Received: September 2025
DOI 10.17677/fn20714807.2025.05.02
Fluorine Notes, 2025, 162, 3-4
ION SERIES OF MASS SPECTRA OF FOUR PERFLUOROBICYCLOISOMERS C10F16
AND TWO CONFORMERS OF PERFLUORODECALIN C10F18.
N.D. Kagramanov
A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 119991, GSP-1, Moscow, B-334, Vavilov St. 28
e-mail: ndkagram@gmail.com
Abstract: This report completes a series of studies on the primary synchronous detachments of one, two and three fluorine atoms with the formation of corresponding ion series in the spectra of n-perfluoroalkanes [1], perfluorotributylamine [2], detachments of three radicals in the spectra of polyoxaperfluoroalkanes and polyoxaperfluoroalkyl halides [3], in the ion series of fluorobenzenes with perfluoroalkyl substituents [4] and derivatives of perfluorocyclohexane and perfluorocyclohexene [5]. The spread of excitation energies of the molecular ion +.М1-3,4 is the cause of primary synchronous detachments of radicals of different energies: -.F, -2.F, -3.F, -CF, -CF2, with the formation of primary ions of the corresponding ionic series with different masses. In the absence of branches of the ionic series, the last significant digit of the masses of all its fragment ions is preserved, since they all arise as a result of successive emissions of the regular fragment group -CF2 (-50 Da). If the series do not branch, this rule applies to all ionic series, which facilitates their interpretation. When the ionic series branches, in the initial or final stage of fragmentation, “non-standard” secondary detachments of fluorine, carbon or CF atoms usually occur. As a result of the “non-standard” detachment of the radical, the last significant digit of the ion mass changes. And then, when detachments occur, CF2 (-50 Da) is preserved until a new branch occurs. Mass spectrum analysis allows us to determine all fragmentation paths - ion series of the spectrum, establish branching of the series and qualitatively evaluate the ratios of the energy of the primary synchronous detachments of fluorine atoms in the corresponding ion series. It should be noted that similar primary synchronous detachments of three radicals:.CH3 and two hydrogen atoms, .С2H5 and two hydrogen atoms and subsequent emissions of the regular group СH2=СH2-28 occur in the ion series of the spectra of linear n-alkanes [6].
Keywords: ionic series of four perfluorobicycloisomers C10F16 and two conformers C10F18.
Ion series mass spectra of four perfluorobicycloisomers C10F16
This report presents mass spectra of four perfluorobicycloisomers of C10F16 from NIST libraries. One of them is perfluorobicyclodecene, and the other three isomers are tetradecafluoroindene derivatives with the CF3 group in the 3-, 8-, and 2-position. Figure 1 the mass spectrum of perfluorobicyclodecene and Figure shows its ion series.
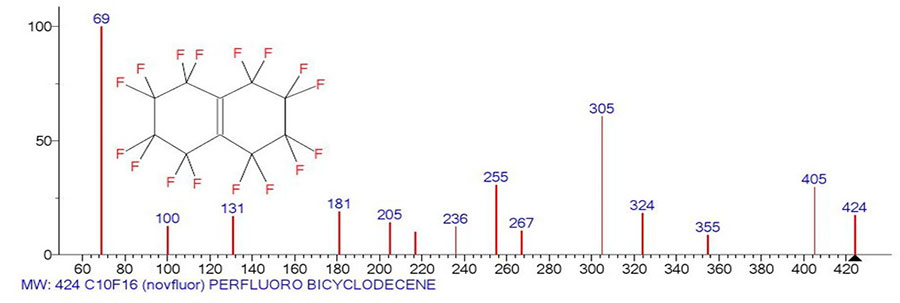
Figure 1. Mass spectrum of perfluorobicyclodecene C10F16 MW 424 INEOS USSR Academy of Sciences.
