Fluorine Notes, 1999, 5, 5-6
Reactions of some polyfluoroaromatic compounds with potassium hydrosulfide.A.M Maksimov, V.E. Platonov N.N. Vorozhtsov Novosibirsk Institute of Organic Chemistry, the Russian Academy of Science, Siberian branch 9, Lavrentyeva ave., Novosibirsk 630090, Russia Fax: +7 383 234 4752; e-mail: platonov@nioch,nsc.ru
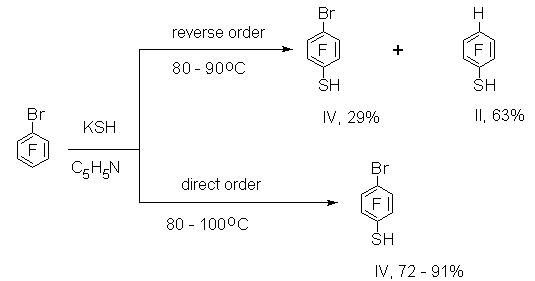
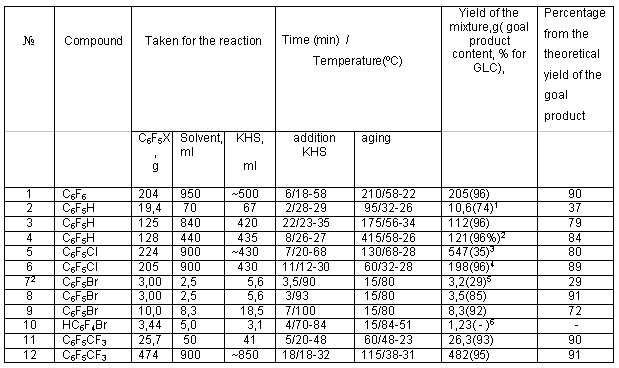
Recently polyfluoroarenethiols have attracted more and more attention for diverse conversions with the purpose to synthesize a wide range of various derivatives of polyfluoroaromatic compounds. For example, polyfluorodiaryldisulfides [1], polyfluoroaryl sulfenyl chlorides [2,3] and polyfluoroaryl sulfonyl chlorides are produced from polyfluoroarenethiols. Interaction of pentafluorothiophenol with haloid alkyls in a medium of DMF results in formation of pentafluorophenyl alkyl sulfides [5]. Reaction of polyfluorinated 4-substituted thiophenols with difluorochloromethane in the presence of alkali gives polyfluoroaryl difluoromethyl sulfides [6]. On the basis of polyfluorocontaining thiophenols, syntheses of series of sulfur-containing polycyclic compounds with thiophene skeleton[7,8] and a carbocyclic compound such as perfluoroindan [9] have been carried out. Coordination compounds of zinc, cadmium and mercury [10] have been produced using polyfluoroarenethiols . Taken into account the significant interest in polyfluoroarenethiols, development of easy and convenient methods to synthesize this type of compounds seemed expedient. It is well known that interaction of hexafluorobenzene with NaSH solution at boiling in pyridine gives pentafluorothiophenol (I) in 66% yield [1]. Thiophenol I [11] is produced in 69-72% yield from C6F6 and KSH in absolute pyridine at 70oC. In this case KSH solution is prepared by passing hydrogen sulfide into KOH solution in anhydrous ethylene glycol. A solution of C6F6 in absolute pyridine is added at heating to the KSH prepared in such a way (reverse order of mixing). At comparison of [1] and [11] a necessity to use anhydrous solvents in the latter remains unclear. In order to simplify the procedures of synthesis of polyfluorothiophenols, we have studied reactions of some polyfluoroaromatic compounds with KSH using commercial non-dried solvents (ethylene glycol, pyridine). The solution of potassium hydrosulfide was prepared by passing hydrogen sulfide into a solution of KOH in ethylene glycol. In some cases we tried to replace pyridine with more available DMF. It was shown earlier that thiophenol I [12] was also produced in 77% yield at boiling of C6F6, NaSH in DMF. We managed to carry out the interaction of hexafluorobenzene with KSH in DMF at more mild conditions at a temperature of 58oC and less. In this case thiophenol I is produced in a yield up to 90% ( table1, exp.1) In the case of pentafluorobenzene at the temperature of 32oC and less the reaction does not run to completion and the starting compound is present in the reaction mixture (table1, exp.2). A better result is attained at increasing the reaction mixture temperature up to 58oC. Here the content