Received: November 2025
DOI 10.17677/fn20714807.2025.06.03
Fluorine Notes, 2025, 163, 5-6
POLYMERIZATION OF PERFLUORO-4-FLUOROSULFONYL-PHENYL-VINYL ETHER, THE NEW POLYMER FOR PROTON-CONDUCTING MEMBRANES
A.A. Tyutyunova, I.O. Goriachukb, E.V. Poluninc, V.I. Sokolovb, G.A. Khromova, S.M. Igumnova
aA.N. Nesmeyanov Institute of Organoelement Compounds of Russian Academy of Sciences, Moscow, Russia
bNRC "Kurchatov Institute", Moscow, Russia
cN.D. Zelinsky Institute of Organic Chemistry RAS, Moscow, Russia
e-mail: tuytuynov@rambler.ru
Abstract: It is demonstrated that perfluoro-4-fluorosulfonyl-phenyl-vinyl ether polymerizes at the pressure of 1.5-1.6 GPa and 190-225 °С temperature without any radical polymerization initiators. The resulting homopolymer contains ending FSO2- groups and thus could be of interest for the fabrication of proton-exchange membranes for hydrogen fuel cells.
Keywords: fluorosulfonyl-perfluoro-vinyl ethers, polymer proton-exchange membranes, radical polymerization, ultrahigh pressure.
Introduction
Converting chemical energy into electrical energy electrochemical devices and, particularly, hydrogen fuel cells with proton-exchange membranes are of great interest for scientific researches and industry due to their high energy conversion efficiency, low environmental impact, wide applicability in various fields ranging from portable electronic devices to automobiles, trains, vessels, aircrafts and space vehicles [1]. Proton-exchange membrane is one of the key elements of a fuel cell. It supports transport of protons, produced in hydrogen catalytic oxidization at the anode, participates in oxygen reduction with water formation at the cathode and also prevents unimpeded uncontrollable interaction of hydrogen with oxygen [2 - 3]. Currently low temperature fuel cells with proton-exchange membranes made of fluorinated sulfonic-acid polymers acquired significant distribution, for instance, with commercially available membranes of Nafion, Aciplex, Aquivion, Dyneon. Membranes of that type exhibit high chemical, thermic and mechanical stability, have appropriate proton conductivity under high hydration degree [4-10]. An immense number of fluorinated monomers, particularly, with various protogenic groups have been synthesized for the development and the fabrication of proton-exchange membranes [11-20]. Proceeding in this way, we have elaborated a new Nafion-like monomer, namely FSO2C6F4OCF=CF2, in which FSO2- group is tied to CF2=CFO- substitute by perfluorophenyl fragment, rather than perfluoroaliphatic one [21]. The present work deals with the investigation of the polymerization capability of the monomer.
Synthesis of the homopolymer using FSO2C6F4OCF=CF2 monomer
The investigation of the synthesized vinyl ether FSO2C6F4OCF=CF2 [21] has shown, that it possesses low polymerization capability. It doesn`t polymerize neither by heating to 150 °С, nor under long-lasting UV radiation exposure. Its polymerization cannot be accomplished with the help of AIBN or BP (benzoyl peroxide) initiators neither in sealed degassed ampoules at 30-80 °С, nor in RAFT polymerization conditions. The given monomer preserves its initial state and chemical purity during long time storage. These results are consistent with literature information on low polymerization activity of C6F5OCF=CF2 perfluorovinyl ether, which can be polymerized only at the ultrahigh pressure (UHP) conditions, as it was shown by the researches [22, 23].
The polymerization of FSO2C6F4OCF=CF2 monomer (hereafter called E8S) is found to undergo under the pressure of 1.5-1.6 GPa and the temperature of 190-225 °С without the use of any radical polymerization initiators, see Scheme 1. The resulting E8S homopolymer is a white powder-like substance.

Scheme 1.
The synthesized homopolymer doesn’t dissolve in methanol, isopropanol or perfluoro(1,3-dimethylcyclohexane). It poorly dissolves in chloroform, benzene or toluene, while dissolves at room temperature in hexafluorobenzene.
