Received: March 2025
DOI 10.17677/fn20714807.2025.02.01
Fluorine Notes, 2025, 159, 1-2
METHODS FOR OBTAINING FLUORINE-CONTAINING CARBOXYLIC ACIDS.
COMMUNICATION 1
S.V. Vershilov a, V.V. Kornilov, A.S. Tsyrulnikova а, N.V. Lebedev a
а FSBU S.V. Lebedev Scientific Research Institute of Synthetic Rubber
Abstract: This part of the review deals with the following synthesis methods of fluorine-containing carboxylic acids: electrochemical fluorination (ECF), isomerization and anionic polymerization of perfluoroalkene oxides, and oligomerization of perfluoroalkenes in the liquid-phase oxidation processes.
Key words: electrochemical fluorination, perfluoroalkene oxides, perfluoroalkenes, liquid- phase oxidation, anionic polymerization.
Introduction
This review considers some synthesis methods of fluorine-containing carboxylic acids. Despite the fact that in recent years there has been a refusal of using perfluorinated aliphatic carboxylic acids as emulsifiers in the synthesis of fluorinated polymers and copolymers by aqueous emulsion polymerization, fluorine-containing carboxylic acids and their derivatives remain important precursors for the synthesis of various fluorinated materials.
In addition to the traditional fields of application (foaming agents, protective coatings, additives to lubricants, etc.) of fluorine-containing carboxylic acids and their derivatives, compounds of this class are used as additives to electrolytes in lithium-ion batteries (LIBs). This is necessary to improve the characteristics of such power sources at low temperatures [1]. Carboxylic acids are also used in the synthesis of biologically active compounds with fluorinated fragments, which can improve the physicochemical properties (acidity, lipophilicity, permeability) of pharmaceutical substances [2].
1. Electrochemical fluorination (ECF)
The ECF method is used to obtain perfluoroacyl fluorides, which are converted into the corresponding acids by hydrolysis. For a long time, the ECF method was one of the main industrial methods for obtaining these compounds.
This was significantly related to the production of perfluoroheptanoic acid, perfluorooctanoic (PFOA) and perfluorononanoic acids, which were used in the aqueous emulsion polymerization processes to produce polytetrafluoroethylene (PTFE) and other fluorinated polymers and copolymers. In the last decade, due to the environmental requirements, many fluoropolymer manufacturers had refused the use of PFOA and its homologues with more than 6 carbon atoms in polymerization processes. This has somewhat reduced the importance of the ECF method.
The preparation method for perfluorocarboxylic acids via ECF is described in sufficient detail in literature, for example in the review articles [3-5].
The ECF mechanism is described in detail in the paper [6], in which the distribution of products was explained using the examples of the ECF of butyryl chloride and fluoride isomers (iso-butyryl chloride, etc.).
For the synthesis of acids with a number of carbon atoms in the chain of no more than 4, acyl halides (fluorides, chlorides) or esters of the corresponding hydrocarbon acids are used as a starting raw material. With an increase in the number of carbon atoms in the chain, the yield of linear products decreases, and the main products of ECF become perfluorinated cyclic ethers (Scheme 1) [3].
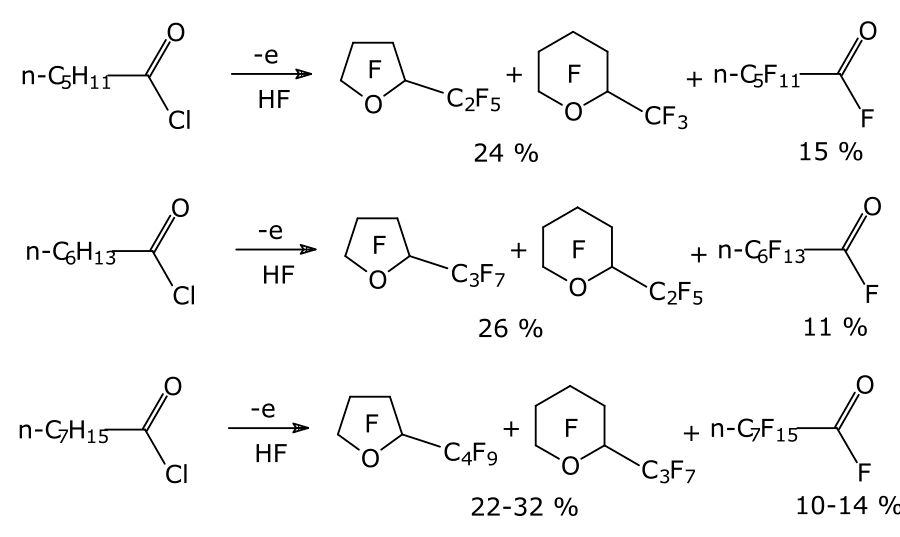
Scheme 1
Therefore, to obtain linear perfluorinated carboxylic acids with a higher number of carbon atoms in the chain for ECF, compounds already containing fluorine atoms were used as raw materials.
