Received: November, 2010
Fluorine Notes, 2011, 75, 5-6
PREPARATION OF 1,4-DIBROMOTETRAFLUOROBENZENE FROM 4-BROMOTETRAFLUOROBENZENETHIOL AND BROMINE. REACTIONS OF 1,4-DIBROMOTETRAFLUOROBENZENE WITH KSH
P.V. Nikul'shin1, A.M. Maksimov1, V.E. Platonov1, A.I. Lotkov2, L.L. Meisner2
1N.N. Vorozhtsov Novosibirsk Institute of Organic Chemistry of the Siberian Branch of Russian
Academy of Sciences,
Novosibirsk, Russia
e-mail: platonov@nioch.nsc.ru
2Institute of Strength Physics and Materials Science of the Siberian Branch of the Russian
Academy of Sciences (ISPMS SB RAS). Tomsk, Russia
Abstract:A process for preparation 1,4-dibromotetrafluorobenzene by pyrolysis of 4 bromotetrafluorobenzenethiol with bromine at 400°C has been developed and reaction of 1,4-dibromotetrafluorobenzene with KSH has been studied that leads mainly to substitution of bromine atoms by hydrogens.
Keywords:pyrolysis, 1,4-dibromotetrafluorobenzene, KSH, halogenophilic reaction
Brominated polyfluoroarenes are important starting materials for syntheses of the wide range of polyfluoroaromatic compounds containing various functional groups [1,2]. The most available bromopolyfluoroarenes are bromopolyfluorobenzenes usually obtained by bromination of the corresponding polyfluorobenzenes with bromine in 65% oleum. Bromopentafluorobenzene (1) [3] and isomeric dibromotetrafluorobenzenes [4, 5, 6] were synthesized by this process. 1,2,4-Tribromotrifluorobenzene (2) was prepared by bromination of 1,2,4-trifluorobenzene with Br2 in the presence of aluminum [7].
Compounds 1, 1,2-, 1,3- (3), and 1,4-dibromotetrafluorobenzenes (4) can be used for preparation of polyfluorinated polyphenylenes [2]. Perfluorinated polyphenylenes were recommended for applications as lubricants and special coatings that protect technological equipment from radiation and aggressive media [8]. Pentafluorophenylmagnesium bromide synthesized from compound 1 and dibromotetrafluorobenzenes 3 and 4 are used to obtain polyfluorinated polyphenylenes; compounds 3 and 4 are involved in the Ullmann reaction for this purpose [2].
Recently we have developed a method for introducing bromine in polyfluoroarenes by substitution of the thiol group by bromine. Compound 1 is obtained by pyrolysis of pentafluorobenzenethiol (5) in the presence of bromine at 500°C [9]. Other monobromopolyfluoroarenes were obtained in a similar way. It seemed expedient to extend this approach to the synthesis of polybromofluoroarenes. The available 4-bromotetrafluorobenzenethiol (6) [10] was used for the pyrolysis in the presence of bromine to prepare compound 4. Thiol 6 was purified by distillation under vacuum before carrying out the reaction. The distilled thiol 6 (98.5%) contained 4-chlorotetrafluorobenzenethiol (~1%) as the main impurity, this impurity is due to the presence of small amounts of chloropentafluorobenzene in compound 1; thiol 6 containing this impurity was prepared from compound 1 by its reaction with KSH [10]. It turned out that the temperature for carrying out the pyrolysis of thiol 6 in the presence of bromine might be decreased to 400°C instead of 500°C used for the preparation of compound 1and other bromopolyfluoroarenes [9]. When thiol 5 was brominated at 400°C, the substitution of the thiol group by bromine did not run to completion to give a mixture of compound 1and decafluorodiphenyl disulfide in the ratio of 4 : 1. The yield of arene 1 reached 42% [9]. Thiol 6 proved to react with bromine at 400°C to give compound 4 in 69% yield. The scheme for the formation of compound 4 is similar to that proposed in work[9].
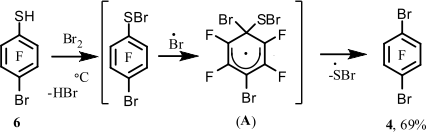
The higher yield of compound 4 in comparison with the yield of arene 1 at 400°C can indicate that the reactivity of thiol 6 is higher than that of thiol 5.
The similar result was obtained in the pyrolysis of an equimolar mixture of thiols 5 and 6 when the deficiency of bromine took place; in this case a mixture of arenes 1 and 4 was formed in the molar ratio of ~1 : 1.8 (according to 19F NMR data).
