Received: October 2025
DOI 10.17677/fn20714807.2025.06.02
Fluorine Notes, 2025, 163, 3-4
PROPERTIES OF COPOLYMERS OF PERFLUOROPROPYL VINYL ETHER AND PERFLUORO(3,6-DIOXA-4-METHYL-7-OCTENE)SULFONYL FLUORIDE SYNTHESIZED AT ULTRAHIGH PRESSURE
V.I. Sokolov1, I.O. Goriachuk1, S.I. Molchanova1, E.V. Polunin2
1 – NRC "Kurchatov Institute", Moscow, Russia
2 – N. D. Zelinsky Institute of Organic Chemistry RAS, Moscow, Russia
Abstract: Amorphous copolymers of perfluoropropylvinyl ether and perfluoro(3,6-dioxa-4-methyl-7-octene)sulfonyl fluoride with various molar contents of sulfonyl fluoride units in the macromolecule were synthesized using an ultrahigh-pressure method without the use of any radical polymerization initiators. The copolymers are capable of film formation and may be of interest in the creation of proton-exchange electrolyte membranes for hydrogen fuel cells. They exhibit high optical transparency in the visible and near-IR spectral regions, low refractive index, and are suitable for use as coatings for optical waveguides and fibers.
Keywords: Perfluorinated vinyl ethers, sulfonyl fluorides, amorphous copolymers, ultra-high pressure polymerization.
Introduction
Fuel cells based on polymer electrolyte membranes (EMs) are being actively studied because they exhibit high efficiency in converting chemical energy into electrical energy, are environmentally safe, and have the potential to become a key component of hydrogen energy devices [1-11]. One of the most important elements of such devices is the proton-exchange EM, which transports protons formed during the catalytic oxidation of hydrogen at the anode and combines them with oxygen at the cathode to form H2O. Of particular interest are fuel cells with EMs based on fluorinated sulfonic acid polymers such as Nafion (Du Pont), Aciplex (Asahi Kasei), Flemion (Asahi Glass), Aquivion (Solvay Solexis), etc. [12]. The first and best known substance from this list is Nafion, a copolymer of tetrafluoroethylene and perfluoro(3,6-dioxa-4-methyl-7-octene)sulfonyl fluoride in which the terminal -SO2F group is replaced by the -SO3H group. On the other hand, amorphous perfluorinated copolymers are of interest for the creation of optical fibers and other waveguide elements of integrated optical devices due to their high optical transparency, low refractive index and low material dispersion [13, 14]. In this work, the possibility of synthesizing amorphous copolymers of perfluoro(3,6-dioxa-4-methyl-7-octene)sulfonyl fluoride with perfluoropropyl vinyl ether is investigated.
Amorphous perfluoropolymers are typically synthesized by radical copolymerization of two or more monomers in solutions or emulsions in the presence of perfluorinated initiators. In contrast, we used an ultrahigh-pressure technique (1.5-1.6 GPa) to synthesize copolymers. This approach allows for the synthesis of copolymers without the use of any radical polymerization initiators and ensures a high yield of the target product [14, 15].
Synthesis of perfluorinated copolymers at ultrahigh pressure
To obtain amorphous perfluorinated copolymers, the following monomers were used: perfluoropropyl vinyl ether E1 produced by PiM-Invest company and perfluoro(3,6-dioxa-4-methyl-7-octene)sulfonyl fluoride E7S (frequently named PSEPVE) [16], which are transparent colorless liquids. Before synthesis, the monomers were purified from oxygen, which is an inhibitor of the radical polymerization reaction, by distillation in an argon atmosphere, and mixed in a given ratio (the molar concentration of E7S monomer in the mixture was =20-80%). The synthesis of the copolymers was carried out in Teflon ampoules with a volume of 1-4 ml in cylinder-piston molds at a pressure of 15-16 thousand atm. and a temperature of 125-145 °C for 72 hours or more. The synthesis scheme is shown in Fig. 1. The product obtained after opening the ampoule was typically a highly viscous, colorless liquid containing, in addition to the linear copolymer, volatile components (unreacted monomers, dimers, etc.). To remove these components, the product was evacuated to a constant weight at 85 °C. The copolymer yield depends on the temperature and duration of the synthesis and reaches 60–70%.
