Received: December, 2011
Fluorine Notes, 2012, 80, 7-8
The new method of preparing 2,2-bis(trifluoromethyl)propionyl fluoride
V.E. Boyko, A.A. Tyutyunov, S.M. Igoumnov
A.N. Nesmeyanov Institute of Organoelement Compounds Russian Academy of Sciences (INEOS RAS),
Russian Federation, 119991, GSP-1, Moscow, V-334, Vavilova St. 28
"P&M-Invest", Russian
Federation, 119991, Moscow, Vavilova st. 28.
e-mail: boykii@mail.ru
Abstract: The new method of preparing 2,2-bis(trifluoromethyl)propionyl fluoride (1) is suggested.
Keywords: 2-trifluoromethyl-3,3,3-trifluoropropionyl fluoride, 2,2-bis(trifluoromethyl)propionyl fluoride, acyl fluorides.
Earlier it was shown that a-hydrohexafluoroisobutyryl fluoride (2) reacts with methyl iodide in the present of triethylamine in a sealed glass tube or autoclave to give 2,2-bis(trifluoromethyl)propionyl fluoride (1) [1-2]; the formation of ammonium salt 3 as the intermediate of the process has been registered by 19F NMR spectrum [1]:
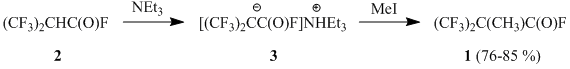
At the same time, acyl fluoride 2 afforded the corresponding cesium salt 4 (similar to 3) under the action of cesium fluoride in diglyme only in the presence of perfluoroisobutene, that performed as an acceptor of hydrogen fluoride [3]. The further alkylation of salt 4 led to the formation of acyl fluoride 1.
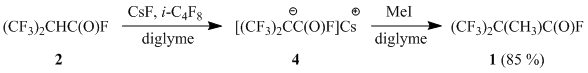
We have found that acyl fluoride 1 can be smoothly obtained by the reaction of acyl fluoride 2 with MeI in acetonitrile in the presence of two equivalents of potassium fluoride. The reaction is performed under atmospheric pressure and can be scaled up easily that allows to consider it as a convenient method of preparing 2,2-bis(trifluoromethyl)propionyl fluoride (1).

Experimental
The 1H and 19F NMR spectra were recorded on a Bruker AM300 spectrometer at 300.13 and 282.4 MHz, respectively, using CDCl3 as external standard. Chemical shifts for 1H spectra were referenced to the residual 1H resonances of the CDCl3 (δ 7.25) and are reported as parts per million relative to tetramethylsilane. Fluorine chemical shifts are reported in ppm relative to CFCl3. Downfield shifts are reported as positive values.
2,2-Bis(trifluoromethyl)propionyl fluoride (1). α-Hydrohexafluoroisobutyryl fluoride (2) (210 g, 1.06 mol), methyl iodide (150 g, 1.06 mol) and KF (123 g, 2.12 mol) were added sequentially to 420 ml of abs. MeCN at -50oC, the reaction mixture was stirred at -50°/30 min, three day at 35-40°C (the reaction was controlled by 19F NMR). When the reaction was completed the reaction product was distilled off and then purified by rectification. 135 g of acyl fluoride (60%) was obtained, b.p. 46-47°C (lit. data: b.p. 47-47.5°C [1]); 1H NMR δ: 2.67 (s, 3 H, CH3); 19F NMR δ: -70.54 (d, 6 F, 4JFF = 11.3 Hz, CF3), 39.18 (m, 1 F, C(O)F).
References
- Yu.A. Cheburkov, M.D. Bargamova, I.L. Knunyants. Russ.Chem.Bull., 1964, 13(2), 339-341
- A.F. Gontar, V.L. Don, E.V. Igoumnova, S.M. Igoumnov. 2,2-Bis(trifluoromethyl)propionic acid. Synthesis and properties. Fluorine notes, 2010, 3(70).
- L.L. Gervits, K.N. Makarov, Yu.A. Cheburkov, I.L. Knunyants. JFC, 1977, 9, 45-52.
Recommended for publication by Prof. Sergei R. Sterlin
Fluorine Notes, 2012, 80, 7-8