One of the methods is to use the corresponding telomer alcohols of the general formula H(CF2-CF2)n-CH2-OH (n=2÷5) as feedstock. Such alcohols are obtained on an industrial scale by the interaction of tetrafluoroethylene with methanol.
This method was used to get perfluorinated pentanoic, heptanoic, nonanoic and undecanoic acids with a high yield [7].
Another method involves the pre-synthesis of compounds with a linear carbon chain containing a fluorocarbon fragment. As an example, take the synthesis of 4-(perfluoro-n-butyl)-n-butanoyl chloride (Scheme 2), which was described by G.P. Gambaretto et al. [8].
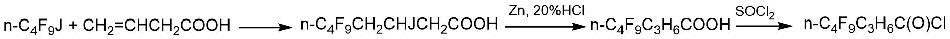
Scheme 2.
Subsequent ECF made it possible to increase the yield of perfluorooctanoyl fluoride to 28% compared to the yield of 12.9% in ECF of n-octanoyl chloride.
The use of 4-(perfluoro-n-butyl)-n-butanoyl fluoride as a raw material in ECF helped to increase the yield of perfluorooctanoyl fluoride to 29% [5].
The increase in the yield of the target linear product when using partially fluorinated raw materials was explained by the fact that in this case the processes of formation of cyclic compounds during ECF were blocked.
When obtaining carboxylic acids by the ECF method, it is necessary to search for conditions for each such process, including the selection of the initial compound and its concentration in electrolyte.
For example, to obtain perfluoropropionyl fluoride, propionyl chloride or telomer alcohol n=1 (HCF2-CF2-CH2-OH) have traditionally been used as the starting material. In article [9], the more inexpensive maleic anhydride was proposed for the synthesis of perfluoropropionyl fluoride with simultaneous obtaining of perfluorosuccinyl fluoride with a high yield (Scheme 3).
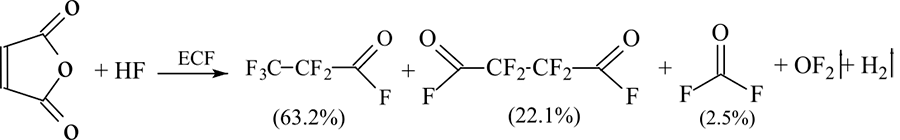
Scheme 3.
In some cases, the introduction of so-called "active additives" is used, which make it possible to stabilize the ECF mode and conduct the process for a long time. As such additives, sulfur-containing compounds (in particular n-butyl mercaptan) or tertiary amines are usually used [10, 11].
2. Isomerization and anionic polymerization of perfluoroalkene oxides, oligomerization of perfluoroalkenes in the liquid-phase oxidation processes
2.1. Isomerization and anionic polymerization of perfluoroalkene oxides
A number of nucleophilic fluorine-containing reagents are capable of opening the epoxide rings in alpha-oxides of perfluoroalkenes with a formation of perfluoroacyl fluorides. Thus, the presence of fluoride ions facilitates rapid opening of these rings while preserving the perfluorinated nature of the products. Nucleophilic attack occurs exclusively at the more substituted carbon with the formation of perfluoroalkoxides, which in some cases can be isolated from the reaction products [12]. As examples, the paper [12] provides alkoxides obtained from hexafluoropropylene oxides (HFPO) and octafluoro-isobutylene oxide (OFIBO).
|
CF3CF2CF2O-M+(HFPO) |
(CF3)2CFCF2O-M+ (OFIBO) |
Under the certain conditions, these salts lose M+ and F- fragments with a formation of the corresponding acyl fluorides. The latter can be converted to the acids by hydrolysis.
The nature of the fluoride ion and the nature of the M+ ion play a very important role in the opening of epoxide ring and in the stability of the resulting perfluoroalkoxide. The authors of [12] conducted studies of more than 40 fluoride salts in various solvents and found that cesium fluoride in tetraglyme showed the best results.
The resulting alkoxides can further react with perfluoroalkene oxide molecules to produce oligomeric products (Scheme 4) [12].
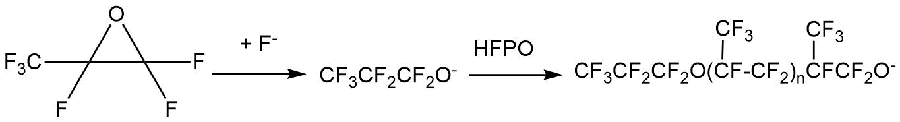
Scheme 4.
In addition, perfluoroalkene oxides can react with other fluorine-containing acyl fluorides and fluorine-containing ketones with a formation of the corresponding acyl fluorides.
The reactions of tetrafluoroethylene oxides (TFEO), hexafluoropropylene (HFPO) and some other perfluoroalkene oxides are covered in literary sources.