Used for the preparing of E8S homopolymer FSO2C6F4OCF=CF2 monomer had been produced by PiM-Invest company and possessed chemical purity more than 99%. The synthesis was performed in Teflon ampoules with the volume of 1-4 ml in cylinder-piston molds at the pressure of 1.5-1.6 GPa at the temperature of 190-225 °C. The reaction time ranged from several days to two weeks. After it the mold was slowly cooled down to the room temperature, the pressure was reduced to the atmospheric one and the ampoule was released. The substance obtained after the ampoule`s opening was a viscous transparent (or slightly colored) viscous liquid containing, in addition to the homopolymer, various additives (initial monomer, dimers, oligomers and other by-products of the reaction). To remove these impurities the obtained substance was vacuumed to a constant weight at 85 °C. A stiff turbid liquid was the result. The homopolymer was reprecipitated with methanol, centrifugated during 30 minutes at 6000 rpm, decanted and dried to a constant weight at 60 °С and 5 mbar vacuum during 10 hours. A white colored powder was obtained as the result. The reaction yield did not exceed 20%. Element analysis was carried out for two samples, for the first one it was found (%): C, 29,31; F, 46,32; S, 9,76; the other one provided (%): C, 28,84; F, 44,87; S, 9,63.
Figure 1 presents the 19F NMR spectra of FSO2C6F4OCF=CF2 monomer and the corresponding homopolymer. They were obtained at «BrukerAM-300» spectrometer (282.40 MHz), the homopolymer was dissolved in hexafluorobenzene.
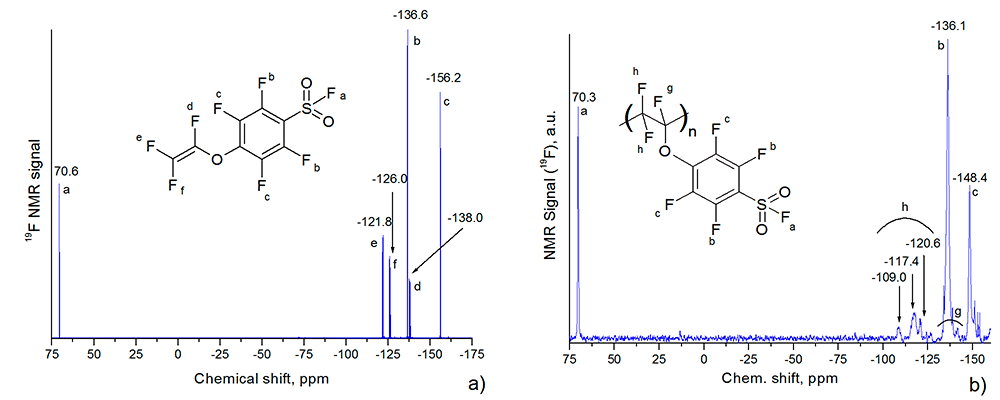
Figure 1. 19F NMR spectra of FSO2C6F4OCF=CF2 monomer (a) and of the corresponding homopolymer (b). The insets demonstrate the monomer structure and the fragment of the homopolymer structure.
The triplet near 70.6 ppm at Fig. 1a corresponds to the fluorine atom of SO2F group. The same broaden peak appears in the homopolymer`s spectrum near 70.3 ppm, see Fig. 2b. On the basis of 19F NMR spectral data for three fluorovinyl ethers [24] one can conclude that the signals near -121.8 and -126.0 ppm in 19F NMR spectrum (Fig. 1a) correspond to two fluorine atoms close to the double bond in trans and cis positions with respect to the oxygen respectively. The signal near -138.0 ppm in Fig. 1a originates from the fluorine atom in =CFO- group of the monomer. The peak -136.6 ppm for the monomer in Fig. 1a (and -136.1 ppm for the homopolymer in Fig. 1b) corresponds to two fluorine atoms of the benzene ring near FSO2- group, while the peak -156.2 ppm in Fig. 1a comes from two other fluorine atoms near this group. In the case of the homopolymer the later peak resides near -148.4 ppm (Fig. 1b) and is more broaden than the signal near -136.1 ppm. The peaks in the region of -109 ÷-121 ppm in Fig. 1b correspond to -CF2- groups in the polymer chain.
