Received: April 2025
DOI 10.17677/fn20714807.2025.02.02
Fluorine Notes, 2025, 159, 3-4
INFLUENCE OF FLUORINE ON THE PROPERTIES OF APATITES AND SYNTHESIS OF FLUORINE-CONTAINING COMPOSITE MATERIALS
V. M. Skachkov1, E. A. Bogdanova2, V. D. Ryabokrys3, O. V. Paporotny3
1 Institute of Solid State Chemistry of the Ural Branch of RAS, 91, Pervomayskaya street, Ekaterinburg, 620108 Russia
2 Joint Stock Company "Giredmet", 2 Elektrodnaya street, Moscow, 111524 Russia
3Ural Federal University named after the first President of Russia B.N. Yeltsin, 19 Mira Street, Ekaterinburg, 620002 Russia
е-mail: skachkov@ihim.uran.ru
Abstract: The work presents the synthesis of fluorine-substituted apatites based on hydroxyapatite and composite materials fluorapatite – non-stoichiometric titanium oxide and fluorapatite – zirconium dioxide. The behavior of components in the Ca10(PO4)6F2–TiOx and Ca10(PO4)6F2–ZrO2 systems is studied in a wide range of temperatures and concentrations. The effect of phase composition based on fluorine content and the amount of additive introduced on the strength characteristics of the material is shown.
Keywords: hydroxyapatite, fluorapatite, zirconium dioxide, non-stoichiometric titanium oxide, composite materials, microhardness.
Introduction
Biomaterial, widely used due to its similarity to the mineral component of bone tissue, hydroxyapatite (HAP) Са10(РO4)6(ОН)2 has variety of practical applications in various fields of medicine [1]. Anionic substitution in the hydroxyapatite structure of the OH− group by SiO44−, F−, Cl− or CO32− leads to a change in the crystal lattice parameters, which affects the crystallinity and, consequently, the solubility of apatites, that can lead to increased bone binding properties, antibacterial activity and osseointegration [2, 3]. HAP has a pronounced osteotropic behavior in biological environments [4-6], however, as a bioceramic material it has low strength characteristics, which does not allow its use for bone tissues that experience regular significant mechanical loads.
The hardness and strength of apatite-based biomaterials can be increased by modifying the structure, including through the mechanosynthesis of crystalline apatites with reinforcing additives (CaF2, SiO2, TiO2, ZrO2, Al2O3 etc.) [7-10]. During heat treatment of composite materials, interactions occur between apatite and the reinforcing phase, often with a change in phase composition due to physical and chemical processes occurring in the systems, changing the microstructure and, consequently, the mechanical and medical-biological properties.
In this paper, we study the properties of ceramic materials based on fluorapatite (Ca10(PO4)6F2) (FAP), with the addition of zirconium dioxide and non-stoichiometric titanium oxide, forming the systems: Ca10(PO4)6F2–ZrO2 and Ca10(PO4)6F2–TiOx, where x=1.5÷1.9, and the properties of apatites with different degrees of anionic substitution in HAP of the OH−-group by F−:
Са10(РO4)6(ОН)2 + xF− = Са10(РO4)6(ОН)(2-x)Fx + xOH− (х=1, 1.5, 2) [11].
Fluorapatite is isomorphic to hydroxyapatite, while the solubility of fluorapatite is lower than the solubility of hydroxyapatite, and fluorine has a stabilizing effect not only in the case of complete but also partial substitution of ОН− groups. Analysis of experimental data (Fig. 1) allows us to conclude that the inclusion of fluorine in the apatite structure helps to obtain a material with improved strength characteristics, since it increases resistance to biodegradation and the effects of acids. The features of the chemical interaction of FAP with reinforcing additives during heat treatment and the effect of additives on strength during annealing were also revealed.
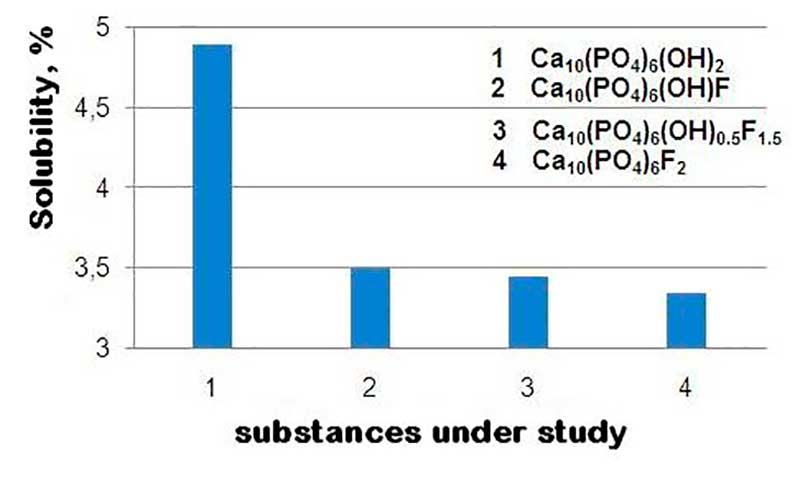
Figure 1. Solubility of apatites depending on composition.
Experimental part
FAP suspensions with different degrees of substitution of the compositions: Са10(РO4)6(ОН)(2-x)Fx (х=1, 1.5, 2) were obtained by the precipitation method from solutions [11] with subsequent filtration and air drying, the powders (Fig. 2 a) were certified by X-ray phase analysis (XRD), which was performed on Shimadzu and DRON-2.0 diffractometers, CuKα radiation, angle range 10°≤2θ≤70°, shooting step 0.03°, time per point 2 seconds, phase identification was carried out using the Powder Diffraction File JCPDSD-ICDD PDF2 (set’s 1-47) card index. Non-stoichiometric titanium oxide (Fig. 2 c) TiOx (х=1.5÷1.9) was obtained by annealing of titanium chips in a furnace at a temperature of 600°C for two hours. Zirconium dioxide (Fig. 2 d) ZrO2 (pure, TU 6-09-2486-77). The components were mixed without adding a homogenizer in a vibration mill (MLW 4000 KM 1) with an agate mortar and ball for 30 min.
Mechanochemical activation allows to increase the dispersed state of the components to achieve a high-strength state in the future; additionally, bonds are broken during the grinding process, which leads to the formation of new chemical compounds as a result of mechanochemical reactions [12]. The composite mixtures and powders obtained as a result of mechanosynthesis with different degrees of fluorine substitution in FAP were formed into tablets weighing 0.5-1.0 g, carried out by uniaxial double-sided pressing without introducing a binder in a cylindrical steel press mold with a diameter of 10 mm on a hydraulic hand press without holding at room temperature and a pressure of 20 MPa. The samples were annealed in a Nabertherm L 9/11 muffle furnace at the temperature range of 25-1200°C with a step of 200°C, the heating rate was 10°C/min with a holding time of 1 hour in an air atmosphere. As a control sample, a HAP (Fig. 2 b) of the composition Ca10(PO4)6(OH)2 [13] was studied in parallel, but its heat treatment was carried out only up to 1000°C, since HA obtained by precipitation from solutions is known to decompose at 800°C with the formation of Ca3(PO4)2 – tricalcium phosphate (TCP) [14, 15].
Microhardness of composite materials was measured by the Vickers method on a PMT‑3M microhardness tester with a load of 0.98 N (100 g) and a loading time of 10 s. The compressive strength was determined at room temperature on a Liangong Group CMT-5L (China) electromechanical universal testing machine with automatic control and data processing on a PC using the MaterialTest 3.0 program, accuracy class 0.5, maximum load 5 kN (~500 kg). Morphological features were studied by scanning electron microscopy (SEM) on a JEOL JSM 6390 LA microscope (Japan), magnification factor from x5 to x300000, resolution 3.0 nm at 30 kV.
|
a |
b |
|
c |
d |
Figure 2. Morphology of the initial materials: a – FAP - Ca10(PO4)6F2 dried at 25°C; b – HAP - Ca10(PO4)6(OH)2 dried at 25°C; c – non-stoichiometric titanium oxide - TiOx; d – zirconium dioxide - ZrO2.
Discussion of results
Results of annealing of fluorine-substituted ceramics
The introduction of fluoride ions into apatites affects the behavior of ceramics during annealing. The presence of fluorine in the crystal lattice, as the FAP content increases, prevents the decomposition of apatites into TCP, structurally stabilizing the fluorine-substituted HAP obtained by precipitation from solution to decomposition temperatures typical of apatites obtained by solid-phase synthesis [14]. During thermal processing of ceramics, sintering occurs, the density of the material increases, sorption and crystalline water are lost, which leads to a change in linear parameters and a decrease in weight (Table 1).
