Communication 1. Application of elemental fluorine for synthesis of organofluorine compounds "
Received: July 2025
DOI 10.17677/fn20714807.2025.04.01
Fluorine Notes, 2025, 161, 1-2
A SERIES OF LECTURES. SOME DIRECTIONS OF FLUORINE CHEMISTRY
COMMUNICATION 1. APPLICATION OF ELEMENTAL FLUORINE FOR SYNTHESIS OF ORGANOFLUORINE COMPOUNDS
V.V. Kornilov
Abstract: Possible mechanisms of fluorination of hydrocarbons and alkenes by elemental fluorine are considered. A brief overview of classical methods of fluorination with elemental fluorine and examples of the use of elemental fluorine on an industrial scale for the synthesis of organofluorine compounds are given.
Key words: elemental fluorine, free-radical processes, recombination of radicals, fluorination, organofluorine compounds.
Introduction
The articles of this series are based on lectures that were prepared in 2018-2019 for presentations to students at Zhejiang Normal University (Zhejiang, Jinhua, China).
The original idea of such lectures belonged to the Professors of the Basic faculty of Lensoviet Leningrad Institute of Technology at SIAC (State Institute of Applied Chemistry) B.N. Maximov, V.G. Barabanov and I.L. Serushkin, who passed on their knowledge in the field of fluorine chemistry to senior students in the 80s of the last century, including the author of the article.
This form of education made it possible to graduate specialists who, by the time they received their higher education diploma, already had a good foundation of knowledge in their specialty. Therefore, I would like to express my gratitude to my teachers for their scientific and educational activities.
Some of the most interesting reports from the author's point of view will be prepared for publication, in which an attempt has been made to take into account both the current level of knowledge in this field and the new directions of application of fluorine and its compounds that have appeared in recent decades.
1. Mechanism of fluorination with elemental fluorine
Fluorination reactions using elemental fluorine have all characteristics of free radical processes, which mechanism is similar to the well-known reaction mechanism of photochemical chlorination (Table 1) [1]:
Table 1.
|
Chlorination |
ΔH kJ/mol |
Fluorination |
ΔH kJ/mol |
|
Chain Initiation: 1. Cl2 + hv→2Cl∙ |
242,83 |
Chain Initiation: 1a. F2 →2F∙ |
157,01 |
|
Chain Propagation and Transfer: 2. RH + Cl∙ → R∙ + HCl 3. R∙ + Cl2→RCl + Cl∙ |
-12,56 -96,29 |
Chain Propagation and Transfer: 2. RH + F∙ → R∙ + HF 3. R∙ + F2→RF + F∙ |
-142,35 -284,70 |
|
Chain Termination: 4. R∙ + Cl∙ →RCl 5. Cl∙ + Cl∙ → Cl2 6. R∙ + R∙ → R–R |
-334,91 -242,83 -347,46 |
Chain Termination 4. R∙ + F∙ →RF 5. F∙ + F∙ → F2 6. R∙ → Fragmentation |
-447÷ -468 -157,01 |
The relatively low value of the dissociation energy of the fluorine molecule means that a significant number of fluorine atoms can participate in reactions even at room temperature. Besides, a low activation energy of the hydrogen abstraction reaction would mean that even this low degree of fluorine molecules dissociation would be sufficient to start the chain process.
Energy isolating at forming of F-H and C-F bonds is spent on dissociation of fluorine atoms. That’s why elemental fluorine reacts violently with organic compounds, at that these reactions are not selective in the majority of cases (Table 2).
Table 2. Selectivity of the action of various radicals (X∙) on primary, secondary and tertiary hydrogen atoms in alkanes.
|
Radical |
– CH3 |
– CH2 - | |
|
X=F∙ |
1 |
1,2 |
1,4 |
|
X=Cl∙ |
1 |
3,9 |
5,1 |
|
X=Br∙ |
1 |
82 |
1600 |
Serious difficulties in using elemental fluorine are also caused of the high heats of formation of C-F and H-F bonds (approximately 456 and 560 kJ/mol, respectively) [2]. This energy exceeds the energy of C-C bond [347,46 kJ/mol] and leads to fragmentation of carbon backbone.
In 1956 William T. Miller [3] suggested a scheme, which was different from common radical-chain mechanism. According to the suggested scheme fluorine molecule reacts to hydrocarbon molecule producing alkyl radical, hydrogen fluoride and a fluorine atom (Scheme 1).
