Received: August 2025
DOI 10.17677/fn20714807.2025.04.02
Fluorine Notes, 2025, 161, 3-4
DEVELOPMENT OF CERAMIC COMPOSITE MATERIALS BASED ON FLUORINE-SUBSTITUTED HYDROXYAPATITE DOPED WITH ZIRCONIUM DIOXIDE
V. M. Skachkov1, E. A. Bogdanova2, V. D. Ryabokrys3, O. V. Paporotny3, N. A. Sabirzyanov1
1 Institute of Solid State Chemistry of the Ural Branch of RAS, 91, Pervomayskaya street, Ekaterinburg, 620108 Russia
2 Joint Stock Company "Giredmet", 2 Elektrodnaya street, Moscow, 111524 Russia
3 Ural Federal University named after the first President of Russia B.N. Yeltsin, 19 Mira Street, Ekaterinburg, 620002 Russia
е-mail: skachkov@ihim.uran.ru
Abstract: In this work the synthesis of fluorine-substituted hydroxyapatite and composite materials based on it, strengthened with zirconium dioxide, was carried out. The behavior of components in systems Са10(РO4)6(ОН)F–ZrO2 and Са10(РO4)6(ОН)0.5F1.5–ZrO2 in a wide range of temperatures was studied. Influence of phase composition and amount of added additive on structural characteristics of the material is shown.
Keywords: fluorine-substituted hydroxyapatite, bioceramics, hardening, zirconium dioxide, composite materials, microhardness.
Introduction
Alongside Са10(РO4)6(ОН)2 (hydroxyapatite – HAP) the isostructurally analogous fluorine-substituted HAP Ca10(РO4)6(ОН)(2-x)Fx, which is applicable for bone scaffolding and as a coating material for metal implants [1], is also used in ceramics creation due to its greater strength and resistance to aggressive environments [2-5].
However, despite the active practical application, a relatively small number of published studies are dedicated to the comparative characteristics of fluorine-containing HAP powders depending on the method and conditions of synthesis, as well as the behavior of ceramics based on it in biological environments. The question of the maximum degree of substitution of ОНֿ groups with fluorine ions without negative biological consequences, in particular fluorosis, has not been resolved. Therefore, additional research is needed both in the field of technology of ceramics based on fluorine-substituted HAP, and in the evaluation of its biological behavior in experiments in vitro and in vivo [2].
In this regard, the synthesis and study of the properties of ceramic materials based on fluorine-substituted hydroxyapatite Са10(РO4)6(ОН)(2-x)Fx (х=0.5÷1.9), strengthened by zirconium dioxide, forming systems Ca10(PO4)6(ОН)(1-0.5)F(1-1.5)-ZrO2 were conducted.
Experimental part
For the purpose of obtaining of fluorosubstituted apatite 0.04 N water solution of calcium hydroxide was used with addition of 0.2 N solution of phosphoric acid with consequential addition of 0.25 N solution of hydrofluoric acid to the resulting suspension with the volumetric ratio of 3.75÷5.55 : 1 : 0.004÷0.088 (0.04 N VCa(OH)2 : 0.2 N VH3PO4 : 0.25 N VHF). рН values were set to 9-11 and the resulting mixture was stirred at room temperature for 10-15 minutes. Suspensions of fluorosubstituted apatite Ca10(PO4)6(OH)2-xFx, where 0.5≤х≤1.9, with different viscosities were obtained.
Strengthening of fluorosubstituted apatite was carried out by adding a zirconia – ZrO2 (pure, TU 6-09-2486-77). The content of the doping component in the composites Ca10(PO4)6(ОН)(1-0.5)F(1-1.5)-ZrO2 was selected on the basis of previous studies conducted by the authors [6], where it was established that the content of ZrO2 in the sample composition should not exceed 10 wt%.
