113,3 (C-F; 1C); 123,7 (C-F; 1C); 125,5 (C-F; 1C); 134,0 (2-C; 1C); 135,0 (3-C; 1C).
MS, m/e (%): 264 ([M-F]+,В 1); 248 ([M-Cl]+; 1); 198 ([M-CF2Cl]+; 20); 148 ([M-C2F4Cl]+; 22); 67 ([C4H5N]+; 100).
5,5,6-Trifluoro-6-perfluorobenzyl-7-azabicyclo[2.2.1]hept-2-enes (4a and 4b)
A mixture of perfluoroallylbenzene (29,8В g; 0,1В mol) and pyrroles (6,7В g; 0,1В mol) was heated at 100-110В°C under argon for 5В days.
The resulting mixture was cooled to room temperature. Unreacted ferfluoroallylbenzene and pyrrole has evaporated at 1В mm (room temperature). The residue was purified by flash chromatography on silica gel (cartridge 800g, spherical particles, sizeВ -В 15Ојm, INTERCHIM) using chloroform as eluent. An analysis fractions has did by GC-chromatography. The first fraction contained 4,78В g 6-exo-perfluorobenzyl-5,5,6-trifluoro-6-perfluorobenzyl-7-azabicyclo[2.2.1]hept-2-ene (4a), followed by 9,27В g 5,5,6-trifluoro-6-endo-perfluorobenzyl-7В azabicyclo[2.2.1]-hept-2-ene (4b).
5,5,6-Trifluoro-6-exo-perfluorobenzyl-7-azabicyclo[2.2.1]hept-2-ene (4a); mp 55В°C.
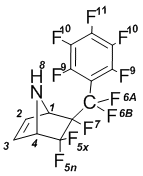
NMRВ 1H (CDCl3): 2,53В brВ s (8-H, 1H); 4,16В dm (4-H, J4-5x = 5,1В Hz, 1H); 4,49В dm (1-H, 1H); 6,57В dm (2-H, J2-3 = 4,5В Hz, 1H); 6,62В dm (3-H, J2-3 = 4,5В Hz, 1H).
NMR 19F (CDCl3): –113,4 dm (5x-F, 1F); –104 d (5n-F, 1F); –99,2 dt (6A-F, 1F);  100,4 dm (6B-F, 1F); –170,0 dq (7-F, 1F); –160,3 t (9-F, 2F); –136,5 dm (10-F, 2F); –148,5 t (11-F, 1F).
NMRВ 13C (CDCl3): 62,4В d (C1H, JВ =В 22В Hz); 64,4В t (C4H, JВ =В 24В Hz); 116,9В d (CF); 118,6В d (CF); 120,2В d (CF); 121,9В d (CF); 123,7В d (CF); 135,5 (CH=CH).
MS, m/e (%): 365 (M+,В 1); 346 ([M-F]+,В 2); 298 ([C9F10]+, 2); 279 ([C9F9]+, 5); 248В ([C8F8]+; 3); 217В ([C7F7]+; 19); 167В ([C6F5]+; 4); 67 ([C4H5N]+; 100).
5,5,6-Trifluoro-6-endo-perfluorobenzyl-7-azabicyclo[2.2.1]hept-2-ene (4b); mp 74В°C.
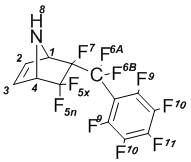
NMRВ 1H (CDCl3): 2,82В brВ s (8-H, 1H); 4,07В dm (4-H, J4-5x = 5,4В Hz, 1H); 4,12В dm (1-H, 1H); 6,56В dm (2-H, 1H); 6,59В dm (3-H, 1H).
NMR 19F (CDCl3): –110,1 dd (5x-F, 1F); –106,2 d (5n-F, 1F);  101,8 ddq (6A-F, 1F); –95,8 dt (6B-F, 1F); –163,96 m (7-F, 1F);  159,9 t (9-F, 2F); –137,4 dm (10-F, 2F); –148,1 t (11-F, 1F).
NMRВ 13C (CDCl3): 64,7В d (C1H, JВ =В 25В Hz); 65,7В d (C4H, JВ =В 23В Hz); 122,3В d (CF); 124,1В d (CF); 125,9В d (CF); 135,5В d (CF); 135,5 (CH=CH), 136,8 (CH=CH).
