Received: April 2025
DOI 10.17677/fn20714807.2025.03.02
Fluorine Notes, 2025, 160, 3-4
THE STRUCTURE OF FREE RADICALS FORMED DURING PHOTOCHEMICAL AND THERMAL AGING OF FLUORINATED POLYURETHANE
I.A. Politsimako, S.V. Kudashev, V. V. Klimov, V.F. Zheltobryukhov
Volgograd State Technical University
28 Lenin Avenue, Volgograd, 400005 Russia
e-mail: kudashev-sv@yandex.ru
Abstract. The modifying effect of polyfluorinated tetraamine synthesized by the interaction of tris-(2-aminoethylamine) and 1H,1H,7H-trihydroperfluoroheptan-1-ol on the properties of polyurethane elastomers modified by it has been studied. The stabilizing effect of the fluorinated modifier used on the properties of elastomers under conditions of photochemical (in the presence of an ozone-air mixture) and thermal aging is shown. The structure of paramagnetic centers formed during the decomposition of polyurethane macromolecules and the features of their binding by fluorocarbon, nitroxyl, and aminyl free radicals are considered.
Keywords: fluoropolymers, polyurethane elastomers, polyfluorinated amines, modification, stabilization, degradation, paramagnetic centers.
Introduction
The operation of polyurethane sports, roofing, and waterproofing coatings causes multifactorial processes of macromolecule destruction of the heterochain polymer under consideration [1,2]. The destructive effects of UV radiation, aggressive media, changing air temperatures, abrasion and biofouling can be reduced by modifying polyurethane materials with poly- and perfluorinated compounds, which contributes to the hydrophobization of the elastomer surface [3-6].
The use of indifferent additives (fluoroalkanes, polytetrafluoroethylene, fluorografite, fluorogectorite and fluoromontmorillonite) leads to their partial migration from the polymer volume during the operation of coatings [3]. The introduction of fluorinated reactive compounds (isocyanates, alcohols and thiols, carboxylic acids, amines, peroxides, and multifunctional compounds) allows chemical modification of polyurethanes [7,8] by «embedding» fluorinated fragments into macromolecules and eliminating possible diffusion of the modifier from the polymer during coating operation.
The combination of amino groups of varying degrees of substitution and polyfluorinated fragments in the structure of the modifier, which can collectively participate in the termination of free radical processes occurring under UV radiation and elevated temperatures, opens up new prospects for stabilizing the properties of polyurethane materials.
The aim of the work is to identify the structural features of paramagnetic centers formed during photochemical (in the presence of an ozone-air mixture) and thermal aging of polyurethane elastomers modified with the bisalkylation product tris-(2-aminoethylamine) 1H,1H,7H-trihydroperfluoroheptan-1-ol.
Experimental part
Preparation of the elastomeric composition
Polymer compositions were obtained using a laboratory mixer by mixing (mixing speed 250 rpm-1) for 10 min. 100 wt. h. oligoesterpoliol (Laprol 5003-2–B10 (hydroxyl number 35 mg KOH/g, mass fraction of water not more than 0.05%, Jiahua Chemical Co., LTD), 1 wt. h. branching chain agent (glycerin (clean for analysis, JSC EKOS-1)), 1.5 wt.h. plasticizer (dioctyladipinate (DOA, basic substance content 99.7% by weight, LLC Vitakhim SPb)), 1.5 wt.h. surfactant (oxyethylated monoalkylphenol (Neonol AF 9-12, mass fraction of water <0.5%, NPK PROMKHIMPLAST LLC), 0.1 wt. h. of urethane catalyst (2.5% solution of di-n-butyldilaurate of tin in white spirit, PTK Neftepromkomplekt LLC) and 1 wt. h. of polyfluorinated tetraamine. Next, 20 wt. h. of isocyanate (Desmodur T80, 2.4-isomer content 80.5%, Wanhua) was added to the reaction mass and mixed again for 7 minutes. The resulting mixture was poured into molds and kept at room temperature (cold curing method) until the Shore A hardness of the elastomer reached a plateau. The [NCO] / [ОН] in the formulation of the composition was 1.50.