Investigation of synthesized homopolymer properties
For the estimation of E8S homopolymer molecular weight the average hydrodynamic diameter of macromolecular globules in hexafluorobenzene was measured. The measurements were carried out by the dynamic light scattering method with the help of 90Plus_Zeta particle/protein analyzer (Brookhaven Instruments Corp., USA) with the laser illumination of 640 nm wavelength. A typical histogram with size distribution of the globules is presented at Fig. 2. Average globule diameter observed equals to <D> = 1.75 nm. Thus, E8S homopolymer, synthesized by radical polymerization under the ultrahigh pressure can be classified as the substance with moderately high molecular weight.

Figure 2. The histogram with size distribution of E8S homopolymer macromolecular globules, measured by the dynamic light scattering method in hexafluorobenzene.
D is the diameter of the globule.The form of the autocorrelation function and the homopolymer structure fragment are shown as the insets.
The measurements of the synthesized homopolymer refractive index n at the wavelength of He-Ne laser (λ=632.8 nm) were accomplished by the help of Metricon2010/M prism coupler (Metriconcorp., USA). For this purpose, light guiding films of E8S homopolymer with the thicknesses of 1 mkm were fabricated on glass substrates by the spin-coating method from the hexafluorobenzene solution. The value of the refractive index n=1.466 of the as-deposited film was determined from m-lines` angular positions in the dependence of reflected ray intensity on the incidence angle.
Fig. 3 presents IR absorption spectrum of E8S homopolymer, deposited on a KBr substrate. The spectrum was measured by Shimadzu 8400S IR spectrometer. It follows from Fig. 3, that E8S homopolymer has significant absorption in the wavelength region above 5 mkm. In the telecom spectral regions near 1.3 and 1.5 mkm the homopolymer`s absorption is small.

Figure 3. Absorption coefficient α of E8S homopolymer on the KBr substrate. The inset presents the fragment of the homopolymer structure.
Obtained by thermogravimetric method E8S homopolymer mass loss dependence on heating temperature is shown in Fig. 4. One can see, that the homopolymer mass loss starts approximately at 250 °С.

Figure 4. Relative mass loss dM/M of E8S homopolymer on the heating temperature T. The inset presents the fragment of the homopolymer structure.
Structural diagnostics of E8S homopolymer was carried out using Rigaku Miniflex600 wide-angle X-ray diffractometer (Cu, λ=1.54184 Å), see Fig. 5. It is found that the diffractogram demonstrates wide “halos” near 2θ ≈ 23.39 and 58.47 deg. and small peaks near 2θ ≈ 11.68 and 31.73 deg. One can conclude from the analysis of Fig. 5 that E8S homopolymer is amorphous with some degree of polycrystallinity.

Figure 5. X-ray diffractogram of E8S homopolymer film.
Conclusions
Perfluoro-4-fluorosulfonyl-phenyl-vinyl ether possesses low polymerization activity, but can be polymerized at temperature of 190-225 °С under the pressure of 1.5-1.6 GPa without the use of any initiators. The homopolymer represents a white powder, which is soluble in hexafluorobenzene. The macromolecule of the homopolymer contains ending FSO2- group in the side substituent and thus is of interest for the fabrication of proton-exchange membranes for hydrogen fuel cells.
Acknowledgments
The work was carried out within the state assignment of NRC "Kurchatov Institute" in terms of the synthesis of the homopolymer at the ultra-high pressure and the study of its optical properties, the state assignment of INEOS RAS in terms of the synthesis of the initial monomer and the study of the chemical properties of the homopolymer.
References
- J. Walkowiak-Kulikowska, J. Wolska, H. Koroniak. Phys.Sci.Rev., 2017, 2, 20170018.
- EG&G Technical Services, Inc. Fuel Cell Handbook. Seventh Edition. U.S. Department of Energy, Office of Fossil Energy, National Energy Technology Laboratory. Morgantown, West Virginia. 2004.
- T. Maiyalagan, S. Pasupathi. Materials Science Forum, 2010, 657, 143-189.
- Yu.E. Kirsh, S.A. Smirnov, Yu.M. Popkov, S.F. Timashev. Perfluorinated carbon-chain copolymers with functional groups and cation exchange membranes based on them: synthesis, structure and properties. Russian Chemical Reviews, 1990, 59, 560-574. DOI: https://doi.org/10.1070/rc1990v059n06abeh003543
- R. Souzy, B. Ameduri. Functional fluoropolymers for fuel cell membranes. Prog. Polym. Sci., 2005, 30, 644-687. https://doi.org/10.1016/j.progpolymsci.2005.03.004
- S.S. Ivanchev, S.V. Myakin. Polymer membranes for fuel cells: manufacture, structure, modification, properties.Russian Chemical Reviews, 2010, 79, 101-117.