The initial components (Fig. 1) were certified by BET method using Gemini VII 2390 V1.03 surface area and porosity analyzer, V1.03 t, particle size was evaluated during the analysis of variance using the Horiba LA-950 universal laser express particle size distribution analyzer, the thermal stability of the samples was investigated using differential thermal and thermal weight analysis (DTA) on a THERMOSKAN-2 thermal analyzer (Analytpribor, Russia) at a heating rate of 10°C/min in thin-walled quartz crucibles up to 800°C in air.
|
|
|
|
|
a |
b |
c |
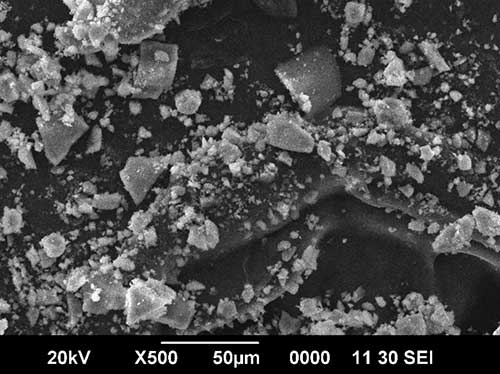
Figure 1. Morphology of original materials: a – fluorine-substituted hydroxyapatite Са10(РO4)6(ОН)F [3]; b –
is fluorosubstituted hydroxyapatite Са10(РO4)6(ОН)0.5F1.5 [3];
c –
zirconium dioxide ZrO2.
In order to obtain a strengthened bioceramics, the components were mixed without the addition of a homogenizer in a vibration mill (MLW 4000 KM 1) with agate mortar and ball for 30 min. The resulting composite mixtures and powders of fluorine-substituted hydroxyapatite, used as reference samples were formed into 0.5-1.0 g tablets by uniaxial double-sided pressing without introduction of binding agent in a cylindrical steel mold with diameter of 10 mm on a hydraulic manual press without holding at room temperature and pressure of 20 MPa.
The samples were sintered in a Nabertherm L 9/11 muffle furnace at 25-1200°C in 200°C increments, the heating rate was 10°C/min, held for 1 hour in an air atmosphere, and cooled with the furnace. Composite materials annealed at various temperatures were qualified by X-ray phase analysis (XRD) on Shimadzu and DRON-2.0 diffractometers (CuKα radiation, angle interval 10°≤ 2θ ≤ 70°, shooting step 0.03°, time per point 2 seconds, identification of phases using Powder Diffraction File JJ CPDSD-ICDD PDF2 (set's 1-47)).
Shrinkage of compacts during sintering was evaluated by changing geometric parameters using a micrometer MK 0-25 mm. Measurement of the microhardness of composite materials by the Vickers method was carried out on a microhardness meter PMT-3M with a load of 0.98 N (100 g) and a loading time of 10 s. The values of microhardness (MPa) by the Vickers (HV) method were calculated by formula:
HV=0.189P/d2×106 (1),
where P is normal load applied to the diamond tip, N; d is the arithmetic mean of the lengths of both diagonals of the print, μm.
Compressive strength was determined at room temperature on a Liangong Group CMT-5L (PRC) electromechanical universal test machine with automatic control and PC data processing by MaterialTest 3.0, accuracy class 0.5, maximum load 5 kN (~ 500 kg). The speed of movement of the loading device beam was 1 mm/min. Compressive strength, σComp, MPa, was calculated using the formula:
σComp=P/S (2),
where P is breaking load, N; S is the sample area, mm2.
Morphological features were examined by scanning electron microscopy (SEM) on a JEOL JSM 6390 LA microscope (Japan), magnification coefficient from x5 to x300,000, resolution 3.0 nm at 30 kV.
Discussion of results
Synthesis of fluorine-substituted hydroxyapatite-based composite ceramic reinforced with ZrO2
Since the present work is aimed at obtaining strengthened bioceramics, and, according to literature data [7-9], it is possible to achieve a high-strength state using nanostructured materials, fluorine-substituted hydroxyapatite Ca10(PO4)6(OH)2-xFx (0.5≤х≤1.9) obtained by precipitation from solution was used as initial component [10]. The selected synthesis method avoids an increase in particle size, a decrease in specific surface area and crystal lattice defects characteristic of solid-phase reactions [9], which further ensures the production of ceramics with a uniform microstructure, open porosity close to zero, small crystal size and increased strength.