The higher reactivity of thiol 6 compared to that of thiol 5 under similar conditions might be explained by the higher stability of intermediate radical σ-complex (А) formed from thiol 6 as compared with the stability of σ-complex (B) resulted from thiol 5.
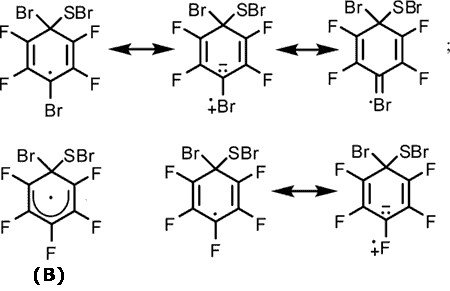
The stability of radical σ-complex (А) appear to increase as a result of involving a bromine atom in delocalization of the unpaired electron. For example, it is well known that the α-chlorine atoms stabilize the radical more effectively than the α-fluorine atoms [11].
Earlier we used 1,4-dichlorotetrafluorobenzene (7) to synthesize 1,2,4-trifluorotrichlorobenzene using transformation of arene 7 into 2,5-dichlorotrifluorobenzenethiol followed by its pyrolysis in the presence of chlorine at ~400°C [12].
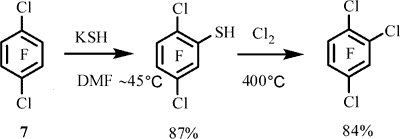
We considered it expedient to use a similar approach to the synthesis of compound 2 from arene 4. However, unlike the reaction of arene 7with KSH, arene 4 reacts with KSH in a complicated manner to give a mixture of various compounds.
The attempt to introduce the thiol group in arene 4 to obtain 2,5-dibromotrifluorobenzenethiol (8) under conditions similar to those used for the similar reaction of arene 7 with KSH [12] have failed. Thiol 8 was not formed (according to 19F and 1H NMR and GC-MS data, Table 1, entry 1). As a result 1-bromo-2,3,5,6-tetrafluorobenzene (9) was predominantly formed in addition to very small amounts of 2,3,5,6-tetrafluorobenzenethiol (10) and traces of thiol 6 and 1,2,4,5-tetrafluorobenzene (11). The formation of well known compounds 6, 9, 10, and 11 was confirmed by 19F and 1H NMR spectra [9,10,13], as well as GC-MS data.
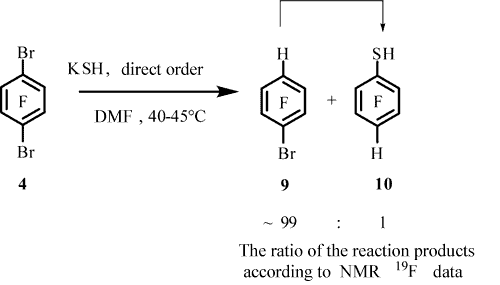
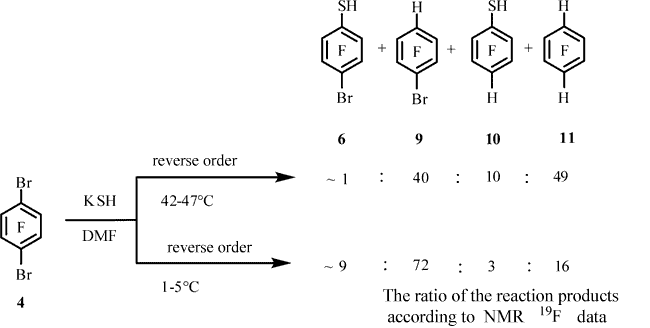
A decrease in the reaction temperature to 1-5°C did not change the main result of the reaction. In this case, in addition to compounds 9, 6, 10, 11,the formation of thiol 8was recorded according to 19F and 1H NMR and GC-MS data. The compound 9 : 6 : 10 : 11: 8 ratio was ~78 : 13 : 3 : 2 : 4 (19F NMR data, Тable 1, entry 2).
Earlier it was shown that thiol 10 was formed in the reaction of compound 9 with с KSH [10].
When the reactants were mixed in the reverse order at 42-47°C, compound 11was predominantly formed in addition to significant amount of compound 9; the resulting mixture also contained thiol 10 and minor amount of thiol 6 (Table 1, entry 3). Compound 9 became the major product of the reaction carried out at 1-5°C, and the content of arene 11 decreased as the content of thiol 6increased; the formation of minor amounts of compounds 10 was observed (Table 1, entry 4).
We can assume that compounds 9 and 11 were formed as a result of halogenophilic reaction [10, 14, 15, 16, 17], while compounds 6 and 10 are the result of nucleophilic reaction of –SH at the C-Br bond of compounds 4and 9 [10, 18].
It was shown that carrying out the reaction of arene 4 with KSH in the presence of azobenzene additive as an inhibitor of the radical chain process [19, 20] at ~45°C in the cases of both direct and reverse order of mixing the reactants did not result in any significant changes in the ratio of products. In the case of direct order of mixing the reactants, the compound 9 : 6 : 10 : 11 ratio was ~91 : 2 : 3 : 4 (Table 1, entry 5), in the case of reverse order of mixing the reactants, the compound 9 : 6 : 10 : 11ratio was ~30 : 1 : 9 : 60, respectively (according to 19F NMR data, Table 1, entry 6); these data might argue for the halogenophilic mechanism of formation of compounds 9 and 11.
Experimental
19F and 1H NMR spectra were recorded on a Bruker AV–300 spectrometer (282.4 MHz for 19F, 300 MHz for 1H) in CCl4 with (CD3)2СO additive. The positive values of chemical shifts correspond the signals in the region downfield from С6F6 and ТМS, respectively; С6F6 and HMDS (0.04 ppm from ТМS) were used as the internal standards. A chromatograph with a mass-selective detector HP G1801A was used for GC–MS. The ionizing electron energy was 70 eV. Separation of substances was carried out on a column of 30 m length and 0.25 мм internal diameter, covered with a HP-5 copolymer film of 0.25 mm thickness; helium was used as a carrier gas with flow rate 1 ml/min, column temperature 50 to 280°С, ion source temperature 173°С. GLC-analysis was carried out on a Hewlett Packard HP 5980 chromatograph fitted with a thermal conductivity detector and a quartz capillary column HP-5 (stationary phase – dimethyldiphenylpolysiloxane), 30 м × 0.52 mm/2.6 mm. Identification of compounds 8, 9, 10, and 11 as well as 1 and 4 was carried out using 19F and 1H NMR and GC-MS analyses of the reaction mixtures.
The starting thiol 6 was prepared using a procedure given in [10] and purified by distillation under vacuum (7 – 8 mm Hg) followed by crystallization from hexane. 19F NMR (δ, ppm): 26.0 m (F2,6), 29.0 m (F3,5). 1H NMR (δ, ppm): 3.98 s (SH) [10 ].
1,4-Dibromotetrafluorobenzene (4). The pyrolysis of thiol 6 in the presence of bromine at 400°C was carried out in a quartz tube (a reactor 400 х 20 mm) heated in an electric tube furnace. The system was blown with argon before bromine and polyfluorobenzenethiol 6 were fed in the reactor under argon (~3 l/h). Thiol 6 (3.87 g, 14.83 mmol) was preliminarily melt and fed simultaneously with Br2 (8.38 g, 52.44 mmol) from separate dropping funnels for 8.3 мин. The products of bromination were collected in a receiving flask cooled with ice water. Afterwards the temperature of the reaction mixture was adjusted to room temperature, and the reaction mixture was treated with a sodium sulfite solution (17.21 g in 70 ml of water) to remove the bromine and then it was steam distilled. A solid product was separated by filtration and dried over CaCl2 to yield 3.16 g of arene 6 of 99% purity (GLC). 19F NMR (d, ppm): 30.6 s [21].
Pyrolysis of a mixture of thiols 5 and 6 in the presence of bromine deficiency.The pyrolysis of a mixture of thiols 5 and 6 in the presence of bromine at 400°C was carried out using a procedure described above. A mixture of thiol 5 (0.98 g, 4.90 mmol) and thiol 6 (1.30 g, 4.98 mmol) was preliminary melt and fed simultaneously with Br2 (0.91 g, 5.69 ммоль) for 2.8 min. The reaction mixture was collected and treated with a sodium sulfite solution (5.10 g in 50 ml of water). The product was separated from water by decantation and dried over CaCl2, as a result 2,14 g of the product was obtained. The molar ratio of arenes 1 and 4was ~1:1.8 (19F NMR). The product also contained decafluoro-, bromononafluoro- and dibromooctafluorodiphenyl disulfides (GC-MS).