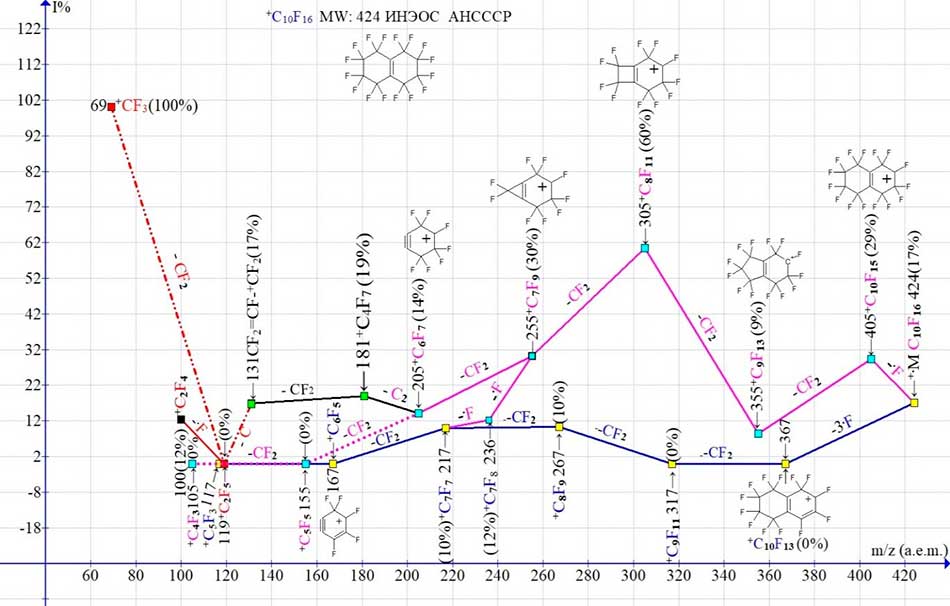
Figure 2. Two main ion series (M -.F and M -3.F) and their four branches in the mass spectrum of perfluorobicyclodecene C10F16 MW: 424.
The mass spectrum of perfluorobicyclodecene shown in (Fig. 1) was probably recorded in 1978 on an MX-1310 mass spectrometer. It contains only fourteen peaks. To date, this mass spectrum is the only spectrum of perfluorobicyclodecene. Its two main ion series (Fig. 2) begin with the primary detachments of one and the synchronous detachment of three fluorine atoms. When one fluorine atom is detached, appears a series of ions with the last significant digit of mass «5», with the most intense peaks, marked in purple. After the primary detachment of three fluorine atoms, appears a second ion series with the last significant digit of mass «7», with less intense peaks, marked in blue in (Fig. 2). In the ionic series, which begins with the primary detachment of a fluorine atom, a secondary sequential detachment of two fluorine atoms also occurs, as a result of which the more intense (purple) series of ions combines with the less intense (blue) ionic series.
When detached from the ion with m/z 205 of the C2 group, the most intense ion series branches to form a perfluoroallyl series consisting of two ions: +C4F7 with m/z 181 and +C3F5 with m/z 131. Since the peak intensity with m/z 119 +C2F5 is zero, the appearance of an intense ion with m/z 69 is apparently the result of a difluoroacetylene C2F2 molecule detachmentfrom the allyl ionCF2=CF-+CF2 with m/z 131. The probablepathway for the formation of the tetrafluoroethylene ion is apparently the detachment of CF from the perfluoroallyl ion +C3F5. Other examples of the formation of the C10F16 ion series are the spectra of three perfluorobicycloisomers with the CF3 group in the third, eighth, and second positions.
Figure 3 shows the ion series of the most intense spectrum [3-methylbicyclo [4,3,0]-nona-1(6)-ene], with the CF3 group in the third position.
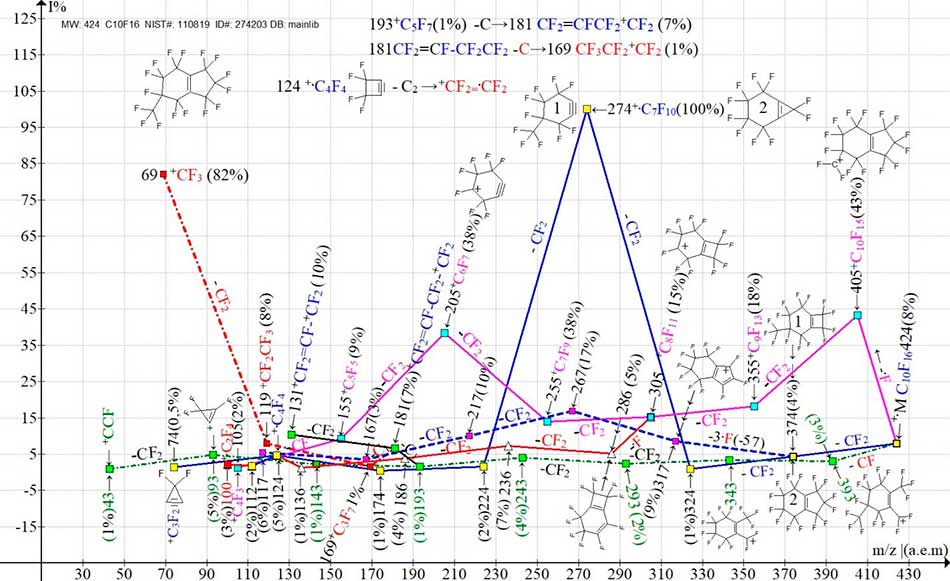
Figure 3. Three main ionic series (M -.F, M -CF, M -CF2) and their branches in the mass spectrum of perfluoro[3-methylbicyclo[4.3.0]-nona-1(6)-en] C10F16 MW: 424 Institute of OrganicChemistry of the USSR Academy of Sciences, 1990 NIST#: 110819 ID#: 274203 DB: mainlib.