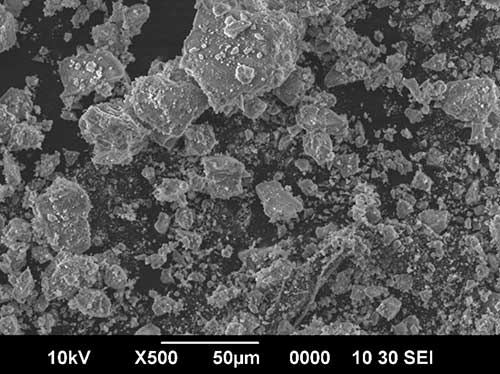
The synthesized fluorapatites Ca10(PO4)6(OH)2-xFx are poorly crystallized and consist of submicron (nanoscale) particles combined into soft agglomerates (Fig. 2, Table 1), which are broken by processing of the dried product in ball mills. In this regard, in order to increase the dispersed state of the components, the reinforcement of the fluorine-substituted HAP with zirconium dioxide ZrO2, actively used as a strengthening component, was carried out by mechanochemical activation of the initial components in a vibration mill, which also contributed to the future achievement of a high-strength state [11].
|
|
|
|
a |
b |
Figure 2. Micrographs of aggregated particles of crystalline fluorine-substituted hydroxyapatite: a – Са10(РO4)6(ОН)F; b – Са10(РO4)6(ОН)0.5F1.5.
Table 1. Characteristics of initial components.
|
Test sample |
Material characteristics |
||||
|
Specific surface area, m2/g |
Pore area, |
Pore volume, |
Particle size, μm (measured) |
Mid-surface diameter, nm (calculated) |
|
|
Са10(РO4)6(ОН)F |
97.53 |
5.77 |
0.0027 |
1.88 |
21.68 |
|
Са10(РO4)6(ОН)0.5F1.5 |
131.82 |
9.25 |
0.0047 |
1.89 |
15.77 |
|
ZrO2 |
7.48 |
- |
- |
0.5-0.8 |
- |
Results of annealing a fluorine-substituted hydroxyapatite-based composite ceramic reinforced with ZrO2
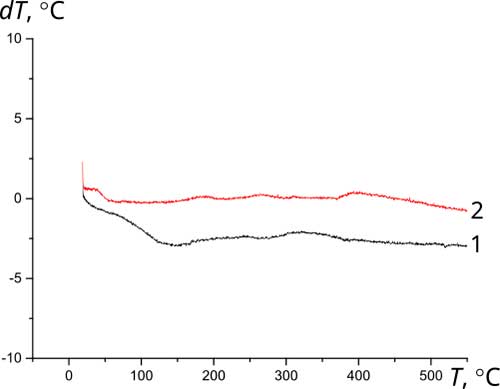
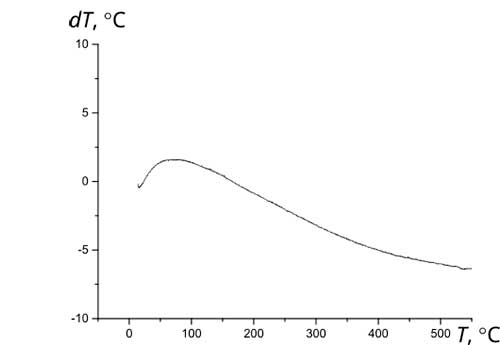
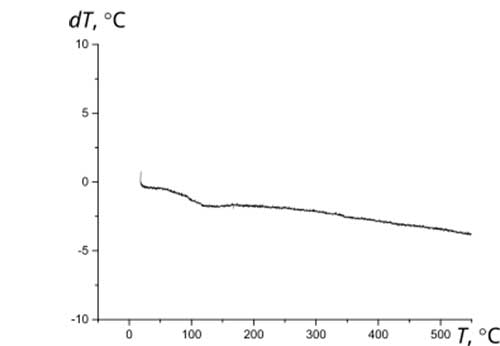
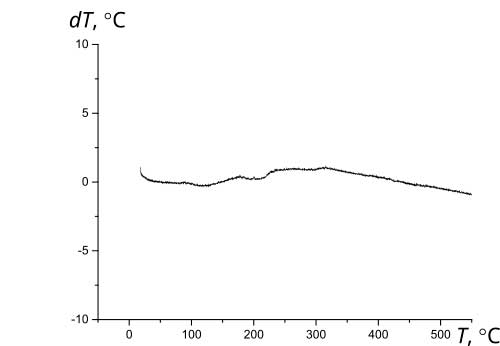
The initial materials used herein as components of Ca10(PO4)6(ОН)(1-0.5)F(1-1.5)-ZrO2 composites are thermally stable. According to the thermogravimetric analysis, their high-temperature annealing is not accompanied by pronounced thermal effects (Fig. 3).