MS, m/e (%): 365 (M+,В 1); 346 ([M-F]+,В 3); 298 ([C9F10]+;2); 279 ([C9F9]+; 6); 248В ([C8F8]+; 4); 217В ([C7F7]+; 21); 167В ([C6F5]+; 4); 67 ([C4H5N]+; 100). +
Perfluorobenz[1,2-e]-2,2,3-trifluoro-6-azatricyclo-[4.3.0.03,7]nonen-8 (5)
A mixture of perfluoroallylbenzene (29,8В g; 0,1В mol) and pyrroles (6,7В g; 0,1В mol) was heated at 120-135В°C under argon for 12В days.
The resulting mixture was cooled to room temperature. Unreacted ferfluoroallylbenzene and pyrrole has evaporated at 1В mm (room temperature). The residue was purified by flash chromatography on silica gel (cartridge 800g, spherical particles, sizeВ -В 15Ојm, INTERCHIM) using chloroform as eluent. An analysis fractions were made by GC-chromatography. The first fraction contained 10,8В g perfluorobenz[1,2-e]-2,2,3-trifluoro-6-azatricyclo[4.3.0.03,7]nonena-8, followed by 0,77В g 6-exo-perfluorobenzyl-5,5,6-trifluoro-6-perfluorobenzyl-7-azabicyclo[2.2.1]-hept-2-ene (4a) and 9,27В g 5,5,6-trifluoro-6-endo-perfluorobenzyl-7-azabicyclo[2.2.1]-hept-2-ene (4b).
Perfluorobenz[1,2-e]-2,2,3-trifluoro-6-azatricyclo[4.3.0.03,7]-nonene-8 (6); mp 53В°C.
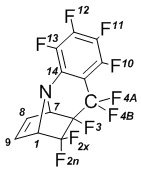
NMRВ 1H (CDCl3): 4,25В dm (1-H, J1-2x = 6,5В Hz, 1H); 4,48В m (7-H, 1H); 6,55В dd (8-H, J8-9 = 6,2В Hz, J7-8 = 2,6В Hz, 1H); 6,67В dd (9-H, J9-1 = 6,2В Hz; J9-8 = 6,2В Hz; 1H).
NMR 19F (CDCl3): –97,6 dd (4A-F, J4A-4B=271 Hz; J10 4A=13 Hz; 1F);  99,8 dt (2X-F, J2х-2n=240 Hz; J2x-1=7 Hz; 1F);  107,7 dd (2n-F, J2х 2n=240 Hz; J=30 Hz; 1F);  120,1 dqd (4B-F, J4A-4B=271 Hz; J=12 Hz; 1F); –139,7 m (11-F, J11-10=21 Hz; 1F); –148,0 dd (10-F, J10 11=21,1 Hz; J10 4A=12,5 Hz; 1F);  148,7 t (12-F; J12-13=20 Hz; 1F);  155,1 t (13-F, J13 12=20 Hz; 1F); –197,6 m (3-F, 1F).
NMRВ 13C (CDCl3): 67,5 (C-7); 70,1 (C-1); 114,2В (Csp3-F); 123,4В (Csp3-F); 128,7 (Csp3-F); 131,5 (C-8); 134,1 (C-9); 139,1В Рґ (Csp2-F); 144,2В (Csp2-F); 146,0 (Csp2-F).
MS, m/e (%): 345 (M+,В 26); 326 ([M-F] +,В 31); 276 ([M-CF3]+;100); 226В ([M-C2F5]+; 21).
References
- I.Yu., Alexeev A.N., Benda A.F., Study pyrrols in reaction with olefines. 5th Youth Scientific School-conference of Organic Chemistry 22-26 april 2002, Ekaterinburg, p.73.
- Jones R.A., Bean G.P. The Chemistry of Pyrroles. A series of monographs: ORGANIC CHEMISTRY, v. 34. Academic Press. 1977, p. 146.
- Trebs A., Michl K.-H. Anlagerung von Acrylsäure und ihren Derivaten an Pyrrole. Annalen der Chemie. B. 589, 1954, p. 163-173.
- Fischer H., Loewe H. Гњber die Anlagerung von Acrylnitril an Pyrrole. Annalen der Chemie.
B.В 615, 1958, p.В 124-136.
- Gonzalez J., Koontz J.I., Hodges L.M., et. al. Osmium-Promoted Dipolal Cycloadditions with Pyrroles: An Efficient, Stereoselective Synthesis of 7-Azanorbornanes. J.В Am. Chem. Soc., 1995, v. 117, pp. 3405- 3421.
Recommended for publication by Prof. A. F. Eleev
В
Fluorine Notes, 2013, 88, 3-4
