Fluorine Notes, 2010, 73, 9-10
Fluorine notes, Vol. 6(73) 2010
Reactions of polyfluoroarenethiols with electrophiles. Synthesis and some transformations of allyl polyfluoroaryl sulfides and -sulfones
(Oral В report at the VIIIth Regular Russian-German-Ukrainian Symposium on Fluorine Chemistry)
R. A. Bredikhin, A. M. Maksimov, V. E. Platonov
N.N. Vorozhtsov Novosibirsk Institute of Organic Chemistry of the Siberian Branch of Russian Academy of Sciences,
Lavrentjev Ave. 9, Novosibirsk, 630090, Russia, E-mail: bred@nioch.nsc.ru
Abstract:
Allyl and benzyl polyfluoroaryl sulfides are produced through the interaction of polyfluoroarenethiols with allyl chloride or bromide or benzyl chloride. The reactions of polyfluoroarenethiols with trihalomethanes result in the formation of dihalomethy lpolyfluoroaryl sulfides, hydropolyfluoroarenes, and ortoformates depending on the nature of polyfluoroarenethiol substituents and that of the halogen in trihalomethane.
Polyfluoroarenesulfonyl bromides are produced from polyfluoroarenethiols under the action of bromine and nitric acid. The reactions of polyfluoroarenesulfonyl bromides with allyl chloride result in the products of adding by the double bond, while their reactions with allyl bromide result in allyl polyfluoroaryl sulfones.
Keywords: polyfluoroarenethiols, polyfluoroarenesulfonyl bromides, allyl polyfluoroaryl sulfides, benzyl polyfluoroaryl sulfides, allyl polyfluoroaryl sulfones, haloforms, allyl chloride, allyl bromide, benzyl chloride, dichlorofluoromethane, dichloromethane, trichloromethane, tribromomethane, Br2, HNO3
Polyfluoroarenethiols have long been known and studied in a number of works. However, their chemical properties are still of interest. Polyfluoroarenethiols are produced through the interaction of polyfluoroarenes with hydrosulfide of an alkali metal - sodium or potassium [1, 2]. We investigate the reactions of polyfluoroarenethiols with electrophilic reagents that occur with the modification of the thiol groups without alteration of aromatic fragments.
A number of approaches to the manufacture of allyl pentafluorophenyl sulfide were offered earlier [3,4,5]. One of them involves the interaction of pentafluorobenzenethiol and allyl bromide in the benzene-water double phase system in the presence of sodium hydroxide and a phase-transfer catalyst [3]. Other approaches make use of organometallic reagents [4, 5].
We found that allyl polyfluoroaryl sulfides are produced in high yields through the reaction of polyfluoroarenethiols and allyl chloride or allyl bromide in the presence of potassium carbonate in a solvent at room temperature. If the reaction is carried out in DMF, the target sulfide is easily separated from the mixture by steam-distillation.
Under similar conditions polyfluoroarenethiols react with benzyl chloride resulting in corresponding benzyl polyfluoroaryl sulfides in high yields (Scheme 1). Target sulfides can be isolated as white precipitates at pouring the reaction mixture in water.
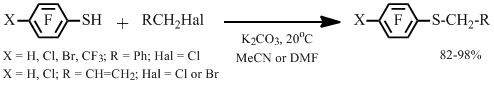
Scheme 1
It is also possible to replace two geminal atoms of chlorine by the arenethiol fragment, as it is exemplified by the reaction of 2,3,5,6-tetrafluorobenzenethiol 1 and dichloromethane (Scheme 2).
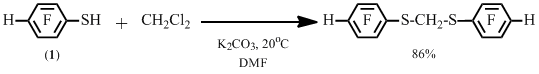
Scheme 2
Our study focused also on the reactions of polyfluoroarenethiols and trihalomethanes. It is known that the interaction of polyfluoroarenethiols and chlorodifluoromethane in the presence of sodium hydroxide gives corresponding difluoromethyl polyfluoroaryl sulfides in high yields [6].
At the same time we have shown that the reactions of polyfluoroarenethiols with dichlorofluoromethane result in the formation a mixture of chlorofluoromethyl polyfluoroaryl sulfide and hydro derivative of polyfluoroarene. The latter may predominate.