|
|
|
|
a |
b |
|
|
|
|
c |
d |
Figure 3 Thermograms of initial materials: a - fluorine-substituted HAP Са10(РO4)6(ОН)F (1 – primary heating; 2 – reheat); b – zirconium dioxide ZrO2; c – composite (1)fluorosubstituted HAP- zirconium dioxid Са10(РO4)6(ОН)F-10%ZrO2; d - composite (1.5)fluorosubstituted HAP- zirconium dioxid Са10(РO4)6(ОН)0.5F1.5-10%ZrO2.
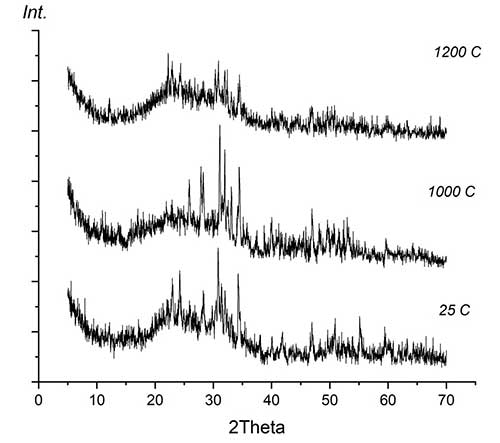
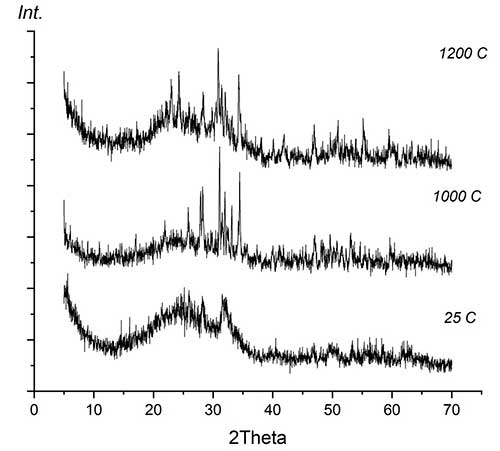
Thermal stability during high-temperature treatment along with lower solubility and greater resistance to aggressive media is a significant advantage of fluorine-substituted HAP for the purpose of obtaining bioactive ceramics compared to stoichiometrically deposited HAP, which begins to decompose at temperatures above 800°C [12]. The presence of fluorine in the crystal lattice prevents the decomposition of apatites into tricalcium phosphate Са3(РO4)2 (TCP), structurally stabilizing the fluorine-substituted HAP [3, 12]. After annealing at 1000°C, HAP, as a rule, contains 30-50% of the TCP phase, completely decomposing at 1200°C [2], while fluorine-substituted HAP only partially decomposes at 1200°C to TCP with the formation of CaF2. According to XRD data (Fig. 4), the phase composition of Ca10(PO4)6(ОН)(1-0.5)F(1-1.5)-ZrO2, composites based on fluorine-substituted HAP remains constant in the temperature range of 25-1000°C. After annealing at 1200°C, the TCP content in the composites Са10(РO4)6(ОН)F-10%ZrO2 и Са10(РO4)6(ОН)0.5F1.-10%ZrO2 did not exceed 20% and 10%, respectively, with an additional formation of ~10% of CaF2.
|
|
|
|
a |
b |
Figure 4 Results of X-ray phase analysis of composite materials based on fluorine-substituted hydroxyapatite at different temperatures:
a – composite Са10(РO4)6(ОН)F–ZrO2; b – composite Са10(РO4)6(ОН)0.5F1.5–ZrO2.