2.1.1. Isomerization and anionic polymerization of tetrafluoroethylene oxide (TFEO)
In 1966, the article by F. Gozzo and G. Camaggi reported the formation of trifluoroacetyl fluoride from tetrafluoroethylene oxide (TFEO) in the presence of potassium fluoride [13] (Scheme 5).
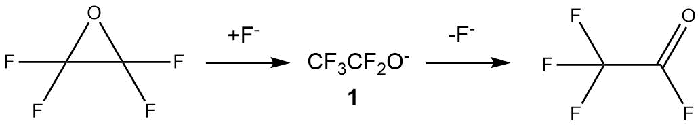
Scheme 5.
In 1967, V.I. Ginzburg and M.I. Vasilyeva also reported in their paper the rearrangement of TFEO into trifluoroacetyl fluoride in the presence of hydrogen fluoride even at -78ºC [14].
In the article by H. S. Eleuterio [15] it was reported that the rearrangement of TFEO to trifluoroacetyl fluoride can be carried out at room temperature on the catalyst surface or in the presence of fluoride ion (F-) sources. In addition, it was noted that the reaction could spontaneously occur even at room temperature, therefore TFEO should be stored only at temperatures below the boiling point (-65 ºC).
As noted above, the resulting alkoxide 1 (Scheme 5) can react further with perfluoroalkene oxide molecules to obtain the addition products.
Patent [16] gives an example of a reaction, which was carried out using activated carbon “Darco” as a catalyst at temperatures of -52÷-30 ºС. Perfluoroethoxyacetyl fluoride (CF2CF2-O-CF2-C(O)F) and further addition products (trimer and tetramer of TFEO) were identified in the reaction products.
The resulting TFEO trimer could be hydrolyzed with water to the corresponding acid CF3-CF2-O-CF2CF2-O-CF2-COOH [16].
Other examples of obtaining TFEO polymer compounds are given in [17]. The process was carried out at low temperatures (-70÷-80 ºС) in the presence of catalysts. Tertiary amines (triethylamine, tributylamine, etc.) and divalent copper salts (Cu2+) were used as catalysts. The products composition depended on the synthesis time: after 24 hours of synthesis, liquid compounds predominated in the products, after 48 hours of synthesis – solid compounds.
The patent [18] describes the addition reactions of TFEO in the presence of catalysts to other acyl fluorides: carbonyl fluoride, trifluoroacetyl fluoride, perfluoropropionyl fluoride, etc. The reactions were carried out in a medium of methylene chloride, dichloromethane, and various linear chlorofluorocarbons (Scheme 6).
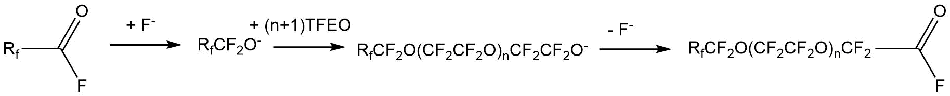
Scheme 6 (Rf = F, CF3, C2F5 et. Al.., n ≥0).
Tetraethylammonium cyanide or bromide were used as catalysts.
Thus, by reacting trifluoroacetyl fluoride (99 g) with TFEO (326 g) at a temperature of -30 °C and a pressure of ≈100÷138 kPa, the products presented in Table 1 were obtained (catalyst - tetraethylammonium cyanide) [18].
Table 1. The composition of reaction products of trifluoroacetyl fluoride with TFEO [18].
|
Structure |
Mass, g |
Boiling point, ºС |
|
C2F5- O-CF2COF |
15 |
0-6 |
|
C2F5-O-C2F4-O-CF2COF |
69 |
65-67 |
|
C2F5-O-(C2F4-O)2-CF2COF |
171 |
99-102 |
|
C2F5-O-(C2F4-O)3-CF2COF |
103 |
134-138 |
|
C2F5-O-(C2F4-O)4-CF2COF |
33 |
167-170 |
The reactions with fluorine-containing acyl fluorides of dibasic acids proceeded similarly. In this case, acyl fluorides containing simple ether bonds in the chain were obtained (Scheme 7) [14].
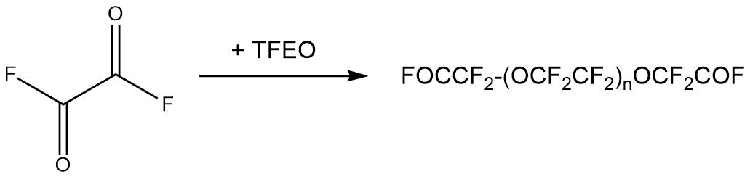
Scheme 7 (n =0÷3[17]).
Perfluoroketones can also react with TFEO to produce the corresponding acyl fluorides. In [18], the reaction of hexafluoroacetone with TFEO to produce acyl fluorides of the general formula (CF3)2-CF-O-(C2F4-O)n-CF2COF (n=0÷4) with the predominant formation of (CF3)2-CF-O-CF2COF was described.
2.1.2. Isomerization and anionic polymerization of hexafluoropropylene oxide (HFPO)
The reactions of hexafluoropropylene oxide (HFPO) are much more well studied compared to TFEO. This is due to the fact that the methods for producing HFPO are more accessible. In addition, HFPO can withstand high temperatures up to 150 ºС inclusive [15].
The process of isomerization of hexafluoropropylene oxide (HFPO) in reactions with nucleophilic agents with a formation of perfluoropropionyl fluoride (2) has been well studied (Scheme 8).
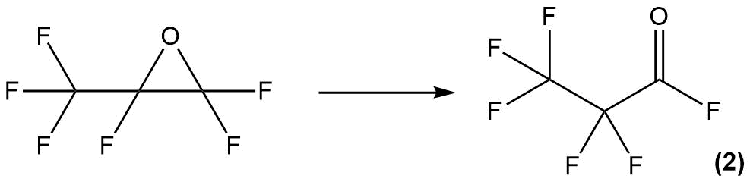
Scheme 8.
Various nucleophiles can be used to carry out this reaction, namely: primary, secondary and tertiary amines (in particular, triethylamine), Me3SiOMe, Me2Si(OEt)2 etc. [19].
There are different versions of the HFPO isomerization mechanism, which are discussed in [19-22].
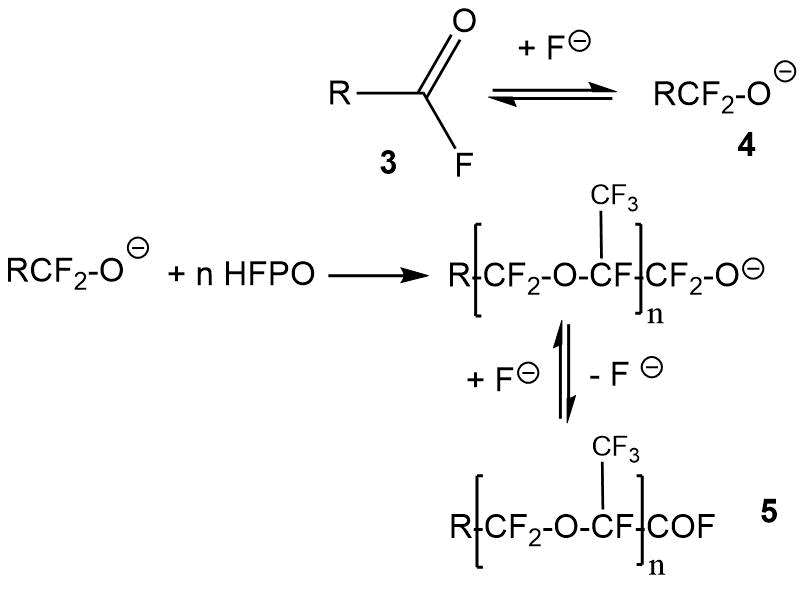
By reacting HFPO with primary acyl fluorides (3), the acyl fluorides of the general formula 5 (Scheme 9) can be obtained [19].
Scheme 9.
The starting acyl fluorides 3 can have different structures. The yield of the products of the general formula 5 depends on the nature of the radical R in acyl fluoride 3 and the stability of fluoroalkoxide 4. A large number of references to examples of such reactions are given in [19].
The use of special catalytic systems promotes the formation of fluoroalkoxides 4. In addition, the use of such catalysts should suppress the formation of perfluoropropionyl fluoride to prevent the side reaction of anionic polymerization of HFPO.
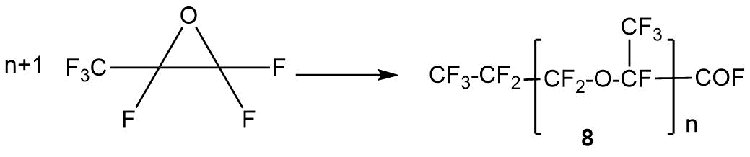
Anionic polymerization of HFPO is a special case of the interaction of HFPO with primary fluoroalkoxides and leads to the production of oligomeric acyl fluoride 8 with a number of n=1÷100 (Scheme 10) [19].
Scheme 10.
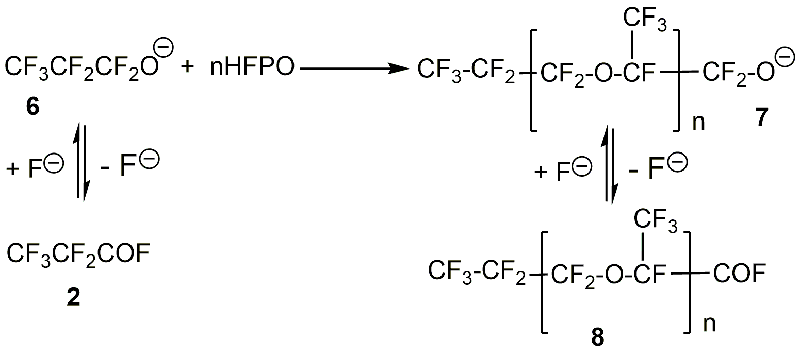
The first step of this process is isomerization of HFPO to perfluoropropionyl fluoride (Scheme 4), which is in equilibrium with perfluoropropoxide ion 6 (Scheme 11). The latter attacks one HFPO molecule to form alkoxide 7 with n=1. In the next step, alkoxide 7 can further react with HFPO molecules to give alkoxides with n>1 or form acyl fluoride 8 after elimination of the fluoride ion [19].