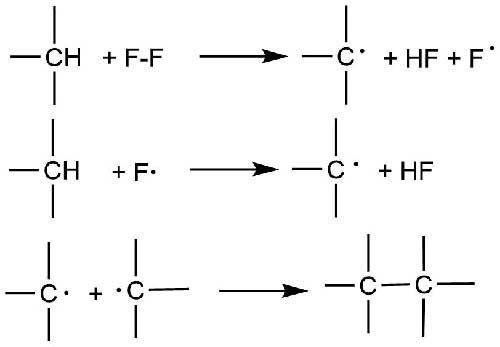
Scheme 1.
This pathway of appearing of fluorine radicals (F∙) is energetically more preferable than the dissociation of fluorine molecule into atoms (16,3 kJ/mol [2] against 157,01 kJ/mol for fluorine dissociation) and may occur quite probably, especially at low fluorination temperature (-40 °С and below). However, no clear experimental evidence has been provided.
2. Interaction of Elemental Fluorine with alkenes
Interaction of fluorine with alkenes can occur by both double bond and via substitution of hydrogen. At that usually a complex mixture of compounds is obtained. However, in case if all hydrogen atoms in alkene are substituted with chlorine and/or fluorine the carrying out of syntheses with obtaining of target products is possible.
Conditions of carrying out such processes were studied and a probable common reaction mechanism was suggested, that explained the composition of obtained products [4]. The reactions were carried out at low temperature and with stirring.
The key feature of the proposed mechanism was the assumption that fluorine molecule quickly reacted with double alkene bond even at low temperature forming fluorinated free radical and fluorine atom (Scheme 2, reaction 1).
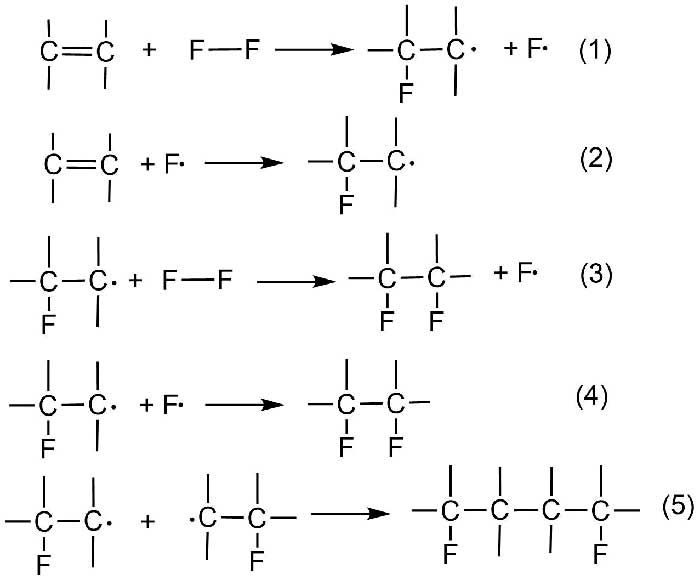
Scheme 2.
This process is formulated as the rate-controlling initiation step 1. Later fluorine atom can react either with new alkene molecule forming new fluorinated free radical (reaction 2) or combine with the first-formed free radical (reaction 4). At that a termination of chain occurs.
Combination of the radicals produced by reactions 1 and 2 yields fluorine dimer addition product (reaction 5). The occurrence of reactions 1 and 2 was shown by experiments with the addition of oxygen and chlorine, in which fluorine served as a free-radical initiator.
Thus, nonreactive mixture of tetrachloroethylene (2,8 mol) and chlorine (1,1 mol) was itself turned into hexachloroethane (with the yield of 85%) over traces of fluorine (0,02 mol) (Scheme 3) [5].
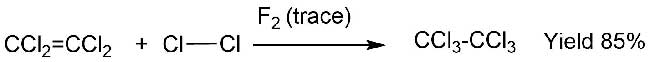
Scheme 3.
The whole scheme of reactions 1-5 was presented by W. T. Miller in article [6] (Figure 1). It was found, that formation of polymers higher than dimers, has not been observed during fluorination (reaction 6 at Figure 1).