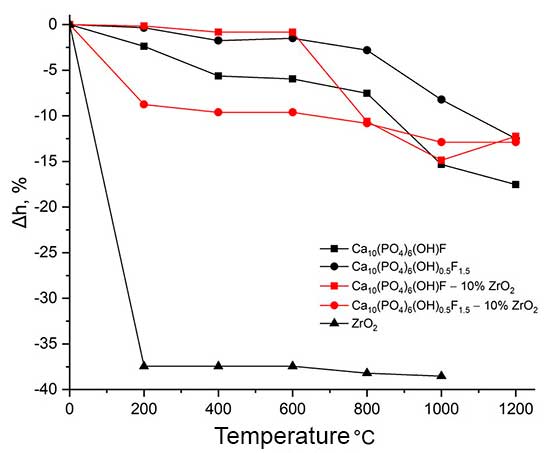
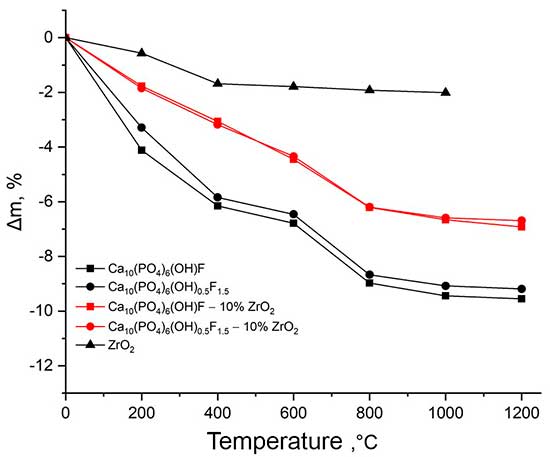
During temperature treatment, ceramic sintering occurs, the density of the material changes, which is accompanied by a change in the linear parameters and weight of the sample. Evaluation of the linear parameters of the studied samples made it possible to establish the correlation between the linear shrinkage of the sintered materials and their composition and firing temperature (Fig. 5).
|
|
|
|
a |
b |
Figure 5. Change of parameters of initial components and composites of
Ca10(PO4)6(ОН)(1-0.5)F(1-1.5)–ZrO2 during sintering: a – linear shrinkage of test samples; b – weight loss of test samples.
The conditions of synthesis of composite materials chosen in the work, aimed at increasing their activity during sintering, made it possible to obtain powder blanks (compacts) with a maximum shrinkage in the range of 800-1200°C and a shrinkage onset temperature of ~ 600°C, which is 100-150°C lower than for HAP obtained by solid-phase synthesis [9]. Judging by the shape of the curve (Fig. 5 a) the impact of the ZrO2 additive on the compaction of the Са10(РO4)6(ОН)0.5F1.5 composite affects the initial stage of heat treatment, while the nature of the following sintering is determined by the presence of fluorine-substituted hydroxyapatite in the sample. For a Са10(РO4)6(ОН)F composite, the change in linear parameters in the low temperature region is consistent with the change in the parameters of the starting fluorine-substituted hydroxyapatite, and the effect of zirconium dioxide on the sintering of the sample is manifested at temperatures above 600°C.
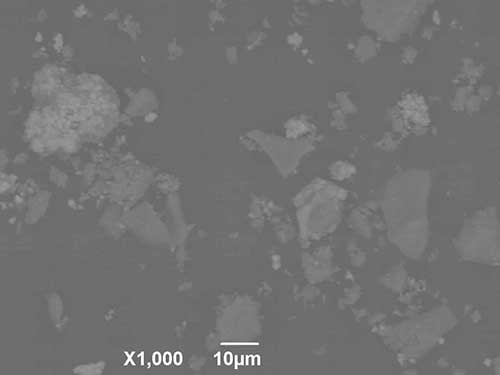
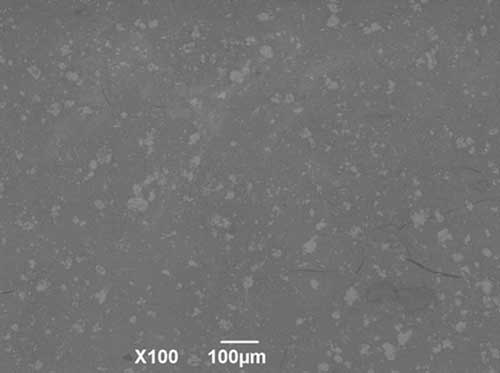
Changes in the linear parameters and weight loss of the test samples due to structural disorder occurring during sintering of composite materials take place uniformly, which allows to avoid cracking and enables to obtain dispersion-strengthened materials with a dense structure and uniform distribution of the reinforcing phase throughout the sample (Fig. 6).