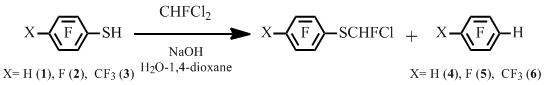
Scheme 3
It turned out that heptafluorotoluene 6 was the main product of the reaction between thiol 3 and trichloro- or tribromomethane. At the same time, the reaction of benzenethiol 1 with trichloro- or tribromomethane resulted mainly in tris(tetrafluorophenylthio)ortoformate 7. The formation of tetrafluorobenzene 4 was also observed alongside (Scheme 4). Earlier the formation of similar ortoformate was observed in the reaction of pentafluorobenzenethiol and triiodomethane В among the products of pentafluorobenzenethiol self-condensation [7].
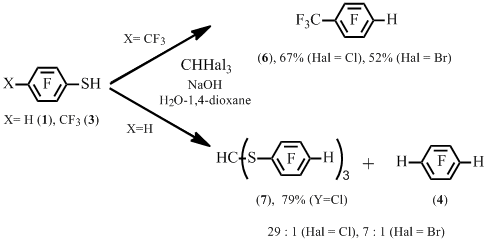
Scheme 4
The reaction of 6-chloro-4-trifluoromethyl-2,3,5-trifluorobenzenethiol 8 with trichloromethaneprovided hydro derivative 9 (Scheme 5). Earlier compound 9 was not obtained by not synthesized via desulfurization of arenethiol 8 under the action of Raney nickel. In this case hydrogen displaced both the thiol group and adjacent chlorine atom.
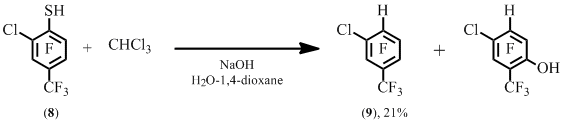
Scheme 5
Therefore, the reactions of polyfluoroarenethiols with haloalkanes in the presence of bases result in various products depending on the corresponding polyfluoroarenethiol and haloalkane structures.
We also studied some transformations of polyfluoroarenethiols under the action of electrophilic reagents known for the oxidizing action and contain sources of bromine.
It is known that non-fluorinated arenethiols can be converted to arenesulfonyl bromides under the action of bromine in acetic acid [8]. At the same time the reaction of 4-chloro-2,3,5,6-tetrafluorobenzenethiol with bromine resulted only in the corresponding diaryl disulfideВ [9].
Earlier the synthesis of pentafluorobenzenesulfonyl bromide from chloropentafluorobenzene via Grignard reagent was described. By this procedure bis(pentafluorophenyl)sulfone was by-produced in significant quantity [10].
Recently we proposed a method for the synthesis of polyfluoroarenesulfonyl bromides based on the reactions of polyfluoroarenethiols and various sources of bromine [11]. Best yields of the desired products were achieved when a mixture of bromine and fuming nitric acid, bromine and concentrated nitric and / sulfuric acids or a mixture of concentrated nitric, sulfuric and hydrobromic acids was used (Scheme 6).
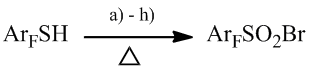
a). Br2 + fuming HNO3; b). Br2 + HNO3 + H2SO4; c). HBr + HNO3 + H2SO4; yield 70-90%;
d). NaBr + HNO3; e). Br2 + HNO3; yield 45-60%;
f). Br2 + H2SO4; g). Br2 + CH3COOH; h). Br2 + H2O;
Scheme 6
At the same time the reaction of pentafluorobenzenethiol 2 with bromine in sulfuric or acetic acid, or in water provided a mixture of pentafluorobenzene sulfonyl bromide 10 and decafluorodiphenyl disulfide 11 in various ratios.
A mixture of disulfide 11 and sulfonyl bromide 10 is also formed at decreasing the temperature of the reaction of pentafluorobenzenethiol 2 with a mixture of concentrated nitric, sulfuric and hydrobromic acids. It was shown separately that disulfide 11 may also be converted to sulfonyl bromide 10 in the conditions of synthesis of sulfonyl bromide 10 from benzenethiol 2 (Scheme 7).
