Received: July, 2010
Fluorine Notes, 2011, 78, 5-6
SYNTHESIS AND PROPERTIES OF 2-HYDRO-1,1,1,2,4,4,5,7,7,8,8- UNDECAFLUORO-5-TRIFLUOROMETHYL-8-SULFONYLFLUORIDE-3,6-DIOXAOCTANE
S.G. Semenov, A.E. Krivoshein, O.M Nesterova, E.I. Cherkas, V.Z. Kaufman, V.V. Kornilov, V.I. Slesareva, S.I.Ozol, D.V. Vinogradov, V.G. Barabanov
FCUE Russian Scientific Center "Applied Chemistry", 197198, Russia, St. Petersburg, Dobrolubov av. 14
e-mail: vbarabanov@rscac.spb.ru
Abstract: It has been proposed the mechanism of formation and have been studied the properties of 2-hydro-1,1,1,2,4,4,5,7,7,8,8-hendecafluoro-5-trifluoromethyl-8-sulfonyfluoride-3,6-dioxaoctane – difficulty separated impurity of industrial monomer-perfluoro (3,6-dioxa-4-methyl-7-octene) sulfonylfluoride.
Keywords:fluoroanhydride, perfluorosulfomonomer, monohydroproduct.
Modern trends of development of science and technology require the wide application of nanotechnology and nanomaterials. The latter include the membrane catalytic polymer systems on basis of perfluorosulformonomers with the prospect of introducing in a number of processes of petrochemical synthesis, as well as in hydrogen energy (electrolysis of water).
One of the most famous and used perfluorosulfomonomers is perfluoro(3,6-dioxa-4-methyl-7octene)sulfonylfluoride(FC-141).
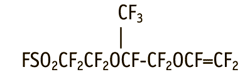
Studying of the mechanisms of formation of this monomer, by-products, their physical and chemical properties promotes the development of methods for synthesis of the high purity monomer and methods for analysis of impurities in the monomer.
The currently used method for the synthesis of the monomer of FC-141 is based on the reaction of fluoroanhydride - 2,5-di(trifluoromethyl)-8-sulfonyl-3,6-dioxaperfluorooctanoylfluoride (FC-161) (I) with a metal carbonate (sodium or potassium) in the presence of a polar aprotonic solvent such as dimethyl ether of diethylene glycol (diglyme) at 20-60°C, followed by pyrolysis of the formed salt (II) at 100-130°C(equation(1))[1].
Carrying out of these processes in the absence of the solvent increases the required pyrolysis temperature up to 200-220°C [2].
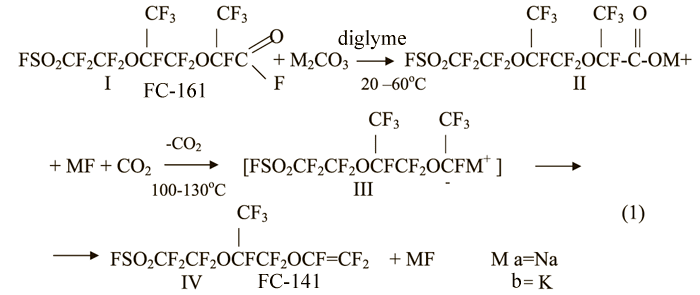
Even the first synthesizes of monomers, including vinyl esters, pyrolysis of the corresponding salts perfluorocarboxylic acids and their derivatives have shown the formation of monohydroproducts as by-products [2], in this case

2-hydro-1,1,1,2,4,4,5,7,7,8,8-hendecafluoro-5-trifluoromethyl-8-sulfonyl-3,6-dioxaoctane (FC-151).
This compound is the result of the reaction of a carboanion complex (III) with water and with a solvent, having in their molecule a mobile hydrogen atom [3,4].
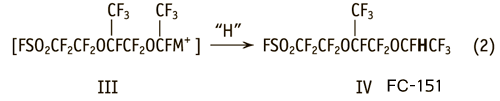
However, above chemical transformations in the reaction mixture (in the case of water) are not completed. We have found that the reduction of the yield of the monomer FC-141 in the presence of water can not be explained only by the formation of hydrogencontaining FC-151.
In order to prove the formation mechanism of these impurities we have synthesized the compound FC-151. For the synthesis of FC-151 avoiding the formation of the perfluorosulfovinyl ether FC-141 in the reactor prepared for the synthesis of the vinyl ester by pyrolysis of fluoroanhydride FC-161 in the presence of sodium carbonate in diglyme was added an excess of water relative to reactants. Indeed, it was managed to evolve the ether FC-151 from the reaction mixture practically without the monomer FC-141.
Investigation of a salt mixture, isolated from the reaction mixture after the separation of FC-151 and other volatile reaction products and solvent (diglyme) showed that besides a sodium fluoride, the reaction residue contained other salt compound. Separated from the sodium fluoride by the extraction with acetone and purified by crystallization the compound was identified by 1H NMR and 19F (Table1).
Table1. Data of IR and NMR 1H and 19F spectra.