Scheme 11.
The oligomerization degree depends on the catalyst system used, the reaction conditions and the purity of the starting HFPO [19].
Thus, when using tetramethyl thiourea ((Me2N)2CS) as a catalyst, a mixture of oligomers of the following composition was obtained in diglyme: 13% with n = 1, 40% with n = 2 and 22% with n = 3 [23].
Table 2 shows examples of the dependence of the oligomerization degree of HFPO on the solvent nature [19].
Table 2. The dependence of the oligomerization degree of HFPO (n) on the solvent nature [19].
|
Solvent |
Yield (%) |
||||
|
n=0 |
n=1 |
n=2 |
n=3 |
n=4 |
|
|
Tetrahydrofuran |
100 |
||||
|
Acetonitrile |
88 |
9 |
3 |
||
|
Monoglym |
71 |
20 |
9 |
||
|
Diglym |
32 |
31 |
25 |
10 |
|
|
Tetraglym |
16 |
17 |
21 |
20 |
10 |
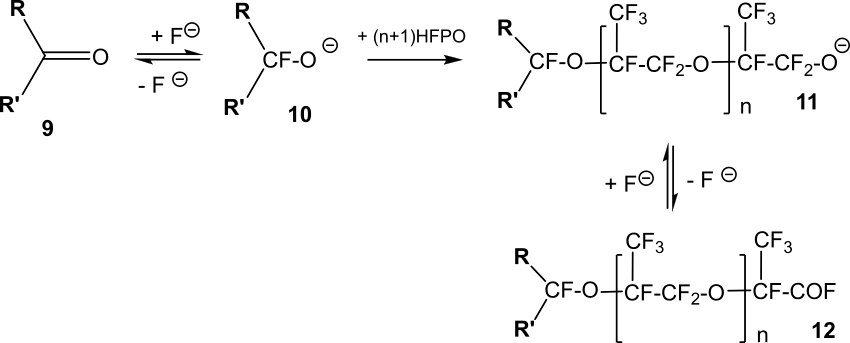
The reactions of HFPO with fluorinated ketones can lead to the production of fluorinated acyl fluorides of the general formula 12 (Scheme 12) [19].
Scheme 12.
Secondary fluorinated alkoxides 10, which are obtained in the presence of fluoride ions from fluorine-containing ketones 9, as a result of interaction with HFPO form alkoxides of the general formula 11 (n=0). The further process proceeds either with the splitting off of fluoride ion with the formation of acyl fluoride 12 (n=0), or with the further addition of HFPO molecules to alkoxide 11 with the formation of acyl fluorides 12 (n≥1).
The patent [24] describes the reaction of HFPO with hexafluoroacetone (HFA), 1,3-dihydroperfluoroacetone, perfluoro-2-pentanone and a number of other perfluoroketones. The process conditions were as follows: initial temperature of -80 ºС followed by heating to 80 ºС, catalyst – cesium fluoride in diglyme. As a result of the reactions, the corresponding acyl fluorides of the general formula 12 were obtained.
In the case of the reaction of HFPO with HFA at a reagent ratio of 1:1, perfluoro-2-isopropoxypropionyl fluoride was predominantly obtained, from which the corresponding acid was obtained in the next step by hydrolysis. If the ratio of HFPO to HFA was 5:1, the compounds with n = 1-6 were found in reaction products 13.
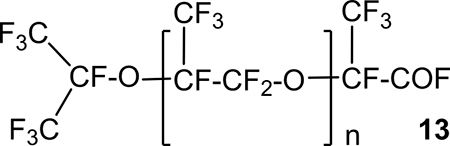
Examples of other syntheses with different radicals R and R’ in ketones 9 (Scheme 12), as well as the reaction conditions of compounds 9 with HFPO (reagents ratio, catalysts) are also given in [19].
2.2. Oligomerization of perfluoroalkenes in liquid-phase oxidation processes
Liquid-phase oxidation of perfluoroalkenes is used in industry to produce perfluoropolyethers (PFPE), which have been known since the 1960s under the trade name Fomblin® (Solvay).
The oxidation process of tetrafluoroethylene (TFE) and hexafluoropropylene (HFP) is carried out with molecular oxygen at low temperature in the presence of an initiator to obtain oligomeric products of the approximate structure 14 and 15 (peroxide PFPE, PO PFPE) in Scheme 13, respectively.
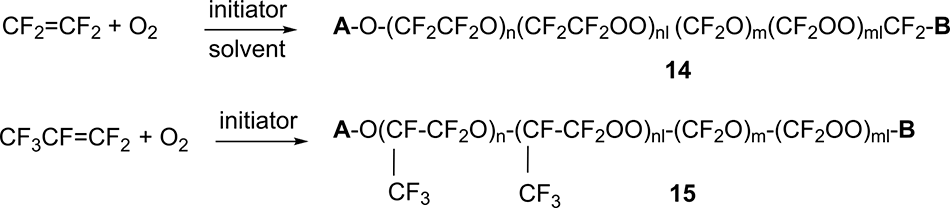
Scheme 13
In the case of liquid-phase oxidation of HFP, the liquid phase is HFP itself, while the TFE oxidation processes require the use of an inert solvent.
The initiators used are either UV radiation or chemical reagents: elemental fluorine, trifluoromethyl hypofluorite, etc.
Groups A and B in Scheme 15 are perfluoroalkyl or acyl fluoride (in most cases, groups -CF3, -CF2CF3, -COF or -CF2COF).
For example, in the case of liquid-phase oxidation of HFP when using UV radiation as an initiator, the ratio of groups A and B was the following: perfluoroalkyl-acyl fluoride: perfluoroalkyl-perfluoroalkyl: acyl fluoride = 2:1:1 [25].
In the resulting PO PFPE 14 and 15 there are the fragments with bonds (-O-O-), the removal of which occurs in the next stage of synthesis.
The PFPE production processes are well described in literature. The main patterns of liquid-phase oxidation of HFP and TFE when initiated by UV radiation are summarized in the paper by Dario Sianesi et al. [25]. The liquid-phase oxidation processes of HFP with chemical initiation (elemental fluorine) are described, for example, in [26, 27]. The features of the liquid-phase oxidation processes of TFE using various chemical initiators, as well as the mechanism and kinetic patterns of the processes are given in articles [28-32]. In addition, there is a large amount of patent literature.
To remove the peroxide fragments (-O-O-), thermal treatment in the temperature range of 150÷300ºC, thermal stabilization with simultaneous UV irradiation [25] or chemical methods [32-34] are used.
As a result, oligomeric compounds 16 and 17 (Scheme 14) are obtained in most cases having at least one acyl fluoride group in their structure, which can be converted by hydrolysis into the corresponding acid.
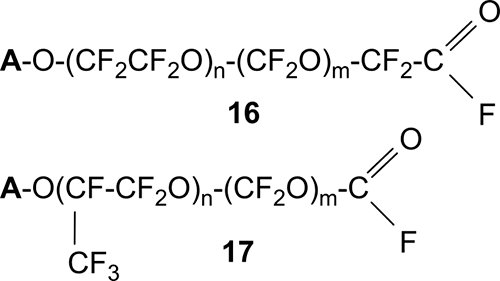
Scheme 14.
By varying the process parameters (temperature, ratio of reactants, initiator nature, method of removing peroxide groups, etc.), the chain length of the resulting compounds and/or the preferred composition of the terminal groups can be adjusted.
For example, carrying out liquid-phase oxidation of HFP at a temperature of about -30ºС (initiator – F2) led to a decrease in the molecular weight of oligomer 15 to MM ≈ 1000 [27], whereas carrying out the process at -50÷-60 ºС leads to obtaining oligomer 15 with MM≈3000÷3500.
The paper [33] describes the production of peroxide PFPE by low-temperature liquid-phase oxidation of TFE (UV irradiation, T= - 40ºС, solvent R227ea) with the simultaneous addition of perfluoroacryloyl fluoride (CF2=CF-COF) to the reactor. In this case, PFPE PO (MM≈2900) of the general formula X1-O(CF2O)n1(CF2CF2O)m1(CF2(CF2)zCF2O)p1 (O)h-X2 were obtained, where the groups X1 and X2 were -CF2-COF or -COF. The destruction of peroxide bonds with 57% aqueous hydroiodic (HI) acid in the presence of methanol led to the formation of the products of the general formula Y1-O(CF2O)n1(CF2CF2O)m1(CF2(CF2)zCF2O)p1-Y2 with an average MM≈490, where Y1 and Y2 were the groups -CF2COOCH3 and/or -CF2COOH.
The work [34] also describes the destruction of peroxide bonds in PO PFPE using a 57% aqueous solution of hydroiodic (HI) acid in the presence of methanol. However, the initial PO PFPE was obtained by low-temperature liquid-phase oxidation of TFE (initiator – F2, T=-82 ºС, solvent dichlorodifluoromethane) without additional ingredients. In this case, the products of the general formula Y-O(CF2O)a(CF2CF2O)b-Y’ with an average MM≈930 were obtained, where Y and Y’ were the groups -CF2COOCH3, -CF3, -C2F5 with a significant predominance of ester group.