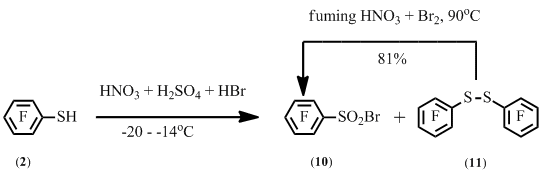
Scheme 7
Thus formed polyfluoroarenesulfonyl bromides are of interest as sources of polyfluoroarenesulfonyl radicals [10]. Radical addition of non-fluorinated arenesulfonyl bromides to double or triple bonds, or their 1,4-addition to conjugated diene systems is well known [12, 13, 14]. These reactions result in the formation of products involving both arenesulfonyl groups and bromine atoms.
We found that polyfluoroarenesulfonyl bromides add to the double bond of allyl chloride on heating or under scattered daylight irradiation at room temperature.
At the same time it was found that the reactions of polyfluoroarenesulfonyl bromides with allyl bromide provide allyl polyfluoroaryl sulfones. Alongside 1,2,3-tribromopropane is also formed (18) (Scheme 8).
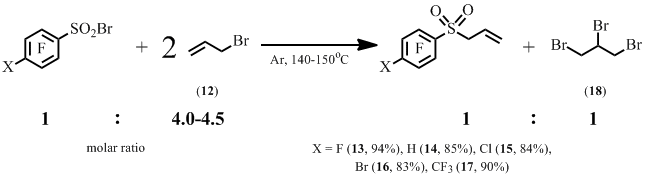
Scheme 8
It was shown that this transformation takes place even at room temperature.
The possible scheme of the process involves the formation of polyfluoroarenesulfonyl radical, its addition to the double bond of allyl bromide, and bromine atom elimination (Scheme 9).
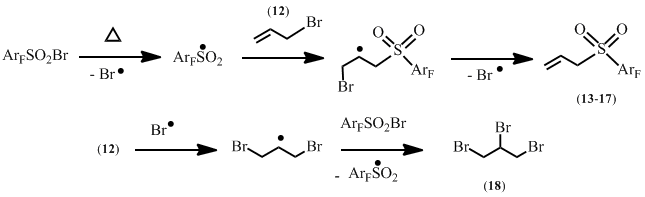
Scheme 9
The reaction slows down in the presence of hydroquinone but accelerates under scattered daylight irradiation or in the presence of copper(I) iodide, thus confirming the radical character of the process.
Recently indium was shown to perform as a catalyst in the reaction of benzenesulfonyl chloride and allyl bromide (12) resulting in the formation of allyl phenyl sulfone [15]. We found that the reaction of sulfonyl bromide (10) and compound (12) in the presence of zinc dust in 1,4-dioxane resulted in allyl pentafluorophenyl sulfone (13) in good yield after 3 hours at room temperature. By this procedure the amount of tribromopropane (18) formed was much lower.
The possible route of the process may include single electron transfer with the formation of polyfluoroarenesulfonyl bromide radical anion and its fragmentation with the formation of bromide-anion and polyfluoroarenesulfonyl radical.
Heating of sulfonyl bromide (10) and allyl sulfone (17) results in the formation of sulfonyl bromide (20) and allyl sulfone (13). At the same time heating of sulfonyl bromide (20) and allyl sulfone (13) results in the formation of sulfonyl bromide (10) and allyl sulfone (17) (Scheme 10).
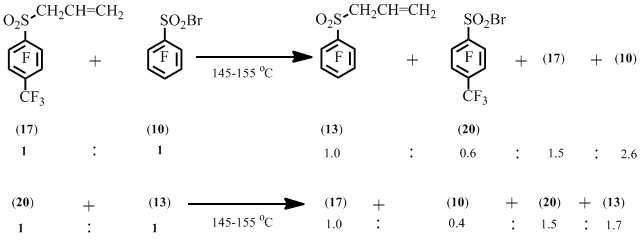
Scheme 10
This result may provide evidence of the reversibility of the allyl polyfluoroaryl sulfones formation in the reaction of polyfluoroarenesulfonyl bromides and allyl polyfluoroaryl sulfones.
References
- Robson P., Stacey M., Stephens R., Tatlow J.C. Aromatic Polyfluoro-compounds. Part VI. Penta- and 2,3,5,6- Tetra-fluorothiophenol // J. Chem. Soc. 1960. N 12. P. 4754-4760.