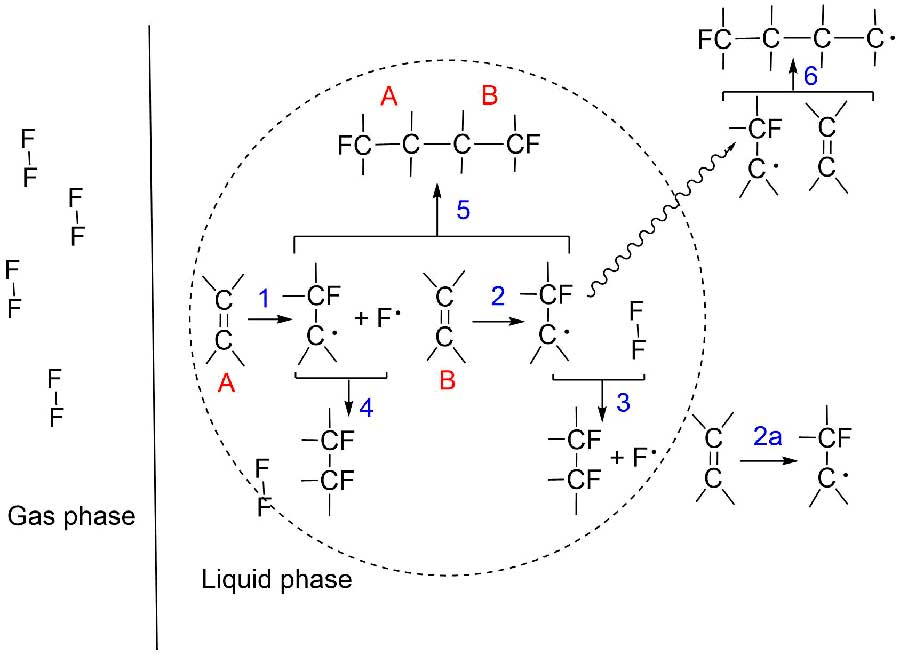
Figure 1. Proposed general fluorine-alkene free radical reaction mechanism [6].
It is considered, that recombination (reaction 5, Fig.1) or fluorination of free radicals which diffuse away from their initial sites is usually much more probable than their addition to new alkene molecule [6].
Thus, by regulating the concentration and feed rate of fluorine and also by altering the temperature of the process carrying out we can direct the process where we need.
The results of fluorination of chlorofluoroethylenes at different temperatures and ratio of fluorine-nitrogen mixture are listed in the Table 3. Rate of F2 addition was 0,8 g/hour in all syntheses.
Table 3. Fluorination of chlorofluoroethylenes [6].
|
Alkene |
N2/F2 Ratio* |
Temp. °C |
Molar Ratio C2F2X4/C4F2X8 |
Dimer Yield (%) |
|
CFCl=CFCl |
8:1 8:1 |
-75 -110 |
0,8 0,5 |
35 52 |
|
CF2=CCl2 |
8:1 45:1 |
-110 -110 |
0,3 0,3 |
77 79 |
|
CF2=CFCl |
8:1 8:1 45:1 |
-110 -150 -150 |
2 0,7 0,4 |
14 48 64 |
Discovered regularities of haloalkenes fluorination processes using elemental fluorine have practical application nowadays (see 4.1).
3. Fluorination methods
The most complete reviews of classical methods of fluorination with elemental fluorine are presented by J. Tedder in the book “Advances in Fluorine Chem.” [7], as well as in Houben-Weyl Methods of Organic Chemistry [8].
The main task at direct fluorination is heat elimination. It is reached the following ways:
1) passing of fluorine and inert gas mixture through cooled liquid,
2) carrying out the reaction over fine metal packing,
3) carrying out the reaction in vapor phase at diluting mixture with big amount of inert phase.
In the first case mixture of fluorine and inert gas or pure fluorine is bubbled through organic compound solved in inert solvent or in the absence of solvent at a low temperature.
Application of inert solvents (chlorofluorocarbons, fluorocarbons, hydrogen fluoride and others) makes the process easier, but brings up additional difficulties. For example, carbon tetrachloride is a fine solvent, but it is not inert enough, at the same time though fluorocarbons are inert they are weaker solvents of organic compounds.
The second method involves passing vapors of organic compounds and fluorine, diluted with nitrogen, through a reactor with finely dispersed metal packing. The purpose of metal packing is to dissipate the heat of reaction and that’s why the fluorination passes at low or moderate temperature. If fluorination is carried out at high temperature, then we can’t exclude the fact, that the layer of metal fluorides is formed on the surface of metal and the fluorinated surface can act as fluorinating agent.