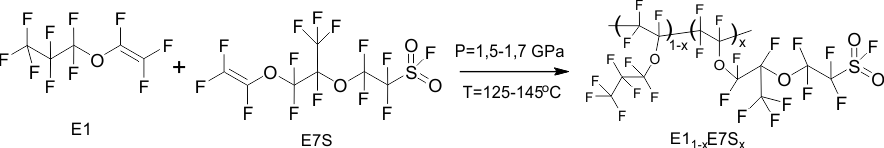
Figure 1. Scheme of synthesis of copolymers of perfluoropropyl vinyl ether E1 with perfluoro(3,6-dioxa-4-methyl-7-octene)sulfonyl fluoride E7S by radical polymerization at ultra-high pressure.
x is the molar concentration of E7S monomer units in the copolymer macromolecule.
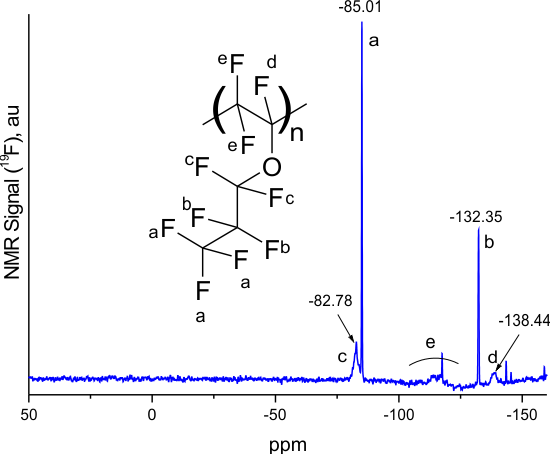
Figure 2 shows the 19F NMR spectra of E1 and E7S homopolymers, as well as the spectrum of the E11-xE7Sx copolymer obtained from the reaction mixture with =50%. All spectra were measured using Bruker AM-300 spectrometer (282.40 MHz) in hexafluorobenzene.
|
|
|
Figure 2. 19F NMR spectra of E1 (a), E7S (b) homopolymers and E1E7S copolymer obtained from the reaction mixture with =50% (c). The insets show the fragment of the structure of the homopolymers and that of the copolymer.
The signal near -85.01 ppm in Fig. 2a corresponds to three fluorine atoms in the trifluoromethyl group of E1 homopolymer (the same line is present in the copolymer, -84.48 ppm in Fig. 2c). The assignment of other signals in E1 homopolymer is given in [17] and is shown in Fig. 2a. The signal near 42.39 ppm in Fig. 2b corresponds to the fluorine atom in the -SO2F group of the side substituent of E7S homopolymer (the same line is present in the copolymer, 42.34 ppm in Fig. 2c). The content of E1 and E7S monomer units in E11-xE7Sx copolymer was estimated from the ratio of the integrals of these signals. Analysis of Fig. 2c shows that the ratio of units in this copolymer is x = 0.47.
The x value can also be estimated from the ratio of the integrals of the 42.34 ppm fluorine atom signal in the -SO2F group of the sulfonyl fluoride unit in the copolymer (Fig. 2c) and the ‑131.8 ppm signal, which corresponds to two fluorine atoms near the trifluoromethyl group of the perfluoropropyl unit. Calculations reveal that the x value is 0.54. It can be concluded that the ratio of perfluoropropyl E1 units to sulfonyl fluoride E7S units in the E11-xE7Sx copolymer is x 0.5 and is close to the molar ratio of the initial E1 and E7S monomers in the reaction mixture ( = 50%). This indicates that the polymerization activities of these monomers are similar.
Let's calculate the equivalent mass of the macromolecule per one -SO2F group. For the E10.5E7S0.5 copolymer, it is 712 g/mol, which is close to the equivalent mass of 1100 g/mol per -SO3H group in the Nafion 212 polymer.