As a result of the primary CF2 detachment and two subsequent CF2 emissions, the base peak +C7F10 of the mass spectrum (Fig. 3) with m/z 274 appears. The primary CF2 detachment can occur either from the CF3 substituent or from the five-membered ring. Two possible structures of this ion (1 and 2) are presented in (Fig. 3).
The more probable structure is apparently structure 1, which occurs as a result of the detachment of the five-membered cycle. It should be noted that in this same series (blue) after the primary CF2 detachment, a secondary synchronous ejection of three fluorine atoms also occurs with the formation of a new series with the last significant mass digit «7».
Similar second asynchronous detachments of two and al so three fluorine atoms, caused by the structure of the compounds, in some cases occur in the spectra of polyoxaperfluoroalkanes and polyoxaperfluoroalkyls with terminal halogenide atoms [3]. In the mass spectrum (Fig. 3) after the primary detachment of the fluorine atom, an intense ion series with the last significant digit 5 (purple series) is formed. This series branches.
The ion with m/z 305 +C8F11 fragments both with detachments of CF2 and with secondary ejection of the fluorine atom. As a result, a new series (red) with the last significant digit "6" is formed. This series, namely its ion with m/z 136, ejects a carbon atom and combines with the blue series, namely the ion with m/z 124. The least intense series of the spectrum is the series arising after the primary detachment of the CF radical with the formation of an ion with m/z 393. In this ion series with the last significant digit "3" (green dotted line, green square), seven consecutive CF2 emissions occur. After five CF2 emissions, the +C5F7 ion with m/z 193 is formed. The detachment of a carbon atom from it leads to the formation of the first ion of the perfluoroallyl series with m/z 181 +C4F7 (black, green square). The allyl ion with m/z 181 ejects a carbon atom to form the first ion of the alkyl series with m/z 169 (red dotted line, red square). The tetrafluoroethylene ion apparently arises as a result of the detachment of two carbon atoms from the +C4F4 ion with m/z 124 (Fig. 3).
Figure 4 shows the ion series of a less intense spectrum, perfluoro[8-methylbicyclo[4,3,0]-nona-1(6)-ene], with the CF3 group in the eighth position.
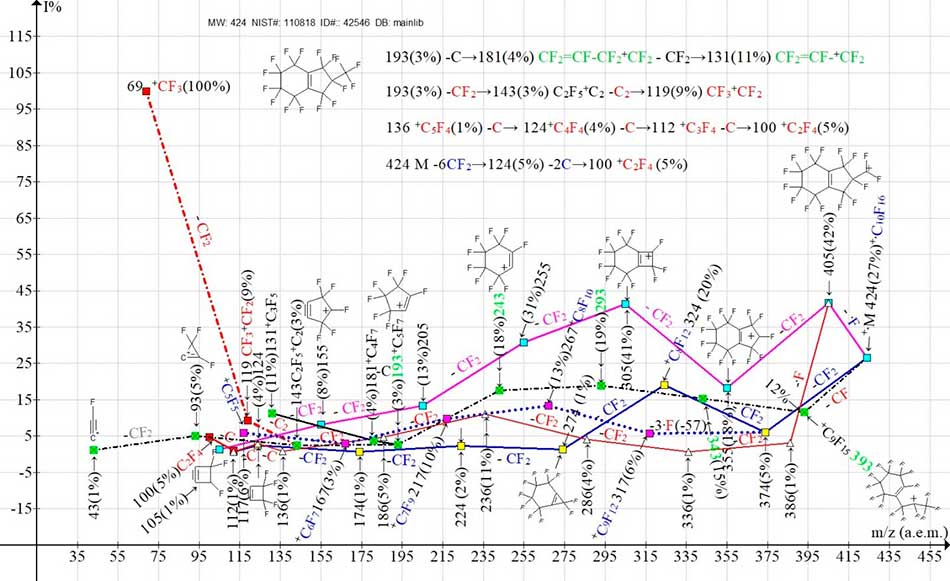
Figure 4. Three main ionic series (M -.F, M -CF, M -CF2) and their branches in the mass spectrum of perfluoro [8-methylbicyclo[4.3.0] nona-1(6)-en] C10F16 MW: 424 Institute of Organic Chemistry, USSR Academy of Sciences NIST#: 110818 ID#: 42546 DB: mainlib.