of 2,3,5,6-tetrafluorothiophenol (II) in the mixture is 95-96% and the yield reaches 79-84% (table1,exp.3-4) Interaction of chloropentafluorobenzene and a number of other polyfluorinated compounds with NaSH was described in patent [12]. The reaction of chloropentafluorobenzene with KSH in DMF at a temperature of 20-68oC resulted in formation of a heterophase reaction mass difficult to separate. We managed to separate the organic layer only after CCl4 addition to the mixture (table 1, exp.5). At the same time a decrease in the temperature to 32oC allowed to produce a reaction mixture almost without liquid phase ( table1, exp.6) and it was easy to separate 4-chloro-2,3,5,6-tetrafluorothiophenol (III). The reverse order of mixing the reagents in the reaction of bromopentafluorobenzene with KSH resulted mainly in the formation of tetrafluorothiophenol II (table1,exp.7). In this connection it seemed expedient to us to try to change the reaction conditions in such a way that the main reaction product would be 4-bromo-2,3,5,6-tetrathiophenol (IV). Indeed, the change of mixing order led to the formation of thiophenol IV mainly (table 1, exp.8-9).
At the same time the reaction of 1-bromo-2,3,5,6-tetrafluorobenzene with KSH (direct order of mixing reagents) does not lead to the formation of bromo-containing mercaptoderivative. According to the data of NMR 19F spectrum, the reaction mixture contains mainly 1,2,4,5-tetrafluorobenzene together with compound (II) (table 1, exp.10).
The process of the bromine atom replacement with hydrogen at interaction of polyfluoroaromatic compounds with KSH takes place probably as a result of halogenophilic reaction [13,14,15,16]. Paper[17] describes the reaction of octafluorotoluene (V) with NaSH in a pyridine medium at 70 њ C, here the yield of 2,3,5,6-tetrafluoro-4-trifluoromethyl-thiophenol (VI) is 52%. We have carried out the reaction of V with KSH. Here ethanol was used instead of pyridine that allowed carrying out the process at room temperature. The yield of thiophenol VI attained 90-91%. The boiling and melting temperatures and data of NMR spectra of the 4-substituted tetrafluorothiophenols produced are given in table 2. Assignment of signals was made on the basis of the analysis of the thin structure of the signals and calculations according to the additional scheme of substitutes. The data on the influence of substitutes were taken from paper [18]. Experimental. 19F and 1H NMR spectra were recorded on a " Varian A56/60A" instrument at 56.4 and 60 Hz respectively. Solutions in CCl4 and CDCl3 (~10mol%) were used to record the spectra of individual compounds on a Bruker WP-200SY instrument. C6F6 and GMS were used as standards. Analyses by GLC method were carried out on a LHM-72 instrument with a thermal conductivity detector with linear temperature programming of 10oC/min. Columns of 4000x4mm (chromosorb W as a solid support), silicones as liquid phase: a) SKTFT-50;b)SKTFV-803; c)E-301;d) QF-1. The ratio of the stationary phase to the solid support was 15:100,a temperature of the columns of 50-250oC, detector temperature of 250oC, helium consumption of 10mL/min.
