Received: December 2024
DOI 10.17677/fn20714807.2025.01.02
Fluorine Notes, 2025, 158, 3-4
SYNTHESIS OF FLUOROSULFONYLOXA DIFLUOROACETYL FLUORIDE BY THERMAL OXIDATION OF PERFLUOROALLYL FLUOROSULFATE WITH MOLECULAR OXYGEN
Chernyavsky G.G., Kulaichenkov S.A., Osipova L.V., Sokolov Y.P., Emelyanov G.A.
Lebedev Institute of Synthetic Rubber (FGBU "NIIISK")
198035, Saint Petersburg, Gapsal'skaya Street, 1
Abstract: The synthesis of fluorosulfonyloxadifluoroacetyl fluoride (FSOAF, FSO₂OCF₂COF) with high yield (over 90 mol%) is described. The process involves thermal oxidation of allyl fluorosulfate (AFS, CF₂=CF-CF₂OSO₂F) using molecular oxygen. The structure of the obtained product, FSOAF, was confirmed by 13C and 19F NMR spectroscopy, as well as IR spectroscopy.
Keywords: allyl fluorosulfate, fluorinated polymers.
In the production of components for modern technology intended for operation under extreme conditions, there is a need for new fluorinated elastomers and fluoropolymers with a broader working temperature range compared to the results achieved so far. The most pressing challenge is the production of bifunctional fluoroorganic compounds — starting reagents for obtaining monomers and comonomers used in the synthesis of fluoroelastomers (FE), fluorinated solid polymers (FSP) — polymeric electrolytes for hydrogen energy, containing functional groups that ensure effective operation, as well as the structuring and necessary modification of the synthesized products.
The development of synthesis technologies for bifunctional fluoroorganic compounds and their derivatives makes it possible to eliminate the shortage of reagents for producing modern thermocryogenic-resistant elastomers and fluorinated solid polymers (FSP) — polymeric electrolytes for hydrogen energy.
In the early 1980s–1990s, the first reports on the synthesis and properties of perfluoroallyl fluorosulfate CF₂=CF-CF₂OSO₂F (AFS), a new and interesting compound, appeared [1–4]
By 2011, a comprehensive review [5] was published, which thoroughly examined various reactivity aspects of the new synthon. In addition to addressing the synthesis of AFS itself, the main focus of the article is on its reactions with nucleophilic and radical reagents. The review emphasizes both the wide range of promising transformations of the initial product and the possibility of achieving high yields of target compounds with good conversion of the starting materials.
To date, a significant amount of data has been accumulated on the involvement of AFS and its derivatives in polymerization processes, leading to the formation of highly interesting products [6]. However, processes related to the oxidation of perfluoroallyl fluorosulfate are significantly less covered in open sources. For instance, patents [7–8] describe the formation of malonyldifluoride as a result of the decomposition of perfluoroallyl fluorosulfate oxide, which is obtained by reacting AFS with oxygen. These issues have been studied in more detail in the work [9].
The processes reflecting the properties of AFS during oxidation by oxygen are concisely shown in Scheme 1.
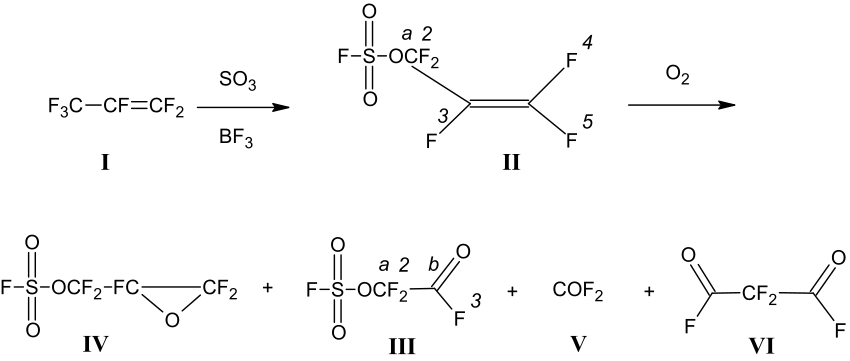
Scheme 1. Processes explaining the properties of AFS during oxidation by oxygen.
During the catalytic sulfotrioxidation of hexafluoropropene (I), perfluoroallyl fluorosulfate AFS CF₂=CF-CF₂OSO₂F (II) is produced [1-4]. This compound, upon oxidation with oxygen, produces oxirane (IV), fluorosulfonyloxadifluoroacetyl fluoride (III), carbonyl fluoride (V), and malonyldifluoride FOCCF₂COF (VI) [7-9].