Table 1. Changes in the parameters of the studied samples of stoichiometric HAP and fluorine-substituted FAP at different temperatures.
|
Sample under study |
Changing linear parameters, ±Δh, % |
|||||
|
200°C |
400°C |
600°C |
800°C |
1000°C |
1200°C |
|
|
HAP Са10(РO4)6(ОН)2 |
-2.17 |
-1.86 |
-2.11 |
-3.23 |
-7.94 |
– |
|
FAP Са10(РO4)6(ОН)F |
-2.38 |
-5.63 |
-5.95 |
-7.54 |
-15.32 |
-17.54 |
|
FAP Са10(РO4)6(ОН)0.5F1.5 |
-0.35 |
-1.75 |
-1.49 |
-2.80 |
-8.22 |
-12.5 |
|
FAP Са10(РO4)6F2 |
-0.34 |
-1.02 |
-1.7 |
-3.06 |
-11.14 |
-14.2 |
|
Change in mass, ±Δm, % |
||||||
|
HAP Са10(РO4)6(ОН)2 |
-4.30 |
-4.78 |
-6.4 |
-8.04 |
-11.8 |
– |
|
FAP Са10(РO4)6(ОН)F |
-4.12 |
-6.15 |
-6.79 |
-8.97 |
-9.44 |
-9.55 |
|
FAP Са10(РO4)6(ОН)0.5F1.5 |
-3.29 |
-5.84 |
-6.46 |
-8.67 |
-9.08 |
-9.19 |
|
FAP Са10(РO4)6F2 |
-3.17 |
-6.10 |
-6.81 |
-9.26 |
-9.82 |
-10.06 |
As the apatites sintered and compacted, their hardness increased. It was experimentally established that fully substituted fluorapatite has the best strength characteristics (Table 2) and has a uniform dense structure, while monosubstituted (Ca10(PO4)6(OH)F) FAP gained maximum hardness after annealing at 1000°C. After heat treatment at 1200°C, a strengthening phase, calcium fluoride, up to 10%, was detected in all FAP modifications.
Table 2. Microhardness of stoichiometric HAP and fluorine-substituted FAP at different temperatures.
|
Sample under study |
Vickers hardness (HV), MPa |
||||||
|
25°C |
200°C |
400°C |
600°C |
800°C |
1000°C |
1200°C |
|
|
HAP Са10(РO4)6(ОН)2 |
52 |
71 |
53 |
75 |
87 |
183 |
– |
|
FAP Са10(РO4)6(ОН)F |
63 |
80 |
142 |
116 |
134 |
406 |
244 |
|
FAP Са10(РO4)6(ОН)0.5F1.5 |
61 |
82 |
108 |
156 |
129 |
179 |
398 |
|
FAP Са10(РO4)6F2 |
65 |
77 |
163 |
143 |
144 |
268 |
473 |
Ceramics made from hydro-chemically obtained HAP cracks already during heat treatment at 1000°C (Fig. 3), but continues to gain hardness.
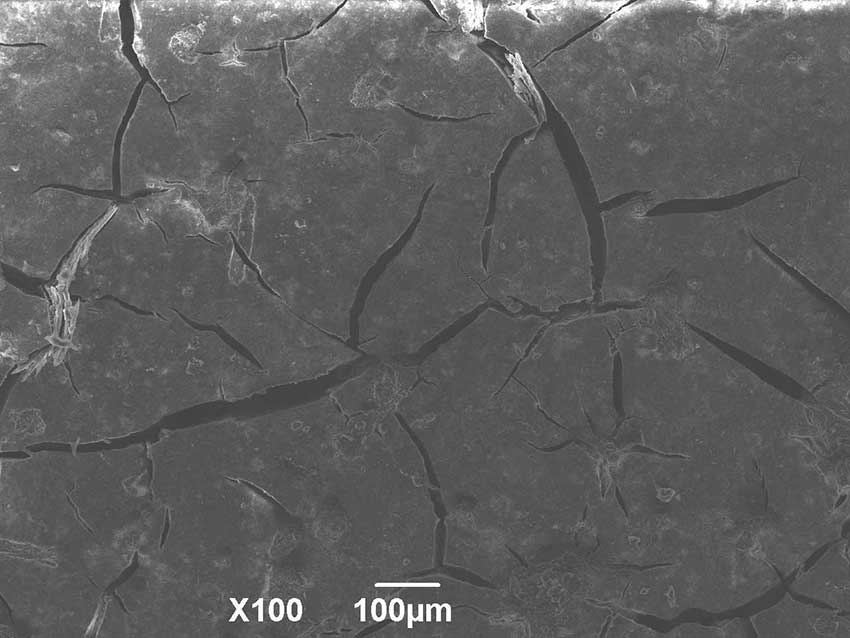
Figure 3. Morphology of the HAP surface after heat treatment at 1000°C.
After annealing of HAP at 1000°C, it contained up to 30% of the TCP phase, while fluorine-substituted FAP only partially decomposed at 1200°C to form CaF2 and Ca3(PO4)2. TCP content, %:
Са10(РO4)6(ОН)F – 20, Са10(РO4)6(ОН)0.5F1.5 – 10, Са10(РO4)6F2 – less than 1.
Annealing results of FAP-TiOx composite ceramics
It is possible to obtain ceramics with high hardness, strength and resistance to high temperatures by introducing reinforcing additives such as titanium and zirconium oxides into its composition. HAP and FAP ceramics with the inclusion of titanium compounds are bioinert materials that do not cause side reactions [16]. Tables 3 and 4 present changes in FAP-TiOx composite materials with different contents of non-stoichiometric titanium oxide during heat treatment.
Composite ceramics made of FAP with reinforcing additives of non-stoichiometric titanium oxide crack during heat treatment (Fig. 4), while continuing to gain hardness. The best values for hardness were achieved with the introduction of 10 and 15% of non-stoichiometric titanium oxide. The phase composition changed as the annealing temperatures increased due to the gradual transition of non-stoichiometric titanium oxide to dioxide, and after annealing at 1200°C, more than 95% of titanium was presented in the form of titanium dioxide, and up to 10% of calcium fluoride released from FAP was also formed.
Table 3. Changes in the parameters of FAP-TiOx composite materials at different temperatures.
|
Sample under study |
Changing linear parameters, ±Δh, % |
|||||
|
200°C |
400°C |
600°C |
800°C |
1000°C |
1200°C |
|
|
FAP -10%TiOx, |
-0.33 |
0.50 |
-7.13 |
-6.30 |
-3.81 |
-3.65 |
|
FAP -15%TiOx, |
0 |
0.96 |
1.28 |
1.44 |
-4.01 |
1.28 |
|
FAP -20%TiOx, |
-0.47 |
1.09 |
-0.31 |
1.87 |
3.12 |
2.18 |
|
FAP -30%TiOx, |
0.37 |
1.10 |
1.66 |
1.10 |
-0.55 |
5.89 |
|
Change in mass, ±Δm, % |
||||||
|
FAP -10%TiOx, |
-6.10 |
-3.07 |
-1.89 |
-0.95 |
-3.71 |
-4.32 |
|
FAP -15%TiOx, |
0.68 |
3.16 |
4.06 |
4.32 |
0.40 |
-0.64 |
|
FAP -20%TiOx, |
-1.35 |
1.00 |
1.85 |
1.56 |
-3.64 |
-5.26 |
|
FAP -30%TiOx, |
1.06 |
3.03 |
3.61 |
2.01 |
-6.26 |
-10.16 |
Table 4. Microhardness of FAP-TiOx composite materials at different temperatures.
|
Sample under study |
Vickers hardness (HV), MPa |
||||||
|
25°C |
200°C |
400°C |
600°C |
800°C |
1000°C |
1200°C |
|
|
FAP -10%TiOx, |
72 |
150 |
182 |
148 |
153 |
260 |
303 |
|
FAP -15%TiOx, |
86 |
117 |
200 |
193 |
130 |
293 |
306 |
|
FAP -20%TiOx, |
60 |
153 |
195 |
156 |
124 |
201 |
282 |
|
FAP -30%TiOx, |
100 |
151 |
150 |
147 |
100 |
120 |
270 |
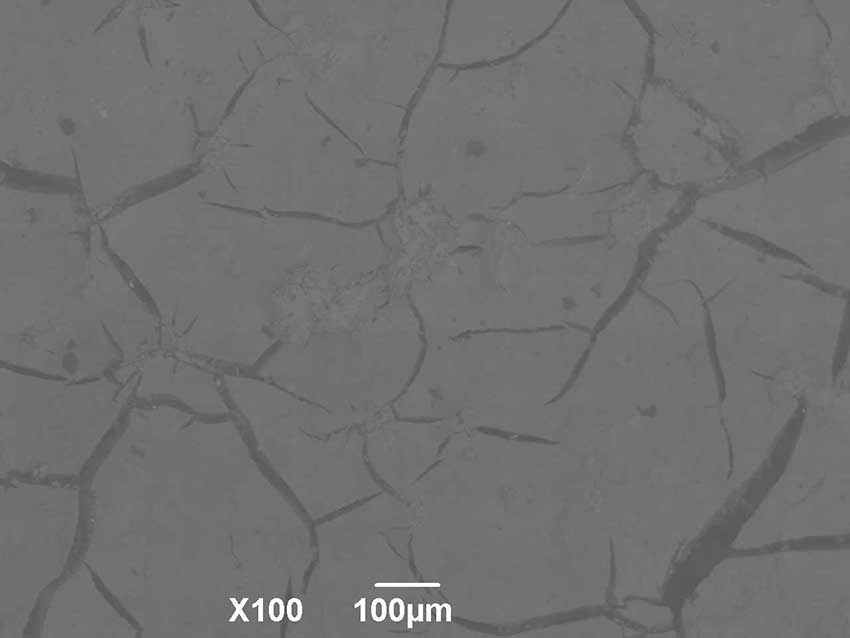
Figure 4. Surface morphology of FAP-15% TiOx after annealing at 1000°С.
Results of annealing of composite ceramics FAP-ZrO2
Tables 5 and 6 show the changes in FAP-ZrO2 composite materials with different zirconium dioxide content and heat treatment.
Table 5. Changes in the parameters of FAP-ZrO2 composite materials at different temperatures.
|
Sample under study |
Changing linear parameters, ±Δh, % |
|||||
|
200°C |
400°C |
600°C |
800°C |
1000°C |
1200°C |
|
|
FAP -5%ZrO2 |
-0.14 |
-0.14 |
-0.29 |
-1.88 |
-16.91 |
-16.91 |
|
FAP -10%ZrO2 |
-0.80 |
-1.92 |
-2.24 |
-2.72 |
-10.72 |
-12.00 |
|
FAP -15%ZrO2 |
0 |
-2.50 |
-2.50 |
-3.33 |
-8.33 |
-14.17 |
|
FAP -20%ZrO2 |
-0.97 |
-2.74 |
-2.90 |
-2.90 |
-9.84 |
-11.29 |
|
Change in mass, ±Δm, % |
||||||
|
FAP -5%ZrO2 |
-1.97 |
-4.85 |
-5.85 |
-8.00 |
-8.06 |
-8.32 |
|
FAP -10%ZrO2 |
-1.7 |
-4.47 |
-5.68 |
-7.28 |
-7.68 |
-7.97 |
|
FAP -15%ZrO2 |
-1.72 |
-4.35 |
-5.44 |
-6.97 |
-7.30 |
-7.70 |
|
FAP -20%ZrO2 |
-1.57 |
-4.05 |
-5.27 |
-6.79 |
-7.13 |
-7.56 |
Table 6. Microhardness of FAP-ZrO2 composite materials at different temperatures.
|
Sample under study |
Vickers hardness (HV), MPa |
||||||
|
25°C |
200°C |
400°C |
600°C |
800°C |
1000°C |
1200°C |
|
|
FAP -5%ZrO2 |
90 |
89 |
253 |
198 |
157 |
473 |
517 |
|
FAP -10%ZrO2 |
76 |
112 |
215 |
214 |
170 |
395 |
312 |
|
FAP -15%ZrO2 |
88 |
111 |
178 |
178 |
199 |
281 |
396 |
|
FAP -20%ZrO2 |
94 |
134 |
167 |
215 |
148 |
375 |
314 |
Ceramics made of FAP with zirconium dioxide remain dense, without cracks (Fig. 5), with high hardness during high-temperature treatment. The micrograph clearly shows inclusions of zirconium dioxide (light areas).