As a result of high-temperature annealing, the strength characteristics of the samples change (Table 2). It has been experimentally established that the reinforcement of fluorine-substituted hydroxyapatite Ca10(PO4)6(ОН)(1-0.5)F(1-1.5) with zirconium dioxide improves the strength characteristics of the material over a wide range of temperatures. In addition, according to XRD data, after heat treatment at 1200°C, another strengthening phase is found in the test samples – calcium fluoride CaF2.
|
|
|
|
a |
b |
Figure 6. Composite surface morphology Ca10(PO4)6(ОН)(1-0.5)F(1-1.5)-ZrO2 after heat treatment at 1000°С: a - powder; b - tableted sample.
Table 2. Microhardness of starting components and composite materials at various temperatures.
|
Test sample |
Vickers hardness (HV), MPa |
||||||
|
25°C |
200°C |
400°C |
600°C |
800°C |
1000°C |
1200°C |
|
|
ZrO2 |
38 |
40 |
74 |
64 |
67 |
101 |
– |
|
Са10(РO4)6(ОН)F |
63 |
80 |
142 |
116 |
134 |
406 |
244 |
|
Са10(РO4)6(ОН)0.5F1.5 |
61 |
82 |
108 |
156 |
129 |
179 |
398 |
|
Са10(РO4)6(ОН)F–10%ZrO2 |
76 |
108 |
173 |
157 |
199 |
215 |
335 |
|
Са10(РO4)6(ОН)0.5F1.5–10%ZrO2 |
83 |
101 |
154 |
159 |
110 |
262 |
350 |
Strength test results
The compressive strength was evaluated depending on the qualitative and quantitative composition of the ceramic samples obtained during research (see Table 3). Cylindrical samples (Ø=10 mm; h=10 mm) after high temperature treatment at 1000 and 1200°С were compressed.
Table 3. Results of evaluation of compressive strength of initial components and composite materials.
|
Test sample |
Compressive strength (σComp), MPa |
|
|
1000°C |
1200°C |
|
|
ZrO2 |
506 |
– |
|
Са10(РO4)6(ОН)F |
448 |
570 |
|
Са10(РO4)6(ОН)0.5F1.5 |
498 |
400 |
|
Са10(РO4)6(ОН)F–10%ZrO2 |
474 |
549 |
|
Ca10(PO4)6(ОН)(1-0.5)F(1-1.5)-10%ZrO2 |
526 |
551 |
Analysis of the obtained data, their comparison with the results of microhardness measuring and XRD data, allows us to conclude that composites Ca10(PO4)6(ОН)(1-0.5)F(1-1.5)-ZrO2 have the best strength characteristics.
The recommended temperature for the production of ceramics, at which it retains the original composition and has the desired functional characteristics, should not exceed 1000°С.
Conclusions
In the course of the research the strengthening and stabilizing effect of the zirconium dioxid ZrO2 doping additive was studied in a wide temperature range of 25-1200°C during mechanochemical reinforcement of fluorine-substituted hydroxyapatite FAP Ca10(PO4)6(OH)2-xFx, (х=1; 1.5), obtained by precipitation from solution. The main characteristics of the obtained materials were determined: phase composition, morphology, linear shrinkage, microhardness, compressive strength. It was experimentally established that in the presence of zirconium dioxide even with high-temperature annealing at 1200°C, the fluorine-substituted HAP decomposes slightly with the formation of TCP and calcium fluoride CaF2. Despite the high thermal stability, the recommended temperature for the production of ceramics Ca10(PO4)6(ОН)(1-0.5)F(1-1.5)ZrO2 is 1000°C, at which the developed materials maintain the desired phase composition with high strength characteristics. The resulting composites have a dense uniform structure with a high degree of crystallinity, which makes them a promising object for further research in order to develop materials for restorative medicine.