Note
1. All samples except the FC-141 were examined in (CD3) 2CO; FC-141 was examined in DCCl3.
2. Double signals in the spectrum 19F in salts of ether of FC-151 (*) indicate the existence of these
salts in the form of two conformers. The presence of this effect and the absence of the formation
of conformers in the transition of FC-141 to the potassium salt of FC-141 are associated with the
characteristic of tetrafluoroethyl group compared with trifluorovinyl group.
Results of the investigation listed in Table 1 prove that, the Na-salt of 2-hydro - 1,1,1,2,4,4,5,7,7,8,8 - hendecafluoro-5-trifluoromethyl-3,6-dioxaoctylsulfonic acid (Na-FC-151) forms in the reaction mixture parallel with the formation of FC-151.
Taking into account the salt formation the reaction of pyrolysis of fluoroanhydride of FC-161 in the presence of water can be described as below equation (3).
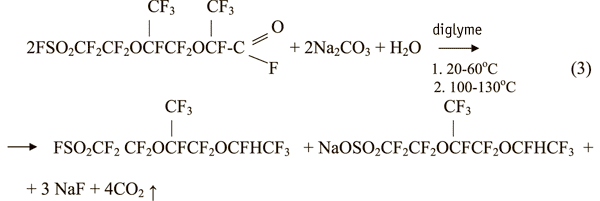
Similar results were obtained in the pyrolysis of fluoroanhydride of FC-161 in the presence of water and potassium carbonate in diglyme.
After the separation from the reaction mixture, ether of FC-151 purified from the traces of diglyme was rectified and the target fraction was separated with a boiling point at 62°C/55 torr. Purity of the ground substance was 99.8% wt. (GLC).
The structure of compound FC-151 is confirmed by the IR and NMR 1H and 19F (see Table 1.).
FC-151 - is a colorless liquid with a slight odor, stable under normal conditions, the density - 1,728 g/cm3 (25°C) and 1,740 g/cm3 (20°C).
Hydrogen-containing impurities and monomers generated during their synthesis by pyrolysis have the close boiling points, therefore, it is difficult to separate them from each other[4] and to produce high-purity monomers.
Because of this, we studied the properties of the compound FC-151 in comparison with the properties of the target monomer FC-141, in particular the dependence of boiling point from pressure (Table 2) and vapor-liquid equilibrium in the system FC-141 - FC-151.
Table2. The dependence of the boiling point from pressure.
|
FС-151 |
FС-141 |
||
|
Pressure, кРа |
Boiling point, °С |
Pressure, кРа |
Boiling point, °С |
|
2.48 |
29.5 |
2.07 |
29.0 |
Regression analysis is presented in Table 3.
Table 3. Regressive analysis.
|
Antuan`s equation |
FС-151 |
FС-141 |
||
|
Coefficents |
Correlation |
Coefficents |
Correlation |
|
|
А=24.083 |
0.9998 |
А=23.822 |
0.99992 |
|
|
А=17.969 |
0.9999 |
А=22.344 |
0.99992 |
Note. Temperature in K and pressure in Pa.
Analysis of the equations shows that the boiling points at 760 mm Hg for FC-151 and FC -141 are 138.0°C and 133.5°C respectively.
Results of the investigation of vapor-liquid equilibrium in the system FC-151 - FC-141 at 150°C are shown in Table. 4 and in Figure 1.
Table 4. Vapor-liquid equilibrium in the system FC-151 - FC-141.

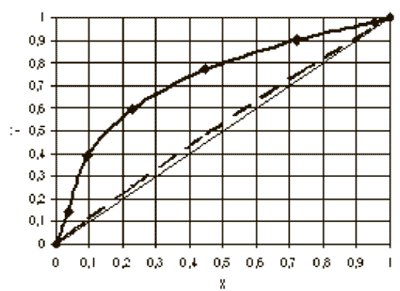
Fig.1. Vapor-liquid equilibrium in the system FC-151 - FC-141.
Note: X and Y - the concentrations of FC-141 in liquid and vapor phases, respectively, the mole fractions.
It is seen from this figure that the curve of VLE (the solid line) differs from the ideal (dashed line), which indicates of the possibility of separating mixtures of these compounds.
The boiling point of FC-151 is above on 4.50 C that the boiling point of FC-141, so it is the main impurity impeding the production of the high-purity monomer FC-141. However, the calculations show that the use of the distillation column with high-efficiency nozzle makes it possible to produce the monomer FC-141 with a purity of 99.95%.
In the laboratory samples of the monomer FC-141 with small amounts of ether FC-151 (up to 5% wt.) can be purified by the azeotropic distillation on less-efficiency columns.
Experimental
1H and 19F NMR spectra were recorded on a Fourier spectrometer "Bruker" at 500
MHz and 471 MHz (for hydrogen and fluorine atoms, respectively).
IR spectra were recorded
on a Fourier transform infrared spectrometer Shimadzu "IR Prestige-21".
Analysis by GLC was carried out on a chromatograph "Tsvet-800" with a thermal conductivity detector on a column 3 m × 3 mm filled Sferohrom-80 (size of particles 0.160-0.200 mm) coated with a stationary phase SKTFT-50-X. Carrier gas - helium, 30 ml/min, temperature 110°C.