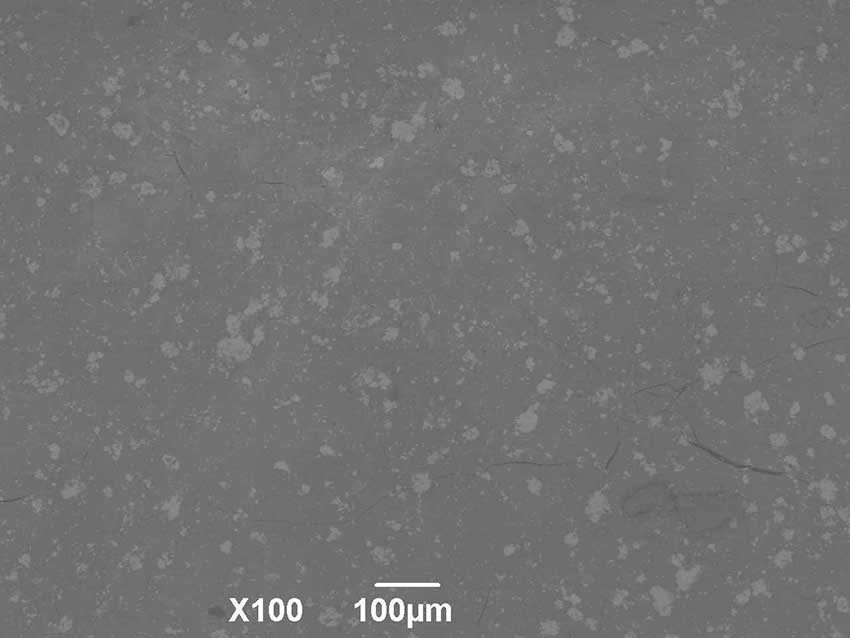
Figure 5. Surface morphology of the composite material FAP-10%ZrO2 after heat treatment at 1000°С.
The phase composition of composite materials changed after annealing at 1200°C, CaF2 was formed in an amount of ~10%, and the crystallinity of the material also increased at high temperatures. Zirconium dioxide acted as a stabilizer for bioceramics, preventing the formation of TCP.
Strength test results
An assessment of the compressive strength limit was carried out depending on the qualitative and quantitative composition of the ceramic samples obtained during the work (see Table 7). Cylindrical samples (Ø=10 mm; h=10 mm) were subjected to compression after high-temperature treatment at 1000 and 1200°C.
Table 7. Results of evaluation of compressive strength limits of HAP, FAP, composite materials and strengthening phases.
|
Sample under study |
Compressive strength (comp), MPa |
|
|
1000°C |
1200°C |
|
|
HAP Са10(РO4)6(ОН)2 |
511 |
– |
|
FAP Са10(РO4)6(ОН)F |
448 |
570 |
|
FAP Са10(РO4)6(ОН)0.5F1.5 |
498 |
400 |
|
FAP Са10(РO4)6F2 |
518 |
549 |
|
FAP -10%TiOx, |
– |
221 |
|
FAP -15%TiOx, |
– |
139 |
|
FAP -20%TiOx, |
– |
214 |
|
FAP -30%TiOx, |
– |
202 |
|
FAP -5%ZrO2 |
– |
559 |
|
FAP -10%ZrO2 |
– |
549 |
|
FAP -15%ZrO2 |
– |
550 |
|
FAP -20%ZrO2 |
– |
442 |
|
CaF2 |
401 |
– |
|
ZrO2 |
506 |
– |
Conclusions
As a result of the work, the strengthening and stabilizing effect of fluorine on bioceramics based on nanostructured precipitated fluorine-substituted HAP was studied, and the strengthening and stabilizing effect was experimentally determined by mechanochemical reinforcement of FAP with titanium and zirconium oxides, even at high-temperature annealing – at 1200°C, FAP decomposes slightly with the formation of less than 10% calcium fluoride, which in turn is also a strengthening phase. The main characteristics of the obtained materials were determined: phase composition, morphology, linear compression, microhardness, compressive strength. It has been experimentally established that the most promising for the development of biocomposites are materials containing 5-10% zirconium dioxide. Contrary to expectations, addition of non-stoichiometric titanium oxides as a strengthening phase in the composite material led to its cracking. Composite material made of FAP – zirconium dioxide has a dense uniform structure with a high degree of crystallinity and strength, which makes it a promising material for further study for use in medicine.
Acknowledgements
The work was carried out in accordance with the state assignment and research plans of the Federal State Budgetary Scientific Institution «Institute of Solid State Chemistry of the Ural Branch of the Russian Academy of Sciences» (No 124020600007-8).
References
- Mondal S., Park S., Choi J., Vu T.T.H., Doan V.H.M., Vo T.T., Lee B., Oh J., Hydroxyapatite: A journey from biomaterials to advanced functional materials, Advances in Colloid and Interface Science, 2023, 321, 103013. https://doi.org/10.1016/j.cis.2023.103013.