- W. Grot. Fluorinated Ionomers. Second Edition. Elsevier Inc. 2011.
- M. Odgaard. The Use of Per-Fluorinated Sulfonic Acid (PFSA) Membrane as Electrolyte in Fuel Cells. In: T. Nakajima, H. Groult (eds.) Advanced Fluoride-Based Materials for Energy Conversion. Elsevier Inc. 2015.
- T. Hirai, Y. Morizawa. Fluorinated Ionomers and Ionomer Membranes: Monomer and Polymer Synthesis and Applications. In: B. Ameduri, H. Sawada (eds) Fluorinated Polymers. Volume 2: Applications. RSC Polymer Chemistry Series No. 24. 2017.
- Kusoglu, A.Z. Weber. New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev., 2017, 117, 987-1104. DOI: 10.1021/acs.chemrev.6b00159
- H.H. Gibbs, R.N. Griffin. Fluorocarbon sulfonyl fluorides/ US pat. № 3041317 (1962).
- M.M. Boudakian, G.A. Hyde, E.H. Kober. US pat. № 3492348 (1970).
- W.G. Grot. CF2=CF-CF2-CF2-SO2F and derivatives and polymers thereof. US pat. № 3718627 (1973).
- R. Beckerbauer. Unsaturated alpha – hydroperfluoroalkyl sulfonil fluorides. US pat. № 3714245 (1973).
- R.J. Cavanaugh, W.H. Calkins. Sulfonyl-Containing Fluorocarbon Winyl Ethers And Ion Exchange Membrane Formed Therefrom. US pat. № 3882093 (1975).
- C.G. Krespan. US pat. № 4275225 (1981).
- G.K. Kostov, St.V. Kotov, G.D. Ivanov, D. Todorova. Study on the Synthesis of Perfluorovinyl-Sulfonic Functional Monomer and Its Copolymerization with Tetraf luoroethylene. J. Appl. Polym. Sci., 1993, 47, 735-741. https://doi.org/10.1002/app.1993.070470417
- M. Herath. Perfluoroalkyl phosphonic and phosphinic acid electrolytes for proton exchange membrane fuel cells. A Dissertation Presented to the Graduate School of Clemson University, for the degree of Doctor of Philosophy Chemistry, 2010.
- B. Ameduri. Chlorotrifluoroethylene Copolymers for Energy-applied Materials. In: B. Ameduri, H. Sawada (eds) Fluorinated Polymers. Volume 2: Applications. RSC Polymer Chemistry Series No. 24. 2017.
- B. Ameduri. Copolymers of vinylidene fluoride with Functional comonomers and Applications therefrom: Recent Developments, Challenges and Future Trends. Prog. Polym. Sci., 2022, 133, pp.101591.
- A.A. Tyutyunov, G.A. Khromov, S.M. Igumnov, Yu.A. Dobrovolsky. Conference abstracts Fundamental and Applied Problems of Solid State Ionics, 2024, 626-627. [In Russian]
- W.J. Pummer, L.A. Wall. 1,2,2-Trifluorovinyl phenyl ether and 2,3,4,5,6-pentafluorophenyl 1,2,2-trifluorovinyl ether and their polymers. SPE Trans., 1963, 3, 220-224.
- D.W. Brown, L.A. Wall. Radiation-Induced Polymerization at High Pressure of n-Tetradecafluoroheptene-1; 1,1,2-Trifluorovinyl Phenyl Ether; and 1,2,3,4,5-Pentafluorophenyl 1,1,2-Trifluorovinyl Ether. SPE Trans., 1963, 3, 300-307. DOI: 10.1002/pen.760030410
- W.S. Brey. 19 F and 13 C spectra of fluorinated and partially fluorinated vinyl alkyl ethers, J. Fluorine Chem. 126 (2005) 389–399. https://doi.org/10.1016/j.jfluchem.2005.01.015
ARTICLE INFO
Received 06 November 2025
Accepted 17 December 2025
Available
online December 2025
Recommended for publication by PhD PhD O.V. Bryzgalova
eLIBRARY Document Number (EDN) MQPXAU

Fluorine Notes, 2025, 163, 5-6