To estimate the molecular weight of the obtained copolymers, the average hydrodynamic diameter D of the macromolecular globules in hexafluorobenzene was measured. The measurements were performed using dynamic light scattering technique on 90Plus_Zeta instrument (Brookhaven Instruments Corp., USA) under illumination with a laser beam with a wavelength of 640 nm. Figure 3 shows a typical histogram of the globule size distribution for the E10.5E7S0.5 copolymer. It is evident that the average globule diameter is <D> = 7.2 nm. Thus, the synthesized copolymers can be classified as high-molecular-weight substances.
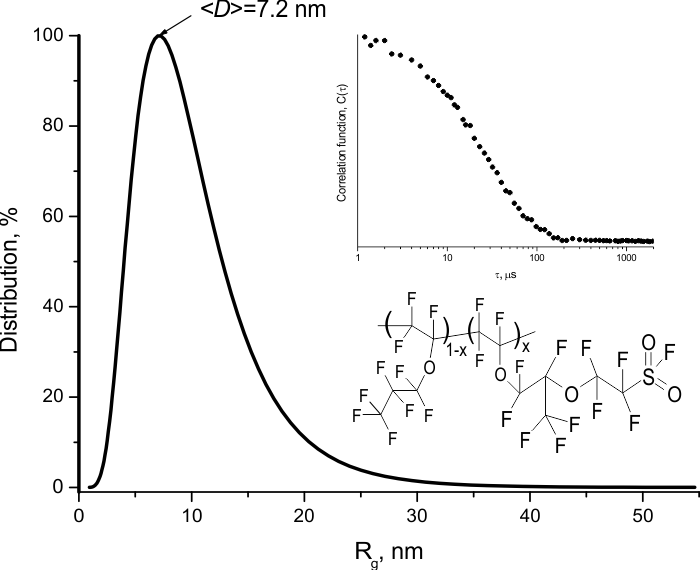
Figure 3. Size distribution of macromolecular globules of the E10.5E7S0.5 copolymer, measured by dynamic light scattering technique in hexafluorobenzene. D is the globule diameter.
The insets show the autocorrelation function and a fragment of the copolymer structure.
The synthesized copolymers are colorless, transparent substances. They dissolve at room temperature in perfluorinated solvents such as hexafluorobenzene, perfluoro-(1,3-dimethyl)-cyclohexane (Flutec PP3).
Structural diagnostics of perfluorinated sulfopolymers
During the synthesis of perfluorinated copolymers of E1 ether and sulfonyl E7S fluoride, several factors lead to disordering of the macromolecular chain. First, the E1 and E7S monomers can be linked to each other in different sequences. Second, the linkages of these monomers can be either head-to-tail or head-to-head. For these reasons, the resulting copolymer should exhibit the properties of an amorphous substance.
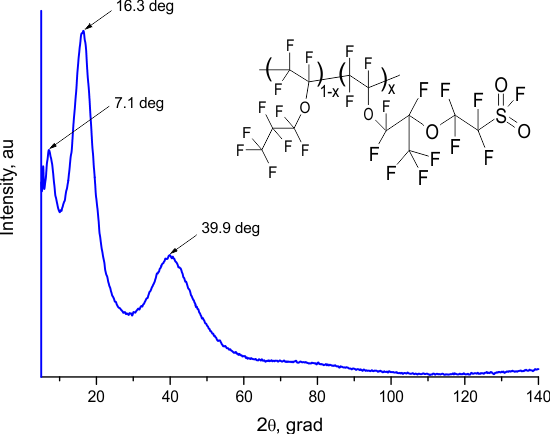
Rigaku Miniflex600 wide-angle X-ray diffractometer (Cu, = 1.54184 Å) was used to study the structure of the copolymers. Figure 4 shows the diffraction patterns of the E11-xE7Sx copolymer ( = 50%) and E7S homopolymer films. The diffraction patterns are clear, lacking sharp peaks and displaying only a few broad "halos", indicating the amorphous nature of these materials.
|
|
Figure 4. X-ray diffraction patterns of the E1E7S copolymer ( = 50%) (a) and the E7S homopolymer (b). The insets show the structure of the copolymer and that of homopolymer, respectively.