- Chkirate K., Azgaou K., Elmsellem H., El Ibrahimi B., Sebbar N.K., Anouar E.H., Hajjaji S.E., Essassi E.M., Corrosion inhibition potential of 2-[(5-methylpyrazol-3-yl)methyl]benzimidazole against carbon steel corrosion in 1M HCl solution: Combining experimental and theoretical studies, Journal of Molecular Liquids, 2021, 321, 114750. https://doi:10.1016/j.molliq.2020.114750.
- Safari-Gezaz M., Parhizkar M., Asghari E., Investigation of the structural properties of Si4+-doped HAP coatings on Ti-6Al-4V substrate as a corrosion barrier in biomedical media, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2024, 699, 134742. https://doi.org/10.1016/j.colsurfa.2024.134742.
- V. М. Bezrukov, А. S. Grigoryan, Hydroxyapatite as a substrate for bone grafting: theoretical and applied aspects of the problem, Stomatologiya, 1996, 75 (5), 7‑12. (in Russian)
- G. V. Akopyan, А. G. Khachatryan, The use of osteoplastic materials in dental implantology, Assotsiatsiya stomatologov v Armenii. Theoretical and practical journal, 2012, 7(1), 10-14. (in Russian)
- Stroganova Е. Е., Novel technologies for production and application of bioceramics in restorative medicine, Steklo i keramika, 2008, 1, 36-38. (in Russian)
- D. I. Pereverzev, E. A. Bogdanova, K. V. Nefedova, Creating biocomposites based on nano-sized hydroxyapatite doped with zirconium oxide and calcium fluoride, Physical and chemical aspects of the study of clusters, nanostructures and nanomaterials, 2020, 12, 697-705. https://doi.org/10.26456/pcascnn/2020.12.697. (in Russian)
- / E. A. Bogdanova, V. M. Skachkov, K. V. Nefedova, Preparation of biocomposites based on nanoscale hydroxyapatite with titanium compounds, Physical and chemical aspects of the study of clusters, nanostructures and nanomaterials, 2022, 14, 521-530. https://doi.org/10.26456/pcascnn/2022.14.521. (in Russian)
- E. A. Bogdanova, I. М. Giniyatullin, D. I. Pereverzev, V. М. Razgulyaeva, Influence of reinforcement additives on sintering and hardening processes of nanoscale hydroxyapatite, Physical and chemical aspects of the study of clusters, nanostructures and nanomaterials, 2019, 11, 548-554. https://doi.org/10.26456/pcascnn/2019.11.548. (in Russian)
- Bogdanova Е.А., Skachkova О.V., Skachkov V.М., Sabirzyanov N.А., Production of hydroxyapatite based fluorine-containing composite materials, Fluorine notes, 2017, 5(114), 3-4. https://doi.org/10.17677/fn20714807.2017.05.02.
- RF Patent 2652193, IPC C01B 25/32, Method for production of hydroxyapatite suspension, N. А. Sabirzyanov; applicant and rightholder – Institute of Solid State Chemistry of the Ural Branch of RAS; publ. 25.04.2018, Bull. No. 12. (in Russian)
- Boldyrev V. V., Mechanochemistry and mechanical activation of solid substances, Uspekhi khimii, 2006, 75(3), 203-216. (in Russian)
- RF Patent 2406693, IPC C01B 25/32, Method for production of hydroxyapatite suspension, N. А. Sabirzyanov; applicant and rightholder – Institute of Solid State Chemistry of the Ural Branch of RAS; publ. 20.12.2010, Bull. No. 35. (in Russian)
- S. М. Barinov, V. S. Komlev, Bioceramics in medicine, Moscow: Nauka, 2005, 284 p. (in Russian)
- Е. А. Bogdanova, N. А. Sabirzyanov, Investigation into thermal stability of fluorine-substituted HAP, Materialovedeniye, 2015, 1, 52-56. (in Russian)
- Placido, F., McLean, A., Ogwu, A. A., & Ademosu, W. (2016). Titanium dioxide coatings for medical devices. In Surgical Tools and Medical Devices, Second Edition (pp. 81-92). Springer International Publishing. https://doi.org/10.1007/978-3-319-33489-9_3.
ARTICLE INFO
Received 04 April 2025
Accepted 15 April 2024
Available online
April 2025
Recommended for publication by PhD V.L. Don
eLIBRARY Document Number (EDN) RSEOHF

Fluorine Notes, 2025, 159, 3-4