Fluoroanhydride FC-161 was synthesized as described in [5]. Monomer of FC-141 was synthesized as described in [1]. Diglyme was dried by vacuum distillation over metallic sodium followed by the treatment with anhydrous zeolite NaA. The residual moisture in diglyme is at least 0.05% by weight. (GLC). Carbonates of sodium and potassium have been dried at a temperature of 350°C for 3 hours.
Synthesis of the FC-151 and its salts
The glass reactor with capacity of 0.25 l, equipped with a stirrer, a thermometer, a dropping funnel under argon was charged with 50 ml of diglyme, 10.6 g (0.1 M) of sodium carbonate 51.2 g (0.1 M) of fluoroanhydride FC-161 was dropped in the reactor. After the cessation of the release of carbon dioxide and holding for 0.5 hours, 2 ml of water was added to the, the dropping funnel was replaced by descending condenser and the temperature in the reactor was raised up to 110-130°C. Under lower pressure the reaction products were collected with a small impurity of diglyme. After separation of diglyme with a separating funnel, product layer was washed with water until the total absence of diglyme (GLC), dried anhydrous magnesium sulfate and rectified.
Target product - the boiling point at 62°/55 Torr with the purity of 99.8% by weight. (GLC).
The
total yield of FC-151 (all fractions) was 21.4 g (91.4% of theory).
The solid residue in the
reactor was treated with acetone to separate sodium fluoride. After evaporation of the extract and
recrystallization the sample of sodium salt of FC-151 (Na-FC-151) 21.1 g (yield 86.8% of theory)
was separated and identified by 1H and 19F NMR spectra.
Similarly a potassium salt of FC-151 was synthesized and characterized.
Investigation of the properties of FC-151
The density of FC-151 was determined by a pycnometer.
The dependence of boiling point from pressure was measured in a glass apparatus consisting of a flask 50 ml, equipped with a thermometer, pressure gauge and vacuum pump a reflux condenser, in which casing was fed a heat-carrier with a temperature of minus 25°C, a thermometer, manometer and vacuum pump. The flask was charged with approximately 25 ml of fluid, the system was evacuated and then the flask was heated to boiling the liquid by observing the boiling point and the corresponding temperature and pressure. Subsequently the pressure in the system was increased and thus changed the boiling point.
The study of equilibrium vapor-liquid system was carried out at a temperature of 150°C, at which the pressure of the mixture was above an atmospheric pressure, which allows you to analyze a vapor mixture by GLC using an injection valve.
For this research it was used a apparatus consisting of a temperature-controlled ampoule of 10 ml equipped with a valve through which the samples were taken for analysis in a injection-valve of chromatograph. An ampoule, a injection-valve and an analytical column were installed in the chromatograph thermostat.
Both components were loaded to the ampoule by weight that allows you to calculate the liquid phase, the ampoule was thermostatically controlled and of samples were taken from it for analysis.
Analysis was carried out on chromatograph "Tsvet-800", with a thermal conductivity detector, column 3
m×2 mm, filled with sorbent "Porapak Q", carrier gas - helium, 20ml/min, thermostat temperature 150°C.
Conclusions
- It has been synthesized 2-hydroxy-1-,1,1,2,4,4,5,7,7,8,8 hendekafluoro-5-trifluoromethyl-8-sulfonyl-3 ,6-dioxaoctane (FC-151),an impurity difficulty separating from the monomer FC-141.
- It has been suggested the mechanism of formation of FC-151 and its derivatives.
- It has been studied the dependence of boiling point from pressure in FC- 151 and FC-141 as well as vapor-liquid equilibrium in the system FC-151 - FC-141.
- It has been shown the possibility of preparing a high-purity monomer FC-141.
References
- Patent Russian Federation 2272806. Method for the manufacture of 5-trifluoromethyl-36-dioxa-8-sulfonylfluorideperluorooctene.
- Gudlitsky M. Chemistry of organic fluorine compounds. M. GNTI KHL, 1961. 239-241.
- Yuminov V.S. Polyfluoroalkykvinyl esters. I. Kinetics and mechanism of formation polyfluoroalkykvinyl ester of sodium perfluoro-2-methyl-3-oxahexanoat sodium. Zh.Org. Khimii., 1995,V. 31, Is. 8, p.1142-1144.
- Yuminov V.S.Polyfluorinated ethers. IV Products of side reactions of synthesis of polyfluoroalkykvinyl esters in the medium of a solvating solvent. Zh.Org.Khimii. 1998, v.34, Is. 12, p. 1786-1792.
- Zatsepina N.N. and Kaufman V.Z., Krivoshein A.E. Barabanov V.G. The method for the manufacture of 2,5-di(trifluoromethyl)-3,6-dioxa-8-(sulfonylfluoride)perfluorooctanoyl fluoride. Fluorine notes, Vol. 3(76) 2011.
Recommended for publication by V. Kornilov
Fluorine Notes, 2011, 78, 5-6