Catalytic N-polyfluoroalkylation according to Scheme 1 of tris-(2-aminoethylamine) (Boiling point 114 °C (15 mm Hg), d 0.976 g/ml, n20D 1.497, Keyingchem) 1H,1H,7H-trihydroperfluoroheptan-1-ol (basic substance content 99 wt%, Joint-stock company HaloPolymer) was carried out in the presence of catalytic amounts of montmorillonite (base substance content 99% by weight, TOO B-Clay) in a sealed glass ampoule at 80 ° C for 2 hours at an ultrasound frequency of 40 kHz, followed by heating to 120 ° C for 6 hours. The bisalkylation product (polyfluorinated tetraamine) was yellow oily substance with t. kip. 129-132 °C (15 mmHg).
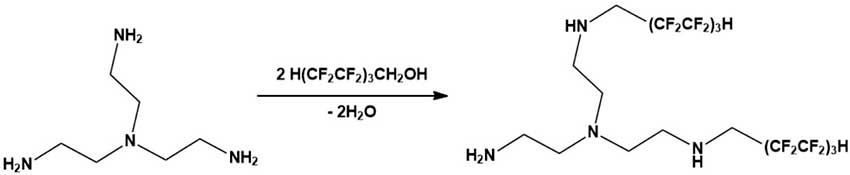
Scheme 1.
Investigation of polymer properties
Photochemical degradation of polyurethanes was carried out at room temperature using a quartz lamp Ogonek OG–LDP14 UV+Ozone (254 nm, 15 W). The distance from the lamp to the sample was 35 cm. Test conditions: 30 min exposure / 60 min rest, 15 days aging. The ozone concentration at a distance of about 1 mm from the surface of the tested polymers was at least 50 mg/m3. Thermal aging of polymer samples was carried out daily (15 days, 8 hours aging/16 hours rest) at a temperature of 40 ± 2 ° C in a Snol drying cabinet with natural air convection.
The physico-mechanical properties of polymer materials were tested on a Zwick/Roell 5.0 kN bursting machine according to State standard 21751-76 and State standard 270-75. Shore A hardness was determined in accordance with State standard. The results were processed using mathematical statistics methods using the IBM SPSS Statistics 21 program.
The wetting edge angle was determined using an OCA 15 EC device from DataPhysics with integrated SCA 20 software. The measurements were carried out by applying drops of test liquid (deionized water (Joint-stock company Lenreactive) with a volume of 5-7 µl to the surface of the elastomer and calculating the wetting angle of the sedentary droplet using the Young–Laplace method. 6-8 parallel measurements were performed and the arithmetic mean of the edge angles was calculated.
Investigation of the structure of paramagnetic centers
Quantum chemical studies in the approximation of an isolated particle in the gas phase with geometry optimization in all parameters using the DFT–PBE0/6–311g** and ab initio methods based on STO–3G** were performed in the GAMESS and Gaussian 09 software products.
Discussion of the results
Spatially hindered phenols, aromatic, aliphatic, fatty aromatic, and heterocyclic amines, and complex compounds of metals with variable valence have been described in the literature as stabilizers of polyurethane properties [9, 10]. The process of photochemical and thermal degradation of polyurethanes is quite complex [11-14] and begins mainly with the homolytic rupture of the N–C and C–O bonds of the urethane group according to the Scheme 2.
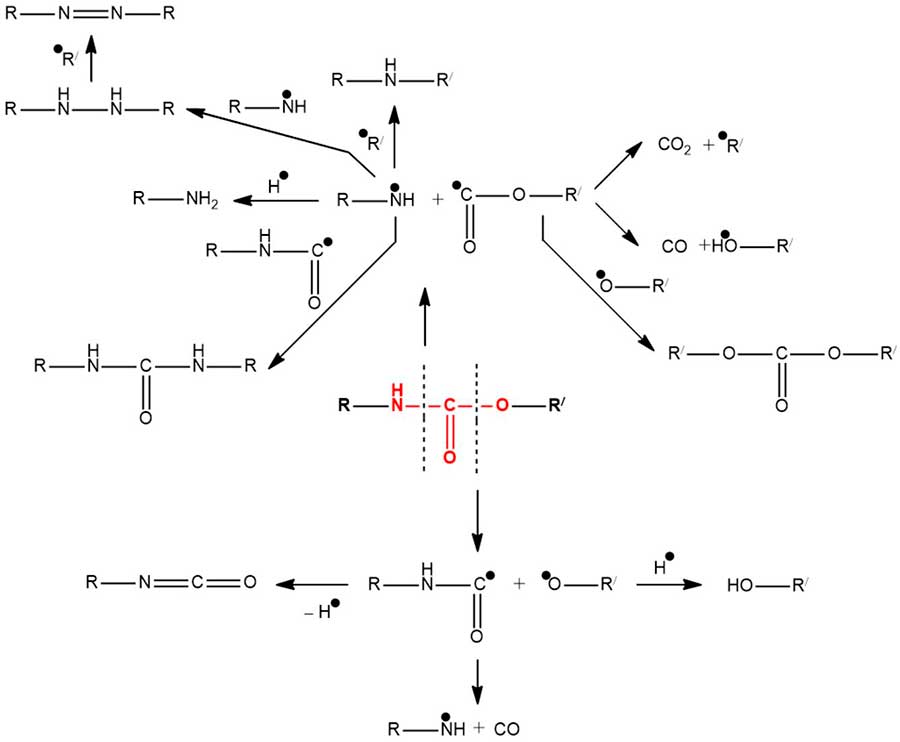
Scheme 2.