Reactions of 1,4-dibromotetrafluorobenzene (4) with potassium hydrosulfide (typical procedure)
In the case of the direct order of mixing the reactants, compound 4 was dissolved in DMF and a solution of KSH in ethylene glycol (~4- 4.2 mol/l) was added to the resulting solution of compound 4 with stirring. In the case of the reverse order of mixing the reactants, compound 4 was dissolved in DMF (5 ml) and the resulting solution was added to a KSH solution diluted with DMF. When the reactants were mixing, spontaneous heating up of the reaction mixture started. If necessary, the temperature of the reaction mixture presented in Table 1 was maintained by placing the flask in a cooling bath. The time of mixing the reactants and the changes in the temperature of the reaction mixture that occur in this case are given in a column titled "mixing the reactants". The reaction mixture was stirred for the period of time that is given in a column titled "exposure". Then the reaction mixture was poured into a mixture of concentrated hydrochloric acid (15 ml) and ice (20-25 g), its temperature was adjusted to room temperature, and the reaction mixture was steam distilled. The organic layer was separated, dried over CaCl2 and analyzed by 19F and 1H NMR, GC-MS, and GLC methods.
Table 1. Reactions of 1,4-dibromotetrafluorobenzene (4) with potassium hydrosulfide
| Entry no. |
Amounts taken for reacting | Time (min)/ Temperature (°C) |
Yield of mixture, g | |||
| Compound 4, g (mmol) | DMF, ml | KSH, ml | mixing the reactants | exposure | ||
| 11 | 1.04 (3.38) | 10 | 1.7 | 5.7/40-45 | 120/42-45 | 0.61 |
| 21 | 1.20 (3.90) | 11 | 2.0 | 5.6/1-5 | 180/1-5 | 0.71 |
| 32 | 1.45 (4.71) | 5+10 | 2.2 | 5.8/42-45 | 135/42-47 | 0.74 |
| 42 | 1.31 (4.26) | 5+9 | 2.0 | 5.7/1-5 | 190/2-5 | 0.70 |
| 51a | 1.08 (3.51) | 10 | 1.8 | 5.1/42-47 | 130/44-47 | 0.65 |
| 62a | 1.10 (3.57) | 4+8 | 1.8 | 5.6/41-48 | 130/46-48 | 0.64 |
1 Direct order of mixing
2 Reverse order of mixing
awith azobenzene
additive (4 : PhN2Ph ~ 1 : 0.4)
2,5–Dibromotrifluorobenzenethiol (8).19F
NMR (δ, ppm): 32.7 dd (F4, JFF4-F3 22 Hz, JF4-F61.7 Hz),
34.1 ddd (F3,JF3-F4 22 Hz,JF3-F6 11 Hz, JF3-H 1.5 Hz),
61.3 ddd (F6, JF6-F3 11 Hz, JF6-H 2.5 Hz,JF6-F4, 1.7
Hz). 1H NMR (δ, ppm): 4.30 dd (SH, JH-F6 2.5 Hz,JH-F3 1.5 Hz). Found: [M]+ 320, 2 bromine atoms. Calculated, M 320.
1-Bromo-2,3,5,6-tetrafluorobenzene (9).19F
NMR (δ, ppm): 24.9 m (F3,5), 29.2 m (F2,6). 1H NMR (δ,
ppm): 7.25 tt (H4, JH-F3,5 9.5 Hz, JH-F2,6 7.5 Hz) [9].
2,3,5,6-Tetrafluorobenzenethiol (10).19F
NMR (δ, ppm): 23.5 m, 24.4 m. 1H NMR (δ, ppm): 3.91 s (SH), 6.92 tt
(H4, JH-F3,5 9.5 Hz, JH-F2,6 7.5 Hz) [10].
1,2,4,5-Tetrafluorobenzene(11).19F
NMR (δ, ppm): 23.5 m. 1H NMR (δ, ppm): 7.15 m [13].
This work was financially supported by the Presidium of the Siberian Branch of the Russian Academy of Sciences (Interdisciplinary Project IP no. 57).
References
- Sintezy ftororganicheskih soedinenij / Pod red. I.L. Knunyantsa, G.G. Yakobsona. M.: Khimiya, 1973. 312 s..
- Ftorpolimery / Pod red. I.L. Knunyanca, V.A. Ponomarenko. M.: Mir, 1975. 448 s.
- Pummer W.J., Wall L.A. Reactions of pentafluorohalobenzenes // J. Res. Nat. Bur. Stand. 1959. V. 63a. № 2. P. 167-169.
- Nield E., Stephens R., Tatlow J.C. Fluorocyclohexanes. Part VI. Some hexa- and pentafluorocyclohexenes and their dehydrofluorination // J. Chem. Soc. 1960.№ 10. P. 3800-3806.