IR absorption spectra of copolymers
The optical transparency of the E11-xE7Sx copolymers in the IR spectral range was studied using Shimadzu8400S Fourier spectrometer (Shimadzu, Japan). For this purpose, the polymer films were formed on KBr plates from solutions of these copolymers in hexafluorobenzene using spin-coating method. After deposition onto a substrate, the films were annealed at 100 °C to completely evaporate the solvent. The absorption spectrum α() of the E11-xE7Sx material ( = 50%) is shown in Fig. 5. The calculation of α() was carried out taking into account Bouguer's law T() exp(-αL), where α is the absorption coefficient of the polymer material, L is the film thickness, and T() is the transmittance of the sample.

Figure 5. Absorption spectrum α() of amorphous perfluorinated copolymer E11-xE7Sx ( = 50%) in the IR region.
Figure 5 shows that the most intense absorption bands associated with stretching vibrations in the CF, CF2 и CF3 groups of the copolymer are located in the range of 6-11 μm. In the telecommunication wavelength regions near 1.3 and 1.5 μm (in particular, in the C-range of 1.530‑1.565 μm), the copolymer exhibits a high degree of transparency.
Measurement of refractive index of light-guiding films made of perfluorinated sulfonyl fluoride copolymers
To measure the refractive index of the synthesized copolymers at the He-Ne laser wavelength (λ = 632.8 nm), Metricon2010/M prism coupler (Metricon corp., USA) was used. Light-guiding films with a thickness of 8 - 18 μm were fabricated on silicon substrates using the spin coating method. The refractive index value was determined from the angular position of the m-lines in dependences of the reflected beam intensity on the angle of incidence. Measurements were carried out with TE and TM polarization of the laser beam, which made it possible to determine the refractive indices nTE and nTM in the directions along the film and orthogonal to it, respectively. It was found that for the perfluorinated E10.5E7S0.5 copolymer, nTE and nTM are close to 1.3293 ± 0.0002. This indicates that the films formed from this material are isotropic.
Conclusion
Amorphous copolymers of perfluoropropyl vinyl ether and perfluoro(3,6-dioxa-4-methyl-7-octene)sulfonyl fluoride with various molar contents of sulfonyl fluoride units in the macromolecule were synthesized using an ultrahigh-pressure, initiator-free method. The copolymers are capable of film formation and may be of interest in the fabrication of electrolytic membranes for hydrogen fuel cells. The resulting copolymers exhibit high optical transparency in the telecommunications wavelength ranges near 1.3 and 1.5 µm, have a low refractive index of n = 1.3293 (at a wavelength of 632.8 nm), and are promising for the fabrication of various waveguide elements in integrated optical devices. They can be used, for example, as waveguide coatings and as cladding for optical fibers.
Acknowledgments
The work was carried out within the state assignment of NRC "Kurchatov Institute".
References
- J. Walkowiak-Kulikowska, J. Wolska, H. Koroniak. Polymers application in proton exchange membranes for fuel cells (PEMFCs) // Phys.Sci.Rev. 2017. V. 2. P. 20170018. DOI:10.1515/9783110469745-010
- Fuel Cell Handbook. Seventh Edition. EG&G Technical Services, Inc. U.S. Department of Energy, Office of Fossil Energy, National Energy Technology Laboratory. Morgantown, West Virginia. 2004. 428 p.
- M.O. Gallyamov, A.R. Khokhlov. Polymer membrane fuel cells: materials for a course on fundamentals of fuel cells. 2014. M.: Faculty of Physics, Moscow State University. 72 p. [In Russian].