The resulting isocyanate, hydroxyl, and amino groups are able to participate in further polymer structuring (crosslinking) processes. Carbon oxides, amines, and azo compounds are registered among the volatile degradation products.
A study of the properties of a modified polyurethane elastomer indicates the stabilizing effect of the fluorinated modifier used (Table). There is an increase in the stability of the developed polyurethane materials under conditions of photochemical and thermal aging, their hardening and hydrophobization.
Table 1. Properties of polyurethane elastomers.
|
Sample |
Contact angle, ° |
Shore A hardness, conl. units |
Tensile strength, MPa |
Relative elongation at break, % |
Residual tensile strength*, % |
|
The initial polyurethane elastomer |
89±2 |
40 |
1,6 |
200 |
36 |
|
Modified polyurethane elastomer containing polyfluorinated tetraamine |
110±2 |
45 |
2,0 |
170 |
48 |
* after 30 days of aging: 15 days of photovoltaics in the presence of an ozone-air mixture, followed by thermal aging in the air for 15 days at 40 °C.
The reasons for the change in the properties of fluorinated polyurethanes are the reorganization of the structure of the heterochain polymer under consideration, including the formation of a spatial grid with higher density and lower defects due to the «embedding» of polyfluorinated tetraamine into polyurethane macromolecules according to Scheme 3, which occurs during the migration polymerization of isocyanate and polyol.
The participation of groups of varying degrees of substitution –HNCH2(CF2CF2)3H and >NCH2(CF2CF2)3H in non-covalent intra– and intermolecular interactions (N–H•••O=C, N–H•••O<, N–H•••F–C, C–H•••F-C) contributes to an increase in the proportion of physical bonds in the formed structure of the fluoropolymer.
Schemes 4 and 5 show the possible mechanisms of the participation of the fragment >N–(CH2)2–NH–CH2(CF2CF2)3H, which is part of the macromolecules of the modified polymer, in the binding of alkylhydroperoxides, alkylperoxide, alkoxyl and hydroxyl radicals formed during photochemical and thermal aging of polyurethane. Generation of the nitroxyl radical >N–O• can occur through the stages of formation of nitroxyl esters >N–OR and hydroxylamines >N–OH [15]. Further transformations >N–O• lead to the formation of aminyl radicals >N•. The energy gain (on average, 10-12 kcal/mol) in structures (1)–(6) is facilitated by intramolecular interactions >N•••H–C.
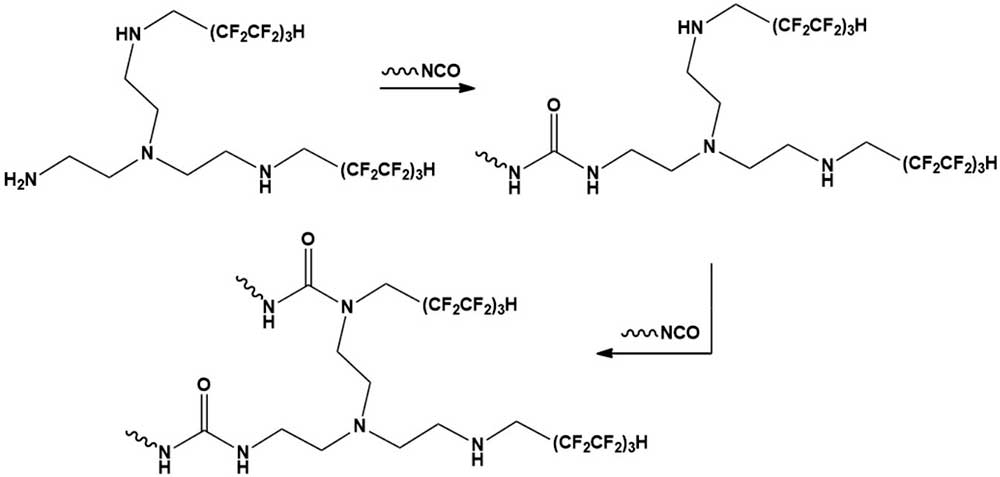
Scheme 3.