As a result of the oxidation of compound (II), a mixture of compounds (III), (V), (IV), (VI), and oligomerization products of oxirane (IV) is obtained. According to the authors of work [9], fluorinated anhydride (III) may be a product of the thermal decomposition of oxirane (IV). In study [9], the process of thermal decomposition of compound (IV) under the effect of various nucleophilic agents was studied in detail, with fluorinated anhydride (III) being spectrally identified among the reaction products, and its yield determined as 0.5 molar %. The boiling point, density, and other physicochemical properties were not established.
In patent [10], a significantly higher yield of the target product (over 90 molar percent) was achieved; however, the harsh process conditions (high temperature of 190–220°C, an excessive amount of oxygen up to five times the stoichiometric requirement) along with the presence of an induction period for the reaction onset made it unsuitable for safe industrial scaling.
To address the aforementioned issues that limit the industrial implementation of the process, in this work, we examined the task of laboratory investigation of the said process, as shown in Scheme 1, to determine the effect of temperature and several other factors on the course of this reaction (see Table 1).
Table 1. Effect of temperature on the yield of products from the thermal oxidation of perfluoroallyl fluorosulfate (II) with molecular oxygena.
|
Temperature, °С |
Yield, % |
|||
|
III |
IV |
олигомеры |
Conversion of compound (II), % |
|
|
60-80b 80-100b 100-120c 120-140c 140-160c 160-165c 160-165de 170-175df |
6 8 14 21 31 52 96 95 |
62 68 74 70 59 41 2 2 |
29 22 17 12 9 6 2 3 |
43 58 73 88 100 100 100 100 |
a – yield, % molar, calculated for the converted compound (II), with a 1.5-fold molar excess of oxygen;
b – an induction period is present;
c – a slight induction period is present;
d – the induction period is virtually absent;
e – 3 molar % of hexafluoropropylene dibromide was added;
f – 7 molar % of tetrafluoroethylene dibromide was added.
As can be seen from Table 1, for the synthesis of compound (III) via thermal oxidation with oxygen, the optimal temperature is 160–165°C when 0.03 molar % of hexafluoropropylene dibromide is added.
In addition to temperature, the choice of solvent is an important factor in developing a synthesis methodology. High-boiling inert solvents, such as C₃F₇OCF(CF₃)CF(CF₃)OC₃F₇ (VII) or other fluoroethers, perfluorocarbons (e.g., perfluorodecalin, perfluorocyclohexane, etc.), are often employed in thermal oxidation processes to suppress chain reactions and mitigate potential excessive exothermic effects.
However, when there is a significant induction period, their use may lead to uncontrollable reaction progress. Upon conducting the target reaction for the synthesis of FSOAF over 4 hours at 195–200°C, it was found that increasing the proportion of solvent (VII) in the charge from 10% to 50% did not significantly enhance the yield of compound (III), while the induction period persisted. Therefore, the use of high-boiling inert solvents like (VII) in this reaction can be omitted.
Among the interesting findings experimentally tested, the possibility of incorporating 3-7 molar % of tetrafluoroethylene dibromide or hexafluoropropylene dibromide into the reaction mixture showed that the induction period during oxidation is virtually absent, as observed in the last two cases presented in Table 1.
Experimental part
Perfluoroallyl fluorosulfate AFS (II) was obtained according to the procedure in [4] with a purity of 95-97% (by monitoring the boiling temperature of the product during purification at the main fraction collection point during rectification at 63.5-64.0°C), and also confirmed using ¹⁹F NMR spectroscopy and GC. The purity of compound (II) was determined by GC on an LСM-80 instrument. The chromatographic analysis conditions for compound (II): column 3000 x 3 mm, stationary phase: 5% mixture of perfluorinated products of hexafluoropropylene oxide polymerization with the general formula CF₃(CF₂)₂O[CF(CF₃)CF₂O]-CF(CF₃)COF on an Inerton AW–HMDS support (particle size 0.25-0.315 mm), thermal conductivity detector, carrier gas helium, flow rate 40 cm³/min, column oven temperature 50°C, injector temperature 100°C, detector temperature 100°C.
The structure of the obtained compound (II) is confirmed by NMR spectroscopy data for ¹⁹F and ¹³C . The ¹⁹F NMR spectrum of compound (II) contains five complex multiplet signals from F nuclei with chemical shifts: δ1 +47.0 t.d., δ2 -73.2 d.d.d., δ3 -192.7 d.d.t., δ4 -103.7 d.d.t., δ5 -90.1 d.d.t., and coupling constants (in Hz): 7.7 (J₁,₂=J₂,₁), 1.5 (J₁,₃=J₃,₁), 27.3 (J₂,₄=J₄,₂), 14.2 (J₂,₃=J₃,₂), 7.4 (J₂,₅=J₅,₂), 118.1 (J₃,₄=J₄,₃, trans), 39.0 (J₃,₅=J₅,₃, cis), 44.5 (J₄,₅=J₅,₄, gem). The numbers corresponding to the atom designations match the labels shown in Scheme 1.