The article [32] describes low-temperature liquid-phase oxidation of HFP, when, in addition to a monomer, oxygen, and an initiator, chlorotrifluoroethylene (CTFE) was introduced into the reactor as a «comonomer». As a result, polyether telomers of the general formula Cl–(C3F6O)n–CF2CFO were obtained. The resulting mixture could be separated into individual compounds by distillation. The obtained polyester telomers with a yield of more than 85% (weight) were converted into the corresponding carboxylic acids: Cl–(C3F6O)n–CF2COOH, where n=1÷5.
The process of obtaining polyether diacyl fluorides of the general formula 16 (group A was also predominantly acyl fluoride) is described in [35]. The initial peroxide PFPE 14 (Scheme 14), which was obtained by low-temperature liquid-phase oxidation of TFE with an average MM≈83400 and contained predominantly one acyl fluorides group, was treated with gaseous hydrogen in the presence of a catalyst (Pd/CaF2) at a temperature of 125÷-130 ºС and a pressure of about 3 atm. The obtained diacyl fluorides did not contain peroxide bonds and had MM≈1500.
The papers [26,36] report on the production of the corresponding acids from acyl fluorides of the general formula 17 (n=8÷50, m=0÷10). They can then be used to obtain various surfactants.
References
- Jinhui Han, Xiaohua Yang, Zhiping Liu, Effect of Fluorinated Carboxylic Acid Ester on Lithium Solvation as an Additive in Electrolyte and Low-Temperature Insight on Battery Performance, Ind. Eng. Chem. Res. 2023, 62, 19, 7682–7692, https://doi.org/10.1021/acs.iecr.3c00417.
- Thibault Alle, Sipak Joyasawal, Killian Oukoloff, Keith Long, Zachary Owyang, Karol R. Francisco, Dominique Cahard, Donna M. Huryn, Carlo Ballatore, Structure-property relationships of fluorinated carboxylic acid bioisosteres, Bioorg. Med. Chem. Lett. 91 (2023), 129363, https://doi.org/10.1016/j.bmcl.2023.129363.
- G.G.Furin, Perfluorinated Carboxylic Acids. Synthesis and Application, Fluorine Notes, Iss. 3(34) 2004 (http://en.notes.fluorine1.ru/contents/history/2004/3_2004/retro/index.html).
- V. М. Berenblit, Electrochemical fluorination of organic compounds, Fluorine Notes, Iss. 1(2)-3(4), 1999 (http://notes.fluorine1.ru/contents/history/1999/1_1999/Notes/retro/index.html).
- Alsmeyer, Y.W., Childs, W.V., Flynn, R.M., Moore, G.G.I., Smeltzer, J.C. (1994). Electrochemical Fluorination and Its Applications. In: Banks, R.E., Smart, B.E., Tatlow, J.C. (eds) Organofluorine Chemistry. Topics in Applied Chemistry. Springer, Boston, MA. DOI: 10.1007/978-1-4899-1202-2_5.
- N.V. Ignat’ev, Electrochemical Fluorination: A Powerful Tool for the Preparation of Organofluorine Compounds. In book: Modern Synthesis Processes and Reactivity of Fluorinated Compounds, pp.71-123, 2017. DOI: 10.1016/B978-0-12-803740-9.00004-4
- B. N. Maksimov, L.N. Kosareva, N.A. Rjabinin, Method of preparing higher perfluoromonocarboxylic acids, Patent RU 2107751, 1993.
- M. Napoli, L. Conte, G.P. Gambaretto, F.M. Carlini, Electrochemical fluorination of 4-(perfluoro-n-butyl)-n-butanoyl chloride, Journal of Fluorine Chemistry, Volume 45, Issue 2, 1989, Pages 213-224, DOI: 10.1016/S0022-1139(00)84147-0
- N.B. Lesnevskaya, E.V. Litvinenko, A.A. Ludikainen, V.A. Matalin, T.V. Mikhailova, Electrochemical fluorination of maleic anhydride, Fluorine Notes, Iss. 6(121) 2018 (http://en.notes.fluorine1.ru/public/2018/6_2018/article_2.html), DOI 10.17677/fn20714807.2018.06.02.
- Pat. US3028321, Electrochemical production of fluorocarbon acid fluorides, 1962.
- G.I. Kaurova, V.A. Matalin, A.A. Krasil’nikov, V.G. Barabanov, B.N. Maksimov, Electrochemical Fluorination in Synthesis of Fluoroorganic compounds, In book: V.I. Manuylova, B.N. Maximov, V.V. Kornilov (Eds.) "Fluorine compounds. Chemistry, Technology, Application", Teza, Saint-Petersburg, 2009, 356 p., ISBN 5-88851-068-8, pp.69-82 (in Russian).
- J. T. Hill and J. P. Erdman, Anionic Polymerization of Fluorocarbon Epoxides, ACS Symposium Series Vol. 59, 1977, pp. 269-284, DOI: 10.1021/bk-1977-0059.ch019.