- Y.A. Dobrovolsky, E.A. Sanginov, N.G. Bukun, A.N. Ponomarev, D.A. Kritskaya, E.F. Abdrashitov. New approaches to the preparation of nanocomposite proton exchange membranes for fuel cells // Nanotechnologies in Russia. 2020. V. 15, N 3. pp. 319-325.DOI: 10.1134/S1995078020030039
- A.A. Belmesov, L.V. Shmygleva, A.A. Baranov, A.V. Levchenko. Proton exchange membrane fuel cells: processes–materials–design in current trends // Russ. Chem. Rev. 2024. V. 93, № 6. P. RCR5121. 10 DOI: 59761/RCR5121
- F. Linden, E. Pahon, S. Morando, D. Bouquain. A review on the Proton- Exchange Membrane Fuel Cell break-in physical principles, activation procedures, and characterization methods // J. Power Sources. 2023. V. 575. P. 233168. DOI: 10.1016/j.jpowsour.2023.233168
- H. Han, H. Miura, Y. Motoishi, N. Tanaka, T. Fujigaya. Development of a proton exchange membrane based on trifluoromethanesulfonylimide-grafted polybenzimidazole // Polym. J. 2021. V. 53. P. 1403–1411. DOI:10.1038/s41428-021-00551-6
- Rukhov A.V., Glazkov Yu.E. Proton-exchange membrane fuel cells for automotive applications // Transport engineering and technology. 2019. № S13. P. 40. [In Russian].
- S. Neelakandan, L. Wang, B. Zhang, J. Ni, M. Hu, C. Gao, W.-Y. Wong, L. Wang. Branched Polymer Materials as Proton Exchange Membranes for Fuel Cell Applications // Polym. Rev. 2022. V. 62:2. P. 261-295. DOI: 10.1080/15583724.2021.1964524
- E. Quartarone, S. Angioni, P. Mustarelli. Polymer and Composite Membranes for Proton-Conducting, High-Temperature Fuel Cells: A Critical Review // Mater. 2017. V. 10. P. 687. DOI:10.3390/ma10070687
- R. S. Raja Rafidah, W. Rashmi, Khalid M., W. Y. Wong, J. Priyanka Recent Progress in the Development of Aromatic Polymer-Based Proton Exchange Membranes for Fuel Cell Applications // Polym. 2020. V. 12. P. 1061. DOI:10.3390/polym12051061
- S. Ebnesajjad. Introduction to Fluoropolymers. Materials, Technology and Applications. 2013. Boston : Elsevier Science & Technology. 344 p.
- B. Ameduri. The Promising Future of Fluoropolymers // Macromol. Chem. Phys. 2020. V. 221 (8). P. 1900573. DOI:10.1002/macp.201900573
- Sokolov V.I., Goriachuk I.O., Molchanova S.I., Zavarzin I.V., Pogodina Y.E., Polunin E.V., Yarosch A.A. New copolymers of perfluoro-2-ethyl-2-methyl-1,3-dioxole and perfluorovinyl ether with low non-monotonic refractive index // Russ. Chem. Bull. 2019. V. 68, Vol. 3. pp. 559-564 DOI: 10.1007/s11172-019-2454-y.
- V. I. Sokolov, I.O. Goryachuk, S.I. Molchanova, E.V. Polunin. Investigation of optical properties of amorphous copolymers of perfluoro-2,2-dimethyl-1,3-dioxol and perfluoro-(2-cyclopentyl) ethylvinyl ether obtained at ultrahigh pressure // Fluorine notes. 2022. № 5(144). P. 3‑4.DOI: 10.17677/fn20714807.2022.05.02
- O.S. Bazanova, A.S. Odinokov, E.V. Irisova, V.G. Barabanov. Methods of synthesis of perfluorosulfonyl vinyl ethers (PSVE) // Fluorine notes. 2024. № 1(152). P. 3-4. DOI: 10.17677/fn20714807.2024.01.02
- N. Belov, Yu. Nizhegorodova, A. Zharov, I. Konovalova, V. Shantarovich, Yu. Yampolskii. A new polymer, poly(perfluoropropylvinyl ether) and its comparison with other perfluorinated membrane materials // J. Membr. Sci. 2015. V. 495. P. 431–438. DOI: 10.1016/j.memsci.2015.08.037
ARTICLE INFO
Received 20 October 2025
Accepted 25 November 2025
Available
online December 2025
Recommended for publication by PhD PhD O.V. Bryzgalova
eLIBRARY Document Number (EDN) GTVYJH

Fluorine Notes, 2025, 163, 3-4