- Maksimov A.M., Platonov V.E., Reactions of some polyfluoroaromatic compounds with potassium hydrosulfide // Fluorine notes, 1999, NВ 4(5), URL: /contents/history/1999/4_1999/letters/index.html.
- Baechler R.D., Filippo L.J.S., Schroll, A. Structural effects upon competitive decomposition pathways of thiosulfoxide intermediates // Tetrahedron Lett., 1981, V. 22, N 52, P. 5247-5250.
- Ward W.E., Sicree S., Chen B., Tamborski C. Synthesis of polyfluoroarylalkyl sulfide compounds // J.В Fluorine Chem., 1995, V. 73, N 1, P. 73-77.
- Brooke G.M., Wallis D.I., Partially Fluorinated Heterocyclic Compounds. Part 14. Syntheses of 4,5,6,7-Tetrafluoro-2,3-dihydro-2-methyl-1-benzothiophen and 5,6,7,8-Tetrafluorothiochroman from Pentafluorophenyl Prop-2-enyl sulfide via the Claisen Rearrangement Intermediate and the Related Reaction of Prop-2-enyl-2,3,5,6-Tetrafluorophenyl sulfide. Reactions which appear to proceed via Homolytic Fission of an Aliphatic Carbon-Fluorine Bond // J.В Chem.В Soc., Perkin Trans. 1, 1981, N 6, P.В 1659-1664.
- Maksimov A.M., Kireenkov V.V., Platonov V.E. Organofluorine sulfur-containing compounds 3. Preparation of difluoromethylpolyfluoroaryl sulfides. Reactions of difluoromethylpentafluorophenyl sulfide with oxidants // Rus. Chem. Bull., 1996, V. 45, N 1, P. 153-155.
- Wragg R.T. Alkylation reactions of pentafluorothiophenol in dimethylformamide // Tetrahedron Lett. 1971. V. 12, N 27, P. 2475-2473.
- Zincke Th., Frohenberg W. Гњber p-Thiokresol // Ber. 1910., V. 43, N 1, P. 837-848.
- Furin G.G., Shchegoleva L.N., Yakobson G.G. Aromatic fluoro derivatives LXVI. Reaction of polyfluorinated diaryldisulfides with incompletely fluorinated aromatic compounds in the presence of antimony pentafluoride // Zh. Org. Khim., 1975, V. 11, Issue. 6, pp. 1290-1297, Chem. Abstr. 1975, 83:78777.
- Chen Q.Y., Chen M.F. Pentafluorobenzenesulfonyl bromide and its reactions // Chinese Chemical Letters., 1991, V. 2, N 8, P. 597-600.
- Platonov V.E., Bredikhin R.A., Maksimov A.M., Kirienkov V.V. A novel and efficient method for the synthesis of polyfluoroarenesulfonyl bromides from polyfluoroarenethiols // J. Fluorine Chem., 2010, V. 131, N 1, P. 13-16.
- Riggi I.De., Surzur J.-M., Bertrand M.P. Addition radicalaire d`halogenures de sulfonyle cyclization et bifonctionalisation du cyclooctadiene-1,5 et de dienes-1,6 // Tetrahedron, 1988, V. 44, N 23, P. 7119-7125.
- Pelter A., Ward R.S., Little G.M. Approaches to 2,6-Diaryl-3,7-Dioxabicyclo[3,3,0]Octane Lignans via Asymmetric Synthesis of Dihydro- and Tetrahydro-furan Derivatives // J. Chem. Soc. Perkin Trans. 1, 1990, N 10, P. 2775-2790.
- Tanaskov, M.M., Stadnichuk, M.D. Reaction of tert-butylbutadiene and its silicon- and germanium-containing analogs with sulfonic acid halides // Zh. Obshch. Khim., 1977, V. 47, Issue 8, pp. 1813-1822, Chem. Abstr. 1977, 87:152312.
- Wang L., Zhang Y. Indium-mediated Coupling Reaction of Sulfonyl Chloride with Alkyl Bromides in Water. A Facile Synthesis of Sulfones // J. Chem. Res. (S)., 1998. V. 9, P. 588-589.
Fluorine Notes, 2010, 73, 9-10