- F. Gozzo, G. Camaggi, Oxidation reactions of tetrafluoroethylene and their products-I: Auto-oxidation, Tetrahedron, 1966, Vol. 22, pp. 1765-1770.
- P. Tarrant and E. Stamp, Zh. Vses. Khim. O-va.,1970, 15, 34.
- H. S. Eleuterio, Polymerization of Perfluoro Epoxides. Journal of Macromolecular Science: Part A - Chemistry, 1972, 6(6), pp. 1027–1052, DOI:10.1080/10601327208056884.
- US Patent 3125599, Polymers of fluorocarbon epoxides, 1964.
- US Patent US3380937, Process for polymerizing tetrafluoroethylene epoxide, 1968.
- US Patent 3250806, Fluorocarbon ethers of tetrafluoroethylene epoxide, 1966.
- H. Millauer, W. Schwertfeger, G. Siegemund, Hexafluoropropene Oxide - A Key Compound in Organofluorine Chemistry, Angew. Chem. Inr. Ed. Engl., 1985, Vol. 24, Iss. 3, pp. 161-179, DOI: 10.1002/anie.198501611.
- D. Sianesi, A. Pasetti, F. Tarli, J. Org. Chem., 31, 1966, p. 2312.
- I. L. Knunyants, V. V. Shokina, I. V. Galakhov, Khim. Geterotsikl. Soedin. 1966, p. 873.
- R. A. Bekker, G. V. Asratyan, B. L. Dyatkin, Zh. Org. Khim., 1973, 9, p. 1644.
- US Patent 4118421, Process for the manufacture of perfluoro-alkoxy-propionic acid fluorides, 1978.
- US Patent 3274239, Fluorocarbon ethers, 1966.
- Sianesi, D., Marchionni, G., De Pasquale, R.J. Perfluoropolyethers (PFPEs) from Perfluoroolefin Photooxidation. In: Banks, R.E., Smart, B.E., Tatlow, J.C. (eds) Organofluorine Chemistry. Topics in Applied Chemistry. Springer, Boston, 1994, MA. DOI: 10.1007/978-1-4899-1202-2_21.
- B. N. Maksimov, V. V. Kornilov, B. A. Melnychenko, and L. N. Kosareva, Perfluoropolyethers. Synthesis and Application, Russian Journal of Applied Chemistry, 2009, Vol. 82, No. 9, pp. 1706−1710.
- RU Patent 2046127, Process for preparing polyperfluoropropylene oxide, 1995
- P.A. Guarda, E. Barchiesi, G. Fontana, S. Petricci, M. Pianca, G. Marchionni, Peroxidic perfluoropolyether from tetrafluoroethylene oxidation: micro structural analysis by NMR spectroscopy and mechanistic considerations, Journal of Fluorine Chemistry, 2005, Vol. 126, Iss. 1, pp 141-153, doi:10.1016/j.jfluchem.2004.11.012.
- P.A. Guarda, E. Barchiesi, G. Fontana, S. Petricci, M. Pianca, G. Marchionni, Peroxidic perfluoropolyether from tetrafluoroethylene oxidation: micro structural analysis by NMR spectroscopy and mechanistic considerations, Journal of Fluorine Chemistry, 2005, Vol. 126, Iss. 4, Pp. 683-695, DOI: 10.1016/j.jfluchem.2005.01.002.
- D. Sianesi, P. A. Guarda, G. Marchionni, A Kinetic and Mechanistic Study of the Low-Temperature Fluorine-Initiated Copolymerization of Tetrafluoroethylene with Oxygen, Macromolecules, 1999, Vol. 32, Iss. 23, pp. 7768-7780, DOI: 10.1021/ma9906985.
- P.A. Guarda, S. Petricci, G. Fontana, G. Marchionni, Synthesis and characterization of poly(tetrafluoroethyleneperoxides), Journal of Fluorine Chemistry, 2009, Vol.130, Iss. 11, pp. 1011-1016, DOI: 10.1016/j.jfluchem.2009.07.021.
- Massimo Malavasia, Dario Sianesi, Novelties and prospects in the synthesis of perfluoropolyethers by oxidative polymerization of fluoroolefins, Journal of Fluorine Chemistry, 1999, 95, pp. 19–25.
- EP 1568730, Peroxidic perfluoropolyethers, 2011.
- EP 0654493, Process for preparing perfluoropolyethers, 1995.
- EP 1388555, A process for the preparation of perfluoropolyethers acyl-fluoride ended by reduction of the corresponding peroxidic perfluoropolyethers, 2004.
- RU Patent 2069673, Antifriction composition for treatment of solid surfaces, 1996.
ARTICLE INFO
Received 27 March 2025
Accepted 21 April 2024
Available online
April 2025
Recommended for publication by PhD A.A. Tyutyunov
eLIBRARY Document Number (EDN) NQGGRN

Fluorine Notes, 2025, 159, 1-2