There are also other methods of fluorination with elemental fluorine (direct aerosol fluorination, etc.). Examples of direct fluorination using modern methods can be found in [2, 9].
The interest in direct fluorination is explained by the fact that in this case, complex multi-stage synthesis is not required to obtain the target compound. It is possible to construct a hydrocarbon molecule, taking available components as a basis, and then try to replace the necessary hydrogen atoms with fluorine.
For example, in the article by S. Rosen [10] the substitution of hydrogen for fluorine at the tertiary carbon atom in complex molecules at low temperatures is described.
Thus, when trans-4-methylcyclohexyl p-nitrobenzoate was treated with 3–4% fluorine in N2, a major product, identified as trans-4-fluoro-4-methylcyclohexyl p-nitrobenzoate, was obtained in 60% yield.
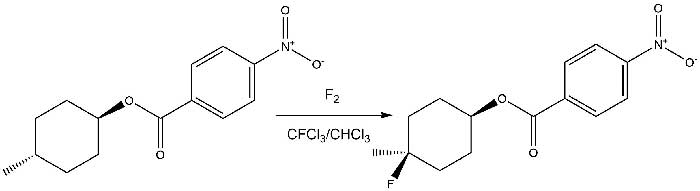
Scheme 4.
Also, in [10] a method for obtaining an important class of fluorinated compounds, namely perfluoroalkyl vinyl ethers (RfOCF=CF2) according to Scheme 5, was proposed.
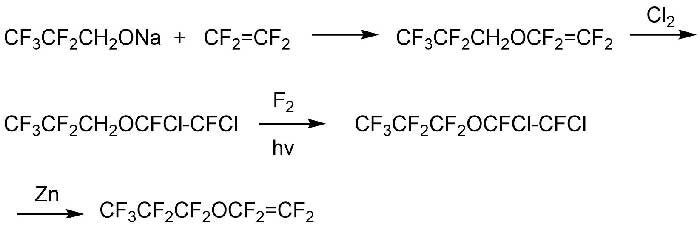
Scheme 5.
The salt had been obtained from 1,1,1,2,2 -pentafluoropropanol and sodium hydride, which was then reacted with tetrafluoroethylene to form a vinyl ether. To protect the double bond, chlorination was carried out by bubbling gaseous chlorine at 10-15 °С.
The obtained product was fluorinated with elemental fluorine under the following conditions:
- temperature -10 °С
- fluorinating reagent: 25–30% F2 diluted with nitrogen
- solvent: perfluoro 2-butyl-THF (or perfluoropolyether Krytox GPL 100) with 5 g of pulverized NaF to absorb the released HF
- irradiation with a 450 W, medium pressure mercury lamp
Yield of perfluoropropylvinyl ether was about 70%.
In spite of method’s perspective and some good results, difficulties of working with elemental fluorine led to the fact, that direct fluorination of organic compounds is being applied not so widely in industry even up to the present.
The fluorination of compounds with functional groups (hydroxyl, carbonyl, amines, etc.) poses particular difficulties, often resulting in the destruction of the original compounds with the loss of such groups.
4. Examples of application of fluorination with elemental fluorine
4.1. Obtaining of perfluoropolyethers (PFPE) via fluorine initiation
Liquid-phase oxidation of perfluoroalkenes is used in industry to produce perfluoropolyethers (PFPE), which have been known since the 1960s under the trade name Fomblin® (Solvay).
The oxidation process of tetrafluoroethylene (TFE) and hexafluoropropylene (HFP) is carried out with molecular oxygen at low temperature in the presence of an initiator to obtain oligomeric products of the approximate structure 1 and 2 in Scheme 6, respectively.
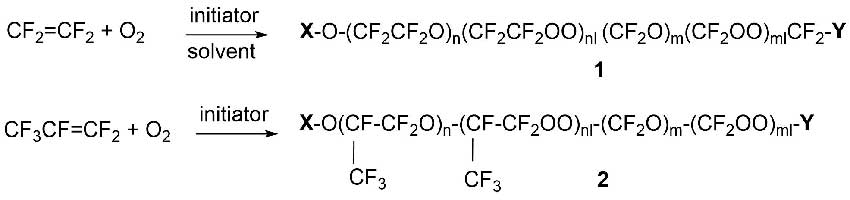
Scheme 6.
In most cases groups X and Y are perfluoroalkyl or acyl fluoride (CF3, -CF2CF3, -COF or -CF2COF).
Elemental fluorine can act as one of the chemical initiators of these processes [11,12].
For example, the oxidation process of HFP is schematically shown in Scheme 7 [11].
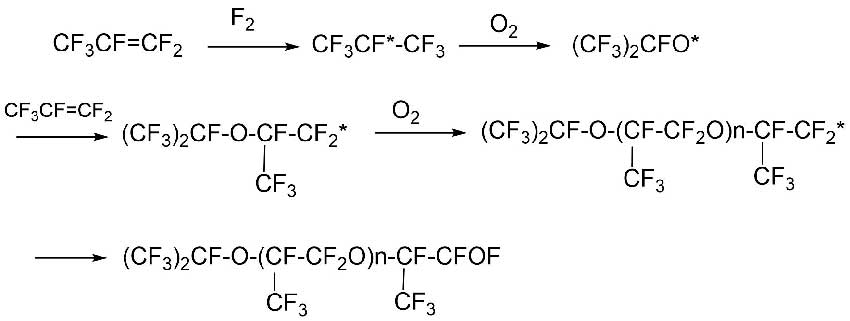
Scheme 7.
4.2. Catalytic Fluorination of Carbon Oxide.
Gas-phase carbon oxide fluorination includes two stages [13]:
CO + F2= COF2 –525 kJ/mol
COF2 + F2= CF3OF –135 kJ/mol
The rate of the second stage of this process can be significantly increased by using a catalyst or initiator.
The obtained trifluoromethyl hypofluorite (TFMHF) can also be used in the processes of PFPE synthesis as an initiator (Scheme 6) [14].
4.3. Purification of perfluorocarbon liquids from unsaturated and hydrogen-containing polyfluorinated compounds by gas-phase fluorination
The paper [13] describes a method for purifying polyfluorinated compounds from residual hydrogen and unsaturated bonds. As an example, the process of fluorination of perfluoro(methylcyclohexane) (PFMCH), which contained impurities of hydrogen-containing and unsaturated compounds, is given (Scheme 8).
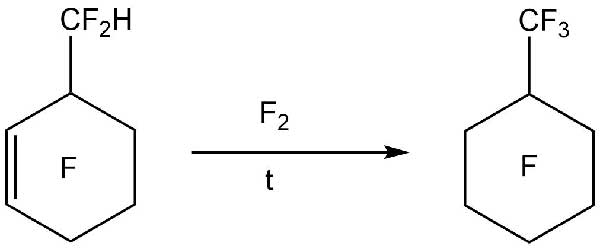
Scheme 8.
The results of PFMCH fluorination at packing of NiF2/α-Al2O3 are listed in the Table 4. (mol. ratio PFMCH:F2 = 1,4).
Table 4. The results of PFMCH fluorination at packing of NiF2/α-Al2O3 [13].
|
# |
Packing |
Temperature, К |
Degree of substrate destruction, % |
Purification Degree (%) |
|
1 |
NiF2/α-Al2O3 |
573 |
0,1 |
99,2 |
|
2 |
-«- |
573 |
0,2 |
99,2 |
|
3 |
-«- |
623 |
0,2 |
99,5 |
|
4 |
-«- |
623 |
0,2 |
99,5 |
|
5 |
-«- |
673 |
0,3 |
99,8 |
|
6 |
-«- |
673 |
0,2 |
99,9 |
4.4. Obtaining neutral perfluoropolyethers (PFPE)
The process of obtaining neutral PFPE consists of replacing the terminal acyl fluoride groups (X and/or Y, Scheme 6), which are obtained during the liquid-phase oxidation of TFE or HFP (see 4.1.), with fully fluorinated ones (Scheme 9) [15].
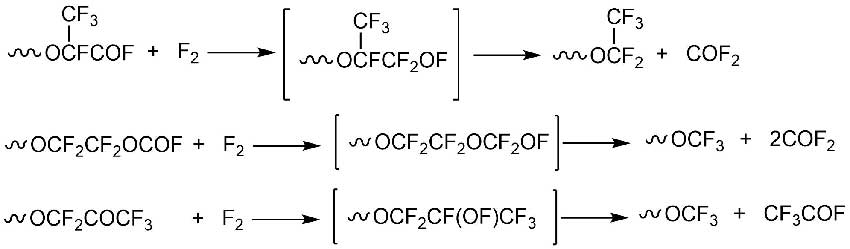
Scheme 9.