In the mass spectrum of the second isomer (Fig.4) the same primary detachments (‑F, ‑CF2, ‑CF) occur as in the spectrum of the isomer (Fig. 3). After the primary detachment of -CF2, as in the spectrum (Fig. 3), a secondary synchronous emission of three fluorine atoms (-3.F) occurs. The main difference between these two isomers is a sharp decrease in the peak intensity with m/z 274 +C7F10 from 100% in the spectrum (Fig. 3) to 1% in the spectrum of the isomer (Fig. 4).
A probable reason for such a decrease in intensity may be the formation of a structure with m/z 274 with a strained three-membered cycle. The perfluoroalkyl ion +C2F5 is probably formed by the detachment of two carbon atoms from the ion with m/z 143 or one carbon atom from the allyl ion +C3F5.
The greatest decrease in the intensities of all fragment peaks occurs in the spectrum of the C10F16 isomer with the CF3 group in position 2 (Fig. 5), which is as close as possible to the five-membered cycle, probably violating the symmetry of the molecule.
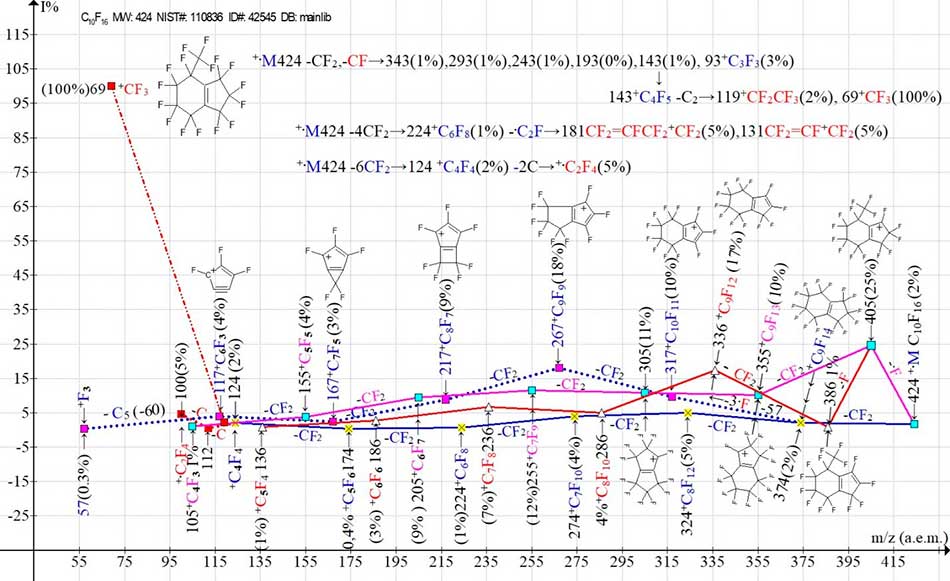
Figure 5. Two main ionic series (M -.F, M -CF2) and their branches in the mass spectrum[2-trifluoromethylperfluorobicyclo[4,3,0]-non-1(6)-en] C10F16 MW: 424 Institute of Organic Chemistry, USSR Academy of Sciences NIST#: 110836 ID#: 42545 DB: mainlib.
In the spectrum (Fig. 5) the number of main ionic series is reduced to two. However, as a result of secondary detachments: a fluorine atom in the +.M -.F series, a secondary ejection of three fluorine atoms in the +.M -CF2 series, and a secondary ejection of CF (not shown in the diagram) in the +.M -CF2 series, the two main series branch out, forming three new series.