Acknowledgements
The work was carried out in accordance with the state assignment and research plans of the Federal State Budgetary Scientific Institution «Institute of Solid State Chemistry of the Ural Branch of the Russian Academy of Sciences» (No 124020600007-8).
References
- A. S. Pankratov, I. S. Fadeeva, V. V. Minaichev, P. O. Kirsanova, A. S. Senotov, Yu. B. Yurasova, Problems of biointegration of micro- and nanocrystalline hydroxyapatite and approaches to their solution, Genes and cells, 2018, 13(3), 46-51. https://doi.org/10.23868/201811032. (in Russian)
- S. М. Barinov, V. S. Komlev, Bioceramics in medicine, Moscow: Nauka, 2005, 284 p. (in Russian)
- Bogdanova E.A., Skachkov V.М., Medyankina I.S. et al. Formation of nanodimensional structures in precipitated hydroxyapatite by fluorine substitution, SN Applied Sciences, 2020, 2(9), 1565. https://doi.org/10.1007/s42452-020-03388-5.
- Chen Y., Miao X. Thermal and Chemical Stability of Fluorohydroxyapatite Ceramics with Different Fluorine Contents, Biomaterials, 2005, 26(11), 1205-1210. https://doi.org/10.1016/j.biomaterials.2004.04.027.
- Zang, M., Li L., Sun X. et al. Characterization, mechanical properties, corrosion behavior and bone-like apatite formation ability of fluorine substituted hydroxyapatite coating, Journal of the Mechanical Behavior of Biomedical Materials, 2024, 151,106364. https://doi.org/10.1016/j.jmbbm.2023.106364.
- E. A. Bogdanova, I. М. Giniyatullin, D. I. Pereverzev, V. М. Razgulyaeva, Influence of reinforcement additives on sintering and hardening processes of nanoscale hydroxyapatite, Physical and chemical aspects of the study of clusters, nanostructures and nanomaterials, 2019, 11, 548-554. https://doi.org/10.26456/pcascnn/2019.11.548. (in Russian)
- N. V. Petrakova, S. M. Barinov, E. V. Evstratov et al. Compaction of hydroxyapatite nanopowders using hydrostatic pressing, Materials science, 2016, 11, 35-41. (in Russian)
- N. V. Bakunova, S. M. Barinov, V. M. Ievlev et al. Influence of heat treatment on sintering and strength of hydroxyapatite nanopowder ceramics, Materials science, 2010, 12, 11-15. (in Russian)
- T. V. Safronova, M. A. Shekhirev, V. I. Putlyaev, Yu. D. Tretyakov, Ceramic materials based on hydroxyapatite, obtained from solutions of various concentrations, Inorganic materials, 2007, 43(8), 1005-1014. (in Russian)
- RF Patent 2652193, IPC C01B 25/32, Method for production of hydroxyapatite suspension, N. А. Sabirzyanov; applicant and rightholder – Institute of Solid State Chemistry of the Ural Branch of RAS; publ. 25.04.2018, Bull. No. 12. (in Russian)
- V. V. Boldyrev, Mechanochemistry and Mechanical Activation of Solids, Advances in Chemistry, 2006, 75(3), 203-216. (in Russian)
- Е. А. Bogdanova, N. А. Sabirzyanov, Investigation into thermal stability of fluorine-substituted HAP, Materialovedeniye, 2015, 1, 52-56. (in Russian)
ARTICLE INFO
Received 18 August 2025
Accepted August 2024
Available online August 2025
Recommended for publication by PhD M.A. Manaenkova
eLIBRARY Document Number (EDN) JWKMSO

Fluorine Notes, 2025, 161, 3-4