Such "fluorine-finishing" reactions are performed under a slight positive pressure of the halogen either thermally at temperatures often exceeding 200°C or photochemically below 100°C. The reactions probably proceed through hypofluorite intermediates that are detectable and even isolable in some instances with photochemical initiation [15].
References
- A Sheppard W.A., Sharts C.M., Organic Fluorine Chemistry, W.A. Benjamin, New York, 1969.
- Chambers R., Fluorine in Organic Chemistry, Blackwell Publishing Ltd, 2004, ISBN 1-4051-0787-1.
- Miller W., Dittman A., The Mechanism of Fluorination. I. Fluorine Sensitized Oxidation of Trichloro- and Tetrachloroethylene// J.Am.Chem.Soc.-1956.- v.78-p. 2793; b)
- Miller W., Koch S. The mechanism of Fluorination III// J.Am.Chem.Soc.-1957.- v.79- p.3084.
- Miller, William T., Koch, Stanley D., McLafferty, Fred W. The Mechanism of Fluorination. II. Free Radical Initiation Reactions. Fluorine-sensitized Chlorination and Oxidation// J.Am.Chem.Soc.-1956.-V. 78., Iss. 19.DOI: 10.1021/ja01600a050
- Miller, William T., Stoffer James, Fuller George and Currie Andrew. The Mechanism of Fluorination IV. The Effect of Temperature and of Fluorine Concentration upon the Olefin Dimerization Reaction. A New Fluorination Apparatus. // J.Am.Chem.Soc.-1964-V. 86 – p.51
- Tedder J.M., The fluorination of organic compounds using elementary fluorine. In: M. Stacey, J. C. Tatlow, and A. G. Sharpe (Eds.). Advances in Fluorine Chem. 2, 104–137 (1961).
- Houben-Weyl Methods of Organic Chemistry Vol. E 10a, 4th Edition Supplement: Organo-Fluorine Compounds - Fluorinating Agents and Their Application in Organic Synthesis, eds. Bernd Baasner, Thieme, 2014, ISBN 3131815442, 9783131815446.
- Okazoe, T. (2018). Perfluorination with F2. In: Hu, J., Umemoto, T. (eds) Fluorination. Synthetic Organofluorine Chemistry. Springer, Singapore, DOI: 10.1007/978-981-10-1855-8_22-1.
- Rozen S., Direct Fluorination of Organic Compounds with Elemental Fluorine. //Efficient Preparations of Fluorine Compounds, First Edition. Edited by Herbert W. Roesky, 2013, John Wiley & Sons, Inc., p. 146.
- B.N. Maksimov, V.V. Kornilov, B.A. Melnichenko, L.N. Kosareva. Perfluoropolyethers. Synthesis and Application. // Russian Journal of Applied Chemistry, 2009, Vol. 82, No. 9, pp. 1706−1710. DOI: 10.1134/S107042720909033X.
- D. Sianesi, P. A. Guarda, G. Marchionni, A Kinetic and Mechanistic Study of the Low-Temperature Fluorine-Initiated Copolymerization of Tetrafluoroethylene with Oxygen, Macromolecules, 1999, Vol. 32, Iss. 23, pp. 7768-7780, DOI: 10.1021/ma9906985..
- Vladimir Y. Zakharov. Chemistry and Technology of Polyfluorinated Organic Compounds Based on New Aggression Resistant Catalysts. // Fluorine Notes, Iss. 4(95), 2014, http://en.notes.fluorine1.ru/public/2014/4_2014/retro/index.html.
- Pat. US5258110, Process for preparing peroxidic perfluoropolyethers, 1993.
- Sianesi, D., Marchionni, G., De Pasquale, R.J. Perfluoropolyethers (PFPEs) from Perfluoroolefin Photooxidation. In: Banks, R.E., Smart, B.E., Tatlow, J.C. (eds) Organofluorine Chemistry. Topics in Applied Chemistry. Springer, Boston, 1994, MA. DOI: 10.1007/978-1-4899-1202-2_21.
ARTICLE INFO
Received 11 July 2025
Accepted 14 August 2025
Available online
August 2025
Recommended for publication by PhD O.V. Bryzgalova
eLIBRARY Document Number (EDN) EAXTYN

Fluorine Notes, 2025, 161, 1-2