The activation barrier for the formation of the transition state (4) is 58 kcal/mol, and for the state (5) it is significantly lower – 45 kcal/mol (dipole moment 4.58 D). Polyfluorinated groups are capable of forming electrophilic free radicals CH2(CF2CF2)3•, which have a linear structure and differ from their non-fluorinated counterparts in that the tetrahedral configuration is preserved (the calculated value of the total g-factor is 2.014).
Thus, the increased stability of polyurethane elastomers modified with the bisalkylation product tris-(2-aminoethylamine) 1H,1H,7H-trihydroperfluoroheptan-1-ol under conditions of photochemical (in the presence of ozone) and thermal aging is combined with the participation of fluorocarbon, nitroxyl, and aminyl free radicals in the binding of paramagnetic centers formed during the decay of macromolecular chains.
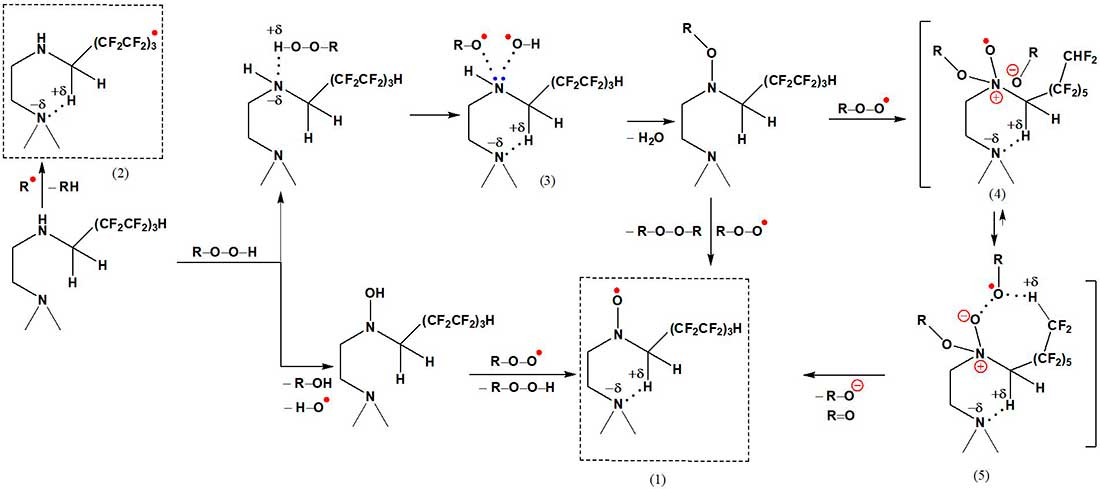
Scheme 4.