As a result of the secondary detachment of a fluorine atom in the +.M -.F series, a new ion series with the last significant digit "6" appears. As a result of the secondary synchronous detachment of three fluorine atoms after the primary detachment of -CF2, a new series with the last significant digit "7" appears. This secondary ion series, beginning with the synchronous detachment of three fluorine atoms (-57), ends with the formation of the +F3 ion with m/z 57, which allows us to conclude that it is relatively stable. This example of the formation of an ion with m/z 57 +F3 is not the only one. This ion is also recorded in the mass spectra (Fig. 12, 13 and 15) of three substituted perfluorocyclohexenes [5].
It should be noted that after the primary detachment of -CF2 in the ion series (Fig. 5) a secondary emission of CF occurs with the formation of a new ion series with the last significant digit "3". Due to the too low peak intensity of this series, with the last significant digit of the masses "3", it is not reflected in the graph of ion series, but is presented as a separate digital sequence of fragmentation (line 1) +.M 424 -CF2, -CF→…). The other two sequences represent the process of formation of perfluoroallyl ions with m/z 181 and 131, as well as the +.C2F4 ion. It is likely that the primary parallel detachments of CF and CF2 occur from different cycles. The primary detachment of CF2 occurs from the only CF3 group of the six-membered cycle, and the primary detachment of CF from one of the CF2 groups of the five-membered cycle. As a result, the five-membered cycle opens with the formation of a new CF3 -group. The reason for the change in the order of CF detachment (instead of primary detachment, secondary) is possibly the violation of the symmetry of the molecule by the CF3 group in position 2.
The peak intensities of four structural isomers of C10F16, arising as a result of primary detachments of the same radicals, are different. The different peak intensity of+C8F11 with m/z 305 of the same ion series of four structural isomers is due to their different structure and different topology of the occurring primary detachments. Thus, the peak intensities of four isomers of the same ion series, beginning with the primary detachment of the fluorine atom and two CF2 emissions with the formation of an ion with m/z 305 +C 8F11 are as follows: (Fig. 2 - 60%), (Fig. 3 - 15%), (Fig. 4 - 41%), (Fig. 5 - 11%). Judging by the minimum intensity of the peak with m/z 305 +C8F11 in the spectrum (Fig. 5 -11%), the least stable isomer is the C10F16 isomer with the CF3 group in the second position (Fig. 5).
Ionic series of two conformers (cis- and trans-perfluorodecalin) C10F18
The mass spectra of two conformers of C1010F18 (cis- and trans-) differ significantly in the peak intensities of their ion series, as well as in the sequences of secondary detachments of three fluorine atoms (Fig. 6, Fig.7).
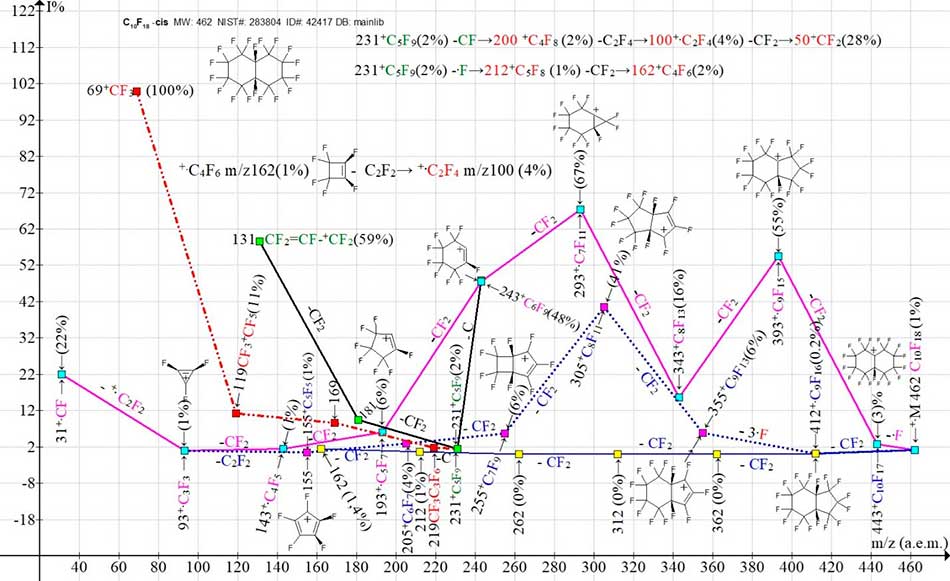
Figure 6. Two main ion series (M -.F, M -CF2) and their branches in the mass spectrum of C10F18 perfluorodecalin, cis- MW: 462 (A. Pleshkova) INEOS USSR Academy of Sciences NIST#: 283804 ID#: 42417 DB: mainlib.