Interaction of polyfluoroaromatic compounds with potassium hydrosulfide. (typical experiment) Polyfluorothiophenols were synthesized in the following way: a specimen of a polyfluoroaromatic compound was dissolved in a certain amount of the solvent (the data on a specific experiment are given in table1) and to this solution at mixing a fixed amount of KSH solution was added (KSH solution was produced by passing hydrogen sulfide in a solution of 2 moles of KOH in 350 ml of ethylene glycol to saturation, the solution concentration was ~4-4.2 mol/L). At the same time the reaction mixture started to spontaneously warm up. The temperature of the reaction mixture stated in table 1 was maintained by placing a flask into a cooling bath if necessary. Duration of KSH solution addition and the change in the reaction mass temperature for this time are indicated in "KSH addition" column. Further the reaction mass was being mixed during the time indicated in the "aging" column, here the reaction mass temperature either remained the same or continued to grow spontaneously and reached the maximum value indicated in the same column. The reaction mass temperature was kept the same for 3-5 min and went down then spontaneously. In experiments using the cooling bath, it was removed at the moment of the start of the temperature drop and the reaction mass was kept stirring in air. The value of the temperature range is indicated in the "aging" column. Then the mixture was poured out into a triple volume of ~20% HCl. The organic layer was separated, dried with CaCl2 and analyzed by GLC method. The product was purified either by distillation or by sublimation (solids) under vacuum of ~12-14 Torr. Experiments 1-6 and 10 were carried out in DMF, experiments 7-9 in pyridine, experiments 11 and 12 in ethanol. Table1. Reactions of polyfluoroaromatic compounds with potassium hydrosulfide.
1. the mixture contains also 24% of the starting compound 2. reverse mixing order 3. the reaction mixture contains 61%CCl4 4. 11g of the liquid phase (containing 44% of the goal product according to GLC) is separated from the reaction mixture as well 5. the reaction mixture contains 44% of compound II according to GLC 6. According to the NMR data the mixture contains 1,2,4,5-tetrafluorobenzene, the starting compound and thiophenol II at a ratio of ~2.5:2:1.
Table2. Boiling [melting] points and data of NMR spectra
(
1 2. According to the GLC data , the main content is 97-98% 3. Acknowledgment. We thank Dr. P.P Rodionov for his experimental assistance. References 1. P.Robson, M.Stacey, R.Stephens, J.C.Tatlow, J. Chem. Soc., 1960, 4754. 2. P.Sartory, A.Golloch, Ber., Bd. 103, 1970, S. 3936. 3. R.J.Neil, M.E.Peach, H.G.Spinney, Inorg. Nucl. Chem. Letters, v. 6, 1970, 509. 4. P.Robson, T.Smith, R.Stephens, J.C.Tatlow, J. Chem. Soc., 1963, 3692. 5. R.T.Wragg, Tetrahedron Lett., 1971, No. 27, 2475. 6. A.M.Maksimov, V.V.Kireenkov, V.E.Platonov, Russ. Chem. Bull., v.45, 1996, 153. 7. G.M.Brooke, Md. Abul Quasem, J. Chem. Soc., C, 1967, 865. 8. V.E.PLatonov, A.M.Maksimov, P.I.Maslovsky, J. Fluor. Chem., v. 75, 1995, 41. 9. N.G.Malyuta, V.E.Platonov, G.G.Furin, G.G.Yakobson, Tetrahedron, v. 31, 1975, 1201. 10. S.V.Larionov, V.N.Kirichenko, V.E.Platonov, A.M.Maksimov, P.P.Rodionov, V.P.Fadeeva, I.M.Oglezneva, V.I.Lisoivan, Zh. Neorg. Khim., v. 37, 1992, 2031. 11. Syntheses of Fluoroorganic Compounds. Ed. I.L.Knunyants, G.G.Yakobson, Moskow, "Chimia", 1973, 188. 12. Neth. Appl., 6605036, 17.10.1966; Ch. A., 1967, v. 66, P65492u. 13. R.Bolton, J.P.B.Sandall, J. Fluor. Chem., v. 7, 1976, 540. 14. R.Bolton, J.P.B.Sandall, J. Chem. Soc., PTII, 1976, 1545. 15. S.Montanari, C.Paradisi, G.Scorrano, J. Org. Chem., v. 58, 1993, 5628. 16. N.S.Zefirov, D.J.Makhon'kov, Chem. Rev., v. 82, 1982, 615. 17. D.J.Alsop, J.Burdon, J.C.Tatlow, J. Chem. Soc., 1962, 1801. 18. L.N.Pushkina, A.P.Stepanov, V.S.Zhukov, A.D.Naumov, Org. Magn. Resonance, v. 4, 1972, 607. |
Fluorine Notes, 1999, 5, 5-6