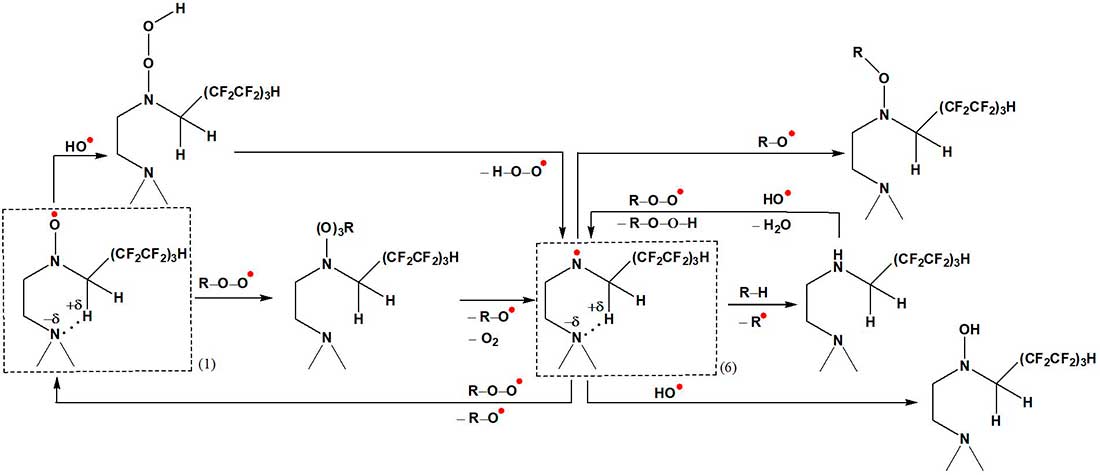
Scheme 5.
References
- Thomas S., Datta J., Haponiuk J. Polyurethane polymers: Composites and nanocomposites. Elsevier, Amsterdam, Netherlands, 2017. 632 р.
- Clemitson I. R. Castable Polyurethane Elastomers. – CRC Press (Taylor & Francis Group), 2015. 272 p.
- Ebnesajjad S., Morgan R. A. Fluoropolymer Additives. Elsevier, William Andrew, 2019. P. 57–66.
- Smirnova O., Glazkov A., Yarosh A., Sakharov A. Fluorinated Polyurethanes, Synthesis and Properties // Molecules. 2016. V. 21. N 7. Р. 1–10. https://doi.org/10.3390/molecules21070904/
- Wu Z., Tang L., Dai J., Qu J. Synthesis and properties of f luorinated non-isocyanate polyurethanes coatings with good hydrophobic and oleophobic properties // J. Coat. Technol. Res. 2019. V. 16. Р.1233–1241. http://dx.doi.org/10.1007/s11998-019-00195-5/
- Li N., Yang R., Tian Y., Lu P., Huang N., Li H., Chen X. Synthesis of durable hydrophobic fluorinated polyurethanes with exceptional cavitation erosion resistance // Tribol. Int. 2023. V. 177. ID. 107973. https://doi.org/10.1016/j.triboint.2022.107973/
- Ponomarenko V.A., Krukovskij S.P., Alybina A.Yu. Ftorsoderzhashchie geterocepnye polimery. – M.: Nauka, 1973. 304 p. (in Russian)
- Malichenko B. F. Ftorsoderzhashchie poliamidy i poliuretany. – Kiev: Naukova dumka. 1977. 231 p. (in Russian)
- Neiman M. B. B. Aging and Stabilization of Polymers (1965th Ed.). Springer, 2012. 376 р.
- Xie F., Zhang T., Bryant P., Kurusingal V., Colwell J. M., Laycock B. Degradation and stabilization of polyurethane elastomers // Progress in Polymer Science. 2019. V. 90 (March). Р. 211-268. https://doi.org/10.1016/j.progpolymsci.2018.12.003.
- Shlyapintoh V. Ya. Fotohimicheskie prevrashcheniya i stabilizaciya polimerov. M.: Himiya. 1979. 344 p. (in Russian)
- Renbi B., Rabek Ya. Fotodestrukciya, fotookislenie, fotostabilizaciya polimerov. M.: Mir. 1978. 677 p. (in Russian)
- Nevskij, L. V. Issledovanie fotodestrukcii poliuretanov: Dis. na soiskanie uchenoj stepeni kandidata himicheskih nauk. Gor'kij, 1967. 124 p. (in Russian)
- Belyakov, V. K. Issledovanie obrazovaniya paramagnitnyh centrov pri destrukcii poliuretanov i ih vliyaniya na razvitie processa: Dis. na soiskanie uchenoj stepeni kandidata himicheskih nauk. Moskva, 1967. 147 p. (in Russian)
- Gijsman P. A review on the mechanism of action and applicability of Hindered Amine Stabilizers // Polymer Degradation and Stability. 2017. V. 90 (November). Р. 211-268.
ARTICLE INFO
Received 17 April 2025
Accepted 06 June 2025
Available online
June 2025
Recommended for publication by PhD V.L. Don
eLIBRARY Document Number (EDN) ZOIGAO

Fluorine Notes, 2025, 160, 3-4