In the spectrum of the cis-isomer (Fig. 6) two ion series appear: a series of intense peaks and a series of low-intensity peaks. The low-intensity series of six peaks with the last significant mass digit "2", formed as a result of six successive emissions of CF2 (blue line-yellow square) is completed by the ion +.С4F6 with m/z 162 (1%). It is likely that when the perfluoroacetylene molecule is detached, it is converted into the ion +.C2F4 (4%). In the same series, after the first detachment of CF2, a secondary synchronous emission of three fluorine atoms occurs, probably from a five-membered cycle. The weak peak series branches to form a new intense series of ions with the last significant mass digit "5". In the new series of ions, marked by the blue dotted line - purple square, an intense ion with m/z 305 (41%) +C8F11 appears. The most intense peak series (purple line - blue square), with the last significant digit "3", appears as a result of the primary detachment of one fluorine atom and seven successive ejections of CF2. Seven successive ejections of CF2 allow us to conclude that the primary detachment of the fluorine atom occurred from the cis- position.
Upon detachment of a carbon atom from the +C6F9 ion with m/z 243, this intense series branches to form a perfluoroalkyl series of ions (black line - green square) with m/z 231 +C5F9, 181 +C4F7 and +C3F5. Upon detachment of a carbon atom from the +C5F9 ion with m/z 231, the allyl series branches to form a perfluoroalkyl series of ions (red dotted line - red square) with m/z 219 +C4F9, 169 +C3F7, 119 +C2F5, 69 +CF3 (100%). The series of the most intense peaks and the series of the weakest peaks combine to form the fragment ion +C3F3 m/z 93 and its fragment ion +CF m/z 31. Two more ion series arising from the detachment of the +CF radical from the ion with m/z 231 to form the perfluoroolefin series of ions, as well as the .F atom to form ions with m/z 212 and m/z 162 are not reflected in the ion series graph (Fig. 6) for reasons that make it difficult to view, but are presented as two digital sequences.
Figure 7 shows weak ion series of the mass spectrum of perfluorodecalin-trans, a less stable conformer than perfluorodecalin-cis.
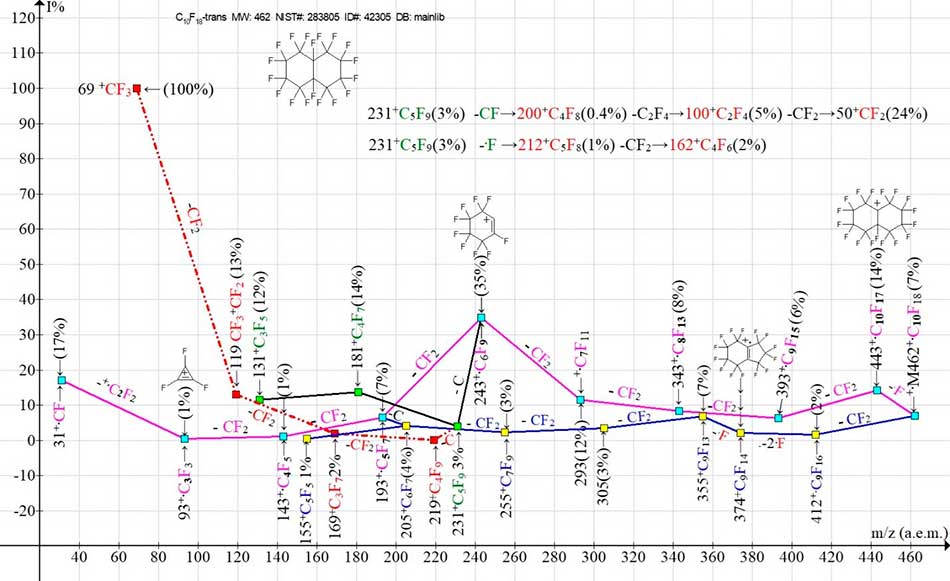
Figure 7. Two main ion series (M -.F, M -CF2) and their branches in the mass spectrum of C10F18 perfluorodecalin, trans- MW: 462 (A. Pleshkova) INEOS USSR Academy of Sciences NIST#: 283805 ID#: 42305 DB: mainlib.
