Fluorine Notes, 2011, 74, 5-6
The Unusual Reaction of (Trifluoromethyl)Trimethylsilane with Sodium Borohydride
A. A. Tyutyunov, V. E. Boyko, S. M. Igoumnov
A. N. Nesmeyanov Institute of Organoelement Compounds Russian Academy of Sciences (INEOS RAS), Russian
Federation, 119991, GSP-1, Moscow, V-334, Vavilova St. 28,
e-mail: tuytuynov@rambler.ru
"P&M-Invest", 28, Vavilova str., Moscow, 119991, Russia
Abstract:It has been established that the reaction of (trifluoromethyl)trimethylsilane (1) with sodium borohydride leads to the formation of (difluoromethyl)trimethylsilane (2) as the main reaction product. Optimal conditions of synthesis 2 have been found, and the reactions of (perfluoralkyl)trimethylsilanes with sodium hydride have been studied
Keywords:(trifluoromethyl)trimethylsilane, sodium borohydride, (difluoromethyl)trimethylsilane
Earlier it was shown that (trifluoromethyl)trimethylsilane enters the reaction with trimethyl borate in the presence of equimolar quantity of potassium fluoride to give potassium (trifluoromethyl)trimetoxyborate (3) in quantitive yield [1-2]. As far as the reaction of (trifluoromethyl)trimethylsilane with four-coordinated borate-anion has resulted in the formation of trifluoromethyl boron derivative it was assumed that the reaction of 1with other borate-anions could lead to the formation of the corresponding trifluoromethyl derivative. Unexpectedly it turned out, that the product of the reaction of 1 with sodium borohydride in THF appeared to be not trifluoromethylated boron derivative (4), but (difluoromethyl)trimethylsilane (2), though the conversion of 1was rather low (10 %, 19F NMR).
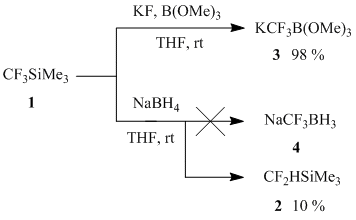
Scheme 1
As far as the direct hydrodefluorination of the trifluoromethyl group in the Ruppert's-Prakash's reagent has not been described earlier, we endeavoured the detailed study of this unusal reaction.
The low conversion of (trifluoromethyl)trimethylsilane (10 %) when the reaction was performed in THF can be explained by very low solubility of sodium borohydride in this solvent. The application of diglyme as a solvent, in which NaBH4 is soluble considerably better (5 g per 100 ml at 25 oC), allows to increase the yield of 2 up to 70 %; the formation of small quantity of FSiMe3 (~5 %) as a by-product is also observed. The solubility of sodium borohydride in DMF is even higher (19 g per 100 ml at 30oC), but under these conditions the reaction is hardly controlled (vigorous exothermic reaction that can result in outburst and even explosion of the reaction mixture), and trifluoromethylation of dimethylformamide is also observed. The products of the reduction of CF3-group haven't been found at all when the reaction was carried out in DMSO whereas the formation of products of trifluoromethylating of dimethylsulfoxide has been registered. When the reaction was conducted in the proton solvent (methanol, water) trifluoromethane was the only reaction product.
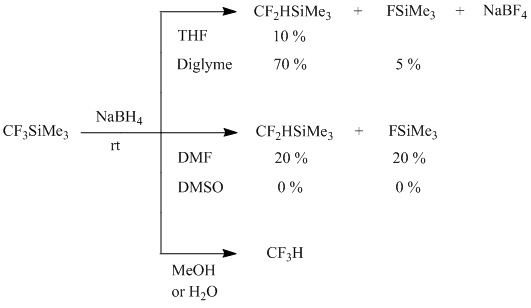
Scheme 2
The complete conversion of 1 can be achieved at mol. ratio 1:NaBH4 = 3:1 that indicates at the participation of three hydride ions in the reduction of 1 by NaBH4. The substitution of KBH4 for NaBH4 results in sharp decrease of the yield of CF2HSiMe3 (up to 5 %), as far as KBH4 is not soluble in diglyme. It should be noted that the reaction of 1 with equimolarquantity of sodium borohydride gives 2 in ~80% yield, but the reaction product isolated from the reaction mixture by distillation inflames in air most probably due to the presence of B2H6 formed as by-product.
We have attempted to involve the higher (perfluoralkyl)trimethylsilanes in the reductive hydrodefluorination by sodium borohydride. Regretfully the reactions of (pentafluoroethyl)- and (n-heptafluoropropyl)trimetylsilanes with sodium borohydride in diglyme at r.t. are characterized by modest conversion of the starting silanes (~30 %), and the formation of perfluoralkylhydrydes has been registered (19F NMR ) as the only reaction products. The reactions conducted at elevated temperature (~100 oC) didn't lead to the significant changes.
Two possible mechanisms of reducing silane 1 with NaBH4 have been considered: the first one assumes the intermediate formation of trimethylsilane and salt 4 - the source of difluorocarbene. The insertion of the latter into Si-H-bond should lead to the formation of the target silane 2.
In order to check this assumption the reaction of 1 with NaBH4 was carried out in the presence of HSiEt3 but the expected HCF2SiEt3 hasn't been detected among the reaction products (1H, 19F NMR). So the concerted mechanism (scheme 3) seems to be most likely.
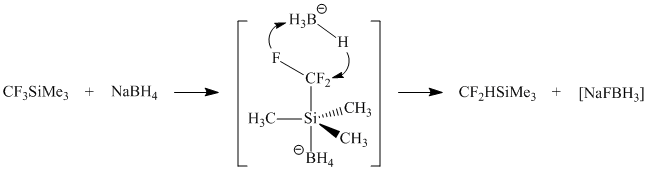
Scheme 3
The driving power of the exchange reaction between silane 1 and NaBH4 is an anionoid mobility of fluorine atom in CF3-group presumably induced by coordination of BH4- with 1.
The suggested method of preparing (difluoromethyl)trimethylsilane 2 is considerably more simple in comparison with the procedures described earlier [3, 4]. It can be readily scaled up that make silane 2 an available reagent.
Experimental
(Difluoromethyl)trimethylsilane 2.Silane 1 (25 g, 176 mmol) is added drop wise to the stirred suspension of sodium borohydride (2.22 g, 59 mmol) in 50 ml of dry diglyme at 20-25oC (the reaction temperature is sustained by external cooling). The reaction products volatile up to ~110 oC are distilled off the reaction mixture.The redistillation of distillate on Vigreux column affords 15.3 g (70 %) of 2, b.p. 65-66 oC; 1H and 19F NMR spectra are identical to that described earlier [4].
Literature
1. A. A. Kolomeitsev et al. TL, 2003, 44, 8273-8277
2. G. A. Molander, B. P. Hoag.
Organometallics, 2003, 22, 3313-3315
3. V. Broicher, D. Geffken. JOMC, 1990,
381, 315-320
4. G. K. S. Prakash, Jinbo Hu, G. A. Olah. JOC, 2003, 68, 4457-4463
Recommended for publication by Prof. Prof. Sergei R. Sterlin
Fluorine Notes, 2011, 74, 5-6