- Burdon J., King D.R., Tatlow J.C. Aromatic polyfluoro compounds–XXXIV.Nucleophilic replacement reactions of some tetrafluorohalogenobenzenes // Tetrahedron. 1966. V. 22. № 8. P. 2541-2549.
- Hellmann M., Bilbo A.J. The preparation of two fluorinated p-dihalobenzenes // J. Am.Chem. Soc. 1953. V. 75. № 18. P. 4590-4591.
- Yakobson G.G., Platonov V.E., Vorozhtsov-jr. N.N. Aromaticheskie ftorproizvodnye. XVI. Poluchenie geksaftorbenzola i poliftorhlorproizvodnyh benzola // ZhOKh. 1965.T. 35. № 7. s. 1158-1161.
- Rahimov A.I. Khimiya i tekhnologiya ftororganicheskih soedinenij. M.: Khimiya, 1986. 271 s.
- Platonov V.E., Maksimov A.M., Dvornikova K.V., Nikul'shin P.V. Ftororganicheskie serosoderzhashchie soedineniya.V. Sopiroliz poliftorarentiolov, -getarentiolov i ih proizvodnyh s chlorom i bromom // ZhOrKh. 2005. T. 41. V. 11. S. 1681–1687.
- Maksimov A.M., Platonov V.E. Reactions of some polyfluoroaromatic compounds with potassium hydrosulfide // Fluorine Notes, 1999. N. 4 .URL:/contents/history/1999/4_1999/letters/index.html (датаобращения: 08.11.2010).
- Nonhibel D., Walton J. Khimiya svobodnyh radikalov. M.: Mir, 1977. 606 s.
- Platonov V.E., Maksimov A.M., Nikul'shin P.V. Sintezy 1,2,4-trihlor-triftorbenzola, 2,4,5-trihlor-3,6-diftorfenola i –tiofenola - bazovyh soedinenij dlya sozdaniya novyh ftorsoderzhashchih pesticidov // II konferenciya «Fundamental'naya nauka v interesah razvitiya khimicheskoj i khimiko-farmacevticheskoj promyshlennosti», Perm' (Russia). 2004. Tezisy dokladov, S. 209.
- Gierczyk B., Wojciechowski G., Brzezinski B., Grech E., Schroeder G. Study of the decarboxylation mechanism of fluorobenzoic acids by strong N-bases // J. Phys. Org. Chem. 2001. V. 14. № 10 P. 691-696.
- Bolton R., Sandall J.P.B. Proto-debromination of some polybromopolyfluorobenzenes // J. Fluor.Chem. 1976. V. 7. № 5. P. 540-542.
- Bolton R., Sandall J.P.B. Nucleophilic displacement in polyhalogenaromatic compounds. Part 2. Kinetics of halogen displacement from bromopolyfluoroaromatic compounds // J. Chem. Soc., PT II. 1976.№ 13. P. 1545-1548.
- Montanari S., Paradisi C., Scorrano G. Thiol anions in nucleophilic aromatic substitution reactions with activated aryl halides. Attack on carbon vs attack on halogen // J. Org. Chem. 1993.V. 58. № 21. P. 5628-5631.
- Zefirov N.S., Makhon'kov D.J. X-Philic reactions // Chem.Rev. 1982. V. 82. № 6. P. 615-624.
- Christopher J.A., Brophy L., Lynn S.M., Miller D.D., Sloan L.A., Sandford G. Synthetic utility of 4-bromo-2,3,5,6-tetrafluoropyridine // J. Fluor.Chem. 2008. V. 129. № 5. P. 447-454.
- Zoltewicz J.A., Oestreich T.M. Heteroaromatic nucleophilic substitution. Inducing a change from an ionic to a radical-chain mechanism with methoxide ion // J. Am.Chem. Soc. 1973. V. 95. № 20. P. 6863-6864.
- Swartz J.E., Bunnett J.F. Reactions of halotoluenes with potassium diphenylphosphide. Evidence for a thermally induced aromatic SRN1 reaction // J. Org.Chem. 1979. V. 44. № 3. P. 340-346.
- Geramita K., Tao Y., Segalman R.A., Tilley T.D. Synthesis and characterization of fluorinated heterofluorene-containing donor–acceptor systems //J. Org.Chem. 2010. V. 75. № 6. P. 1871-1887.
Recommended for publication by Prof. V.E. Platonov
Fluorine Notes, 2011, 75, 5-6