The most intense fragment peak of the mass spectrum of perfluorodecalin-trans is the peak with m/z 243 +C6F9 (35%). Compared with the mass spectrum of perfluorodecalin-cis, the intensity of the peak with m/z 293 +C7F11 decreases from 67% to 12%, and the peak with m/z 243 from 48% to 35%. The ion with m/z 243 fragments both with subsequent CF2detachments, with the formation of an ion with m/z 93 +C3F3 (1%), and with a secondary detachment of the carbon atom, with the formation of a perfluoroallyl series of ions with m/z 231, 181 and 131.
The secondary detachment of a carbon atom from the ion with m/z 231 leads to the formation of a perfluoroalkyl series of ions with m/z 219, 169, 119, 69. The secondary detachments from the ion with m/z 231 of CF, as well as F in (Fig. 7) leading to the formation of a perfluoroolefin as well as an ion series with m/z 212, 162 are shown in (Fig.7) separately from the graph of ion series by two digital sequences.
In the second, weaker ion series, starting with the detachment of CF2 (blue line, yellow square) (Fig. 7), instead of the synchronous detachment of three fluorine atoms (Fig.6), there is a synchronous detachment of only two fluorine atoms, apparently from two trans positions, and then the detachment of one more fluorine atom from the five-membered cycle. This is indirectly confirmed by the subsequent ejections of four CF2 groups, with the formation of the +C5F5 ion.
Comparison of the peak intensities of the ion series graphs of the two C10F18 conformers (Figs. 6-7) allows us to conclude that the trans conformer is less stable than the cis- conformer.
Conclusion
The transformation of the perfluoroorganic compound mass spectrum into its ion series can be implemented using a computer program that transforms the mass spectrum into the corresponding ion series.
The program must determine the digital values of all primary +.M -∆1-N radical detachments that occur. Search for a series of sequences of ion mass decreases of corresponding to ion series, that occur after the primary radical detachments as a result of regular emissions of the CF2 (-50 Da) group with different, but identical last significant digits for each specific sequence (ion series). Establish the termination of regular CF2detachment in a particular ion series as a result of detachment of a non-standard radical (F, C, CF, 2C) instead of a CF2 group. Determine the digital values of all secondary radical detachments +I -∆1-N. Search for new ion series mass sequences after secondary radical detachments as a result of CF2 (-50 Da) emissions and represent the library mass spectrum as its ion series.
To set up the computer program, you can start with the spectra of the ion series that were previously established.
Acknowledgments
The work was carried out with the support of the Ministry of Science and Higher Education of the Russian Federation using scientific equipment of the INEOS RAS Center for Molecular Structure Research.
References
- Kagramanov N.D., Fragmentation algorithms for n-alkanes and n-perfluoroalkanes, Fluorine notes, 2020, 1(128), 3-4.
- Kagramanov N.D., Three ion series of the mass spectrum of perfluorotributylamine (PFTBA), Fluorine notes, 2020, 3(130), 1-2.
- Kagramanov N.D., Synchronous detachment of three radicals in ion series of mass spectra of polyoxaperfluoroalkanes and polyoxa perfluoroalkyl halides, Fluorine Notes, 2024, 2(153), 1-2.
- Kagramanov N.D., Mysov. E.I., Ion series of hexakis (trifluoromethylthio) - and hexakis (trifluoromethylseleno) -benzenes, as well as fluorobenzenes with perfluoroalkyl substituents, Fluorine notes, 2024, 3(154), 1-2.
- Kagramanov N.D., Ion series of mass spectra of perfluorocyclohexanesand perfluorocyclohexenes with perfluoroalkyl substituents, Fluorine Notes, 2025, 1(158), 1‑2.
- Kagramanov N.D., Fragmentation sequences - ion series of mass spectra of n-alkyl halides, α,ω-dihaloalkanes, α,ω-dihaloperfluoroalkanes.Effect of terminal halide mass and chain molecular weight, Fluorine Notes, 2022, 1(140), 5-6.
ARTICLE INFO
Received 10 September 2025
Accepted 2 October 2025
Available online October 2025
Recommended for publication by PhD M.A. Manaenkova
eLIBRARY Document Number (EDN) ZBNKRC

Fluorine Notes, 2025, 162, 3-4