The ¹³C NMR spectrum of compound (II) shows three multiplet signals from the ¹³C nuclei with chemical shifts at δa +118.0 t.d.d.d.d., δb +120.8 d.d.t.d.d., and δc +155.1 d.d.d.t. The coupling constants (in Hz) are as follows: for atom a, there are values of 277.8 t. (Ja,2), 31.0 d. (Ja,3), 7.6 d. (a,s), 6.2 d. (Ja,4), and 1.3 d. (Ja,1); for atom b, the constants are 241.7 d. (Jb,₃), 44.0 d. (Jb,₄), 39.3 t. (Jb,₂), 25.5 d. (Jb,₅), and 1.2 d. (Jb,₁); finally, for atom c, we observe 297.1 d. (Jc,₅), 294.7 d. (Jc,₄), 38.4 d. (Jc,₃), 2.4 t. (Jc,₂), and <0.5 d. (Jc,₁). These notations follow standard conventions in ¹³C NMR spectroscopy, where each signal corresponds to specific carbon atoms within the molecule.
Fluorosulfonyloxadifluoroacetyl fluoride (III) was obtained in a laboratory reactor with a jacket capacity of 0.75 L, equipped with a stirrer. The thermal oxidation process was carried out using approximately a 1.5-fold (molar) excess of the oxidizer, with a reaction duration of 4 hours. The reactor, which was initially evacuated, was then filled with a mixture of compound (II) (230 g) and hexafluoropropylene dibromide (9.3 g, 0.03 mol). After sealing the autoclave, heating and stirring were initiated. At 160°C, with the stirrer operating, oxygen was introduced at a rate of 48 g (1.5 mol) such that the temperature of the reaction mixture did not exceed 165°C.
As the process progresses, the increase in temperature is controlled by the refrigerant circulating within the apparatus jacket. Following the introduction of the oxidizing agent, the charge was maintained at 165°C for a duration of two hours.
After cooling the reactor to room temperature and venting the gaseous products, the crude product was subjected to rectification on a laboratory column with an efficiency of 15-20 theoretical plates, collecting fractions up to 39°C, 39-41°C, 41-63°C, and 63-65°C. More than 90% (by weight) of compound (III) was contained in the 39-41°C fraction. The yield amounted to 182 g (93% mol.).
Fluorosulfonyloxadifluoroacetyl fluoride (FSOAF), FSO₂OCF₂COF, is a transparent colorless liquid with a sharp odor, boiling point 39.4°C, d₂₀⁴ = 1.6279. Found: S 16.3. Calculated: S 16.35.
The structure of the obtained compound (III) is also confirmed by ¹⁹F NMR and ¹³C NMR data. The ¹⁹F NMR spectrum contains three multiplet signals from F nuclei with chemical shifts at δ₁ +50.01 t.d., δ₂ -77.53 d.d., δ₃ +15.76 t.d., and coupling constants (in Hz): 7.25 (J₁,₂ = J₂,₁), 2.90 (J₂,₃ = J₃,₂), 0.70 (J₁,₃ = J₃,₁).
The ¹³C NMR signals of compound (III) exhibit chemical shifts at δa +111.7 t.d.d. and δb +146.0 d.t.d., with coupling constants (in Hz): 289.35 (Jₐ,₂), 94.80 (Jₐ,₃), 1.45 (Jₐ,₁), 372.85 (Jb,₃), 44.55 (Jb,₂), and 1.45 (Jb,₁). The numbers corresponding to the atom designations match those indicated in Scheme 1
The purity of compound (III) was determined using GC on an LСM-80 instrument, in a manner analogous to that performed for compound (II).
The ¹³C and ¹⁹F NMR spectra of compounds (II) and (III) were recorded on a Bruker AM-500 spectrometer (125.8 and 470.6 MHz, respectively) at room temperature using CDCl₃ with hexafluorobenzene as an internal standard.
IR spectrum of FSOAF: C=O: 1980 cm⁻¹; S=O: 1486 cm⁻¹.
Conclusions
An efficient method for the high-yield synthesis of fluorosulfonyloxadifluoroacetyl fluoride (FSOAF), FSO₂OCF₂COF, suitable for scaling up, including industrial application, has been described. The novel synthesis approach involves the thermal oxidation of perfluoroallyl fluorosulfate (AFS), CF₂=CF-CF₂OSO₂F, with molecular oxