Received: September, 2011
Fluorine Notes, 2011, 78, 9-10
Difluoromethylation of Carbonyl Compounds with (Difluoromethyl)trimethylsilane
A.A. Tyutyunov, V.E. Boyko, S.M. Igoumnov
A.N. Nesmeyanov Institute of Organoelement Compounds Russian Academy of Sciences (INEOS RAS), Russian Federation, 119991, GSP-1, Moscow, V-334, Vavilova St. 28
"P&M-Invest", Vavilova str. 28, Moscow, 119991, Russian Federation
e-mail: tuytuynov@rambler.ru
Abstract: It was found that (difluoromethyl)trimethylsilane (1) could be used as difluoromethylating agent towards different carbonyl compounds in the presence of CsF or Cs2CO3 in DMF solution under mild reaction conditions.
Keywords: (Difluoromethyl)trimethylsilane, difluoromethylation, carbonyl compounds.
The successful application of (trifluoromethyl)trimethylsilane (2) as trifluoromethylating agent [1, 2] gave rise to the further study of fluoroaliphatic silicon derivatives as potential tools for introducing fluoroalkyl substituents into different classes of organic compounds. The difluoromethylsilanes (DFS) such as (difluoromethyl)phenyldimethylsilane [3,4], (difluoromethyl)trimethylsilane (1) [5] or bis(trimethylsilyl)difluoromethane [6] – synthetic analogue of 1 – attracted a particular interest because the compounds bearing CF2H-group were reported to possess remarkable physiological activity [5, 7].
The mechanism of trifluoromethylation by 2 can be depicted as the generation of trifluoromethyl anion under the action of nucleophilic anions of different nature – fluoride, alcoholates, carbonate etc. [1, 2, 8]. Generally speaking difluoromethylation by DFS proceeds in a similar way, but only aldehydes and ketones1 could be involved in this reaction, alkali metal fluorides were the only catalysts used and the reaction conditions appeared to be considerably tougher in comparison with that of trifluoromethylation reactions. The less alkylating activity of DFS is evidently connected with lower electrophilicity of Si-atom bearing CF2H-group than Si-atom connected with more electron withdrawing CF3-group and less anionoid mobility of difluoromethyl group in comparison with trifluoromethyl one.
We have shown recently that silane 2 undergoes reductive defluorination under the action of sodium borohydride to give silane 1 in ~70 % yield [13]. The availability of 1 prompted us to reinvestigate the conditions of difluoromethylation reactions with the participation of this compound as a source of difluoromethyl group.
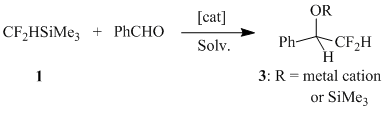
In order to optimize the conditions of the reaction under the question we have studied the reaction of (difluoromethyl)trimethylsilane (1) with benzaldehyde varying the nature of nucleophilic catalyst, solvent and the reaction temperature. The results obtained are summarized in Table 1.
Table 1. Reaction of 1 with benzaldehyde under nucleophilic activation.
Entry |
[cat] |
Solv. |
Temp., °C |
Time,a h |
Yield 3, b % |
1 |
KF |
DMF |
20 |
24 |
< 1 |
2 |
KF |
DMF |
85 |
3 |
57 |
3 |
KF |
DMF |
110 |
3 |
95 |
4 |
KF |
diglyme |
85 |
3 |
0 |
5 |
CsF |
DMF |
20 |
24 |
100 |
6 |
CsF |
MeCN |
20 |
24 |
6 |
7 |
CsF |
MeCN |
55 |
3 |
28 |
8 |
CsF |
diglyme |
20 |
24 |
20 |
9 |
CsF |
diglyme |
55 |
3 |
60 |
10 |
CsF (cat.)c |
DMF |
20 |
24 |
100 |
11 |
K2CO3 (cat.)c |
DMF |
55 |
3 |
0 |
12 |
Cs2CO3 (cat.)c |
DMF |
20 |
24 |
100 |
a not optimized; b determined by 19F NMR; c 10 mol %
It ensued from the data specified in table 1 that the conversion of silane 1 depends not only of the nature of a solvent but to a substantial extent of the nature of metal cation in the composition of nucleophilic catalyst.
The inductivity and solvating ability towards cations and anions are significant characteristics of any solvent. For the solvents used in the given study – MeCN, DMF and diglyme – the values of ε are 37.5, 37.0, 7.2; donor Gutmann’s numbers are 14.1, 26.6, 24.0 and acceptor Gutmann’s numbers are 18.9, 16.0 è 9.9 respectively.
Comparison of solvent characteristics mentioned above and experimental data (table 1) evidently witnesses that the conversion of silane 1 and correspondingly the yield of the reaction product 3 are determined by the ability of the solvent to solvate the cations K+ and Cs+ but not by their polarity. The tougher reaction conditions of silane 1 with benzaldehyde catalyzed with KF indicate that K+ is solvated less effectively than Cs+.
Thus the application of cesium salts as the catalysts and DMF as a solvent that is characterized with high value of donor Gutmann’s number create the optimum conditions for nucleophilic difluoromethylation of carbonyl compounds by silane 1.
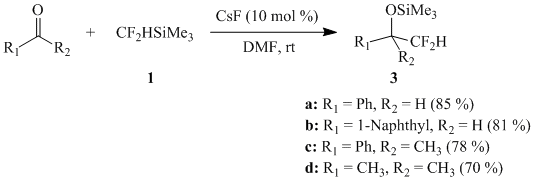
Indeed silane 1 reacts smoothly with a number of aldehydes and ketones in DMF medium under catalysis of CsF (10 mol %) forming the difluoromethylated products 3a-d:
Scheme 1
It appeared unexpectedly that (difluoromethyl)trimethylsilane 1 under catalysis of CsF can be added even to such a weak nucleophile as DMF affording adduct 4 though extremely slow [conversion of 1 ~85 % after the reaction mixture was kept 5 days at ~25 °C (19F NMR)]:
Scheme 2

We failed to involve N-methylpyrrolidone into the reaction with silane 1 even at elevated temperatures (up to 80 °C). At the same time it has been found that the reaction of 1 with DMF accelerated considerably in NMP solution: the conversion of 1 was ~85 % when the reaction mixture was stirred for 24 h/25 °C (19F NMR).
Thus in the given study it was shown that difluoromethylation of carbonyl compounds by silane 1 can be performed under mild reaction conditions; the adducts of 1 with a number of aliphatic and aromatic aldehydes and ketones were synthesized. The results obtained can be used for the preparation of a variety of compounds containing CF2H-group that are attractive from a practical point of view as the potential biologically active substances.
Experimental
The 1H and 19F NMR spectra were recorded on a Bruker AVANCE 300 spectrometer at 300 and 282 MHz, respectively, using D2O as external standard. Chemical shifts for 1H spectra were referenced to the residual 1H resonances of the D2O (δ 4.79) and are reported as parts per million relative to tetramethylsilane. Fluorine chemical shifts are reported in ppm relative to CF3COOH. Downfield shifts are reported as positive values. The boiling points are uncorrected.
The reaction of silane 1 with aldehydes and ketones (typical procedure)
A sample of CsF (1.0 g, 6.58 mmol) was dried in the reaction flask in vacuo at 150 °C/15 min, DMF (20 mL), CF2HSiMe3 (8.2 g, 66.0 mmol) and aldehyde or ketone (66.0 mmol) were added under argon using a syringe, the reaction mixture was stirred at r.t.-re for 1-3 days, poured into water (40 ml), extracted with CH2Cl2 (40 ml), the organic layer was separated, washed with brine, dried over Na2SO4, filtered, the solvent was evaporated and the reaction product was isolated by distillation. The yields and spectral data for the compounds obtained are given below.
1-Trimethylsiloxy-2,2-difluoroethylbenzene (3a)
Yield 85 %; b.p. 96-97 °C (17 Torr); 1H NMR δ: 0.01 (s, 9 H, Si(CH3)3), 4.68 (m, 1 H, CH), 5.52 (td, 1 H, 2JHF = 56 Hz, 3JHH = 4.7 Hz,CF2H), 7.17 (m, 3 H, Ph), 7.31 (m, 2 H, Ph); 19F{1H} NMR δ: -47.7 FA, -49.2 FB (ABq, 2F, 2Jab = 278.8 Hz, CF2H). Found (%): C 57.30; H, 7.12; F, 16.28. C11H16F2OSi. Calcd. (%): C, 57.36; H, 7.00; F, 16.50.
1-Trimethylsiloxy-2,2-difluoroethylnaphthalene (3b)
Yield 81 %; b.p. 112 °C (0.75 Torr); 1H NMR δ: -0.53 (s, 9 H, Si(CH3)3), 5.05 (m, 1 H, CH), 5.39 (td, 1 H, 2JHF = 56 Hz, 3JHH = 4.3 Hz,CF2H), 6.71 (m, 3 H, Naph), 7.04 (m, 2 H, Naph), 7.14 (m, 1H, Naph), 7.62 (m, 1H, Naph); 19F{1H} NMR δ: -46.6 FA, -47.1 FB (ABq, 2F, 2Jab = 277.1 Hz, CF2H). Found (%): C, 64.20; H, 6.31; F, 13.61. C15H18F2OSi. Calcd. (%): C, 64.25; H, 6.47; F, 13.55.
1,1-Difluoro-2-phenyl-2-trimethylsiloxypropane (3c)
Yield 78 %; b.p. 101 °C (12 Torr); 1H NMR δ: 0.04 (s, 9 H, Si(CH3)3), 1.59 (s, 3 H, CH3), 5.44 (t, 1 H, 2JHF = 56.5 Hz, CF2H), 7.16 (m, 3 H, Ph), 7.40 (m, 2 H, Ph); 19F{1H} NMR δ: -50.7 FA, -51.9 FB (ABq, 2F, 2Jab = 270.3 Hz, CF2H). Found (%): C, 58.80; H, 7.35; F, 15.32. C12H18F2OSi. Calcd. (%): C, 58.98; H, 7.42; F, 15.55.
1,1-Difluoro-2-methyl-2-trimethylsiloxypropane (3d)
Yield 70 %; b.p. 130 °C; 1H NMR δ: 0.52 (br s, 9 H, Si(CH3)3), 1.64 (br s, 6 H, CH3), 5.75 (t, 1 H, 2JHF = 57 Hz, CF2H); 19F{1H} NMR δ: -52.8 (s, 2F, CF2H). Found (%): C, 46.45; H, 8.94; F, 20.28. C7H16F2OSi. Calcd. (%): C, 46.12; H, 8.85; F, 20.84.
Dimethyl(1-trimethylsiloxy-2,2-difluoroethyl)amine (4)
Prepared according to typical procedure, but NMP was used as a solvent. Yield 65 %; b.p. 40-41 °C (11 Torr); 1H NMR δ: 0.49 (s, 9 H, Si(CH3)3), 2.63 (s, 6 H, CH3), 4.62 (m, 1 H, CH), 5.97 (td, 1 H, 2JHF = 56 Hz, 3JHH = 4.9 Hz,CF2H); 19F{1H} NMR δ: -47.2 FA, -52.8 FB (ABq, 2F, 2Jab = 285.3 Hz, CF2H).
1 A number of (difluoromethyl)silyl derivatives, that can be prepared by multistage synthesis, show more versatile reactivity as difluoromethylating agents [9-12].
References
- G.K.S. Prakash, A.K. Yudin Chem. Rev., 1997, 97, 757-786.
- R.P. Singh, J.M. Shreeve Tetrahedron, 2000, 56, 7613-7632.
- M. Fujita, T. Hiyama JACS, 1985, 107, 4085-4087.
- T. Hagiwara, T. Fuchikami Synlett, 1995, 717-718.
- J. Hu, W. Zhang, F. Wang Chem. Commun., 2009, 7465-7478.
- A.K. Yudin, G.K.S. Prakash, D. Deffieux, M. Bradley, R. Bau, G.A. Olah JACS, 1997, 119, 1572-1581.
- M.J. Tozer, T.F. Herpin Tetrahedron, 1996, 52, 8619-8683.
- G.P. Stahly, D.R. Bell JOC, 1989, 54, 2873-2877.
- M. Obayashi, K. Kondo Tetrahed. Lett., 1982, 23, 2327-2328.
- G.K.S. Prakash, J. Hu, Y. Wang, G.A. Olah JFC, 2005, 126, 529-534.
- Y.-Y. Qin, X.-L. Qiu, Y.-Y. Yang, W.-D. Meng, F.-L. Qing JOC, 2005, 70, 9040-9043.
- G.K.S. Prakash, J. Hu Acc. Chem. Res., 2007, 40, 921-930.
- A.A. Tyutyunov, V.E. Boyko, S.M. Igoumnov Fluorine notes, 2011, 1(74), (URL: http://notes.fluorine1.ru/public/2011/1_2011/letters/letter2.html).
- V. Broicher, D. Geffken JOMC, 1990, 381, 315-320.
Recommended for publication by Prof. Sergei R. Sterlin
Fluorine Notes, 2011, 78, 9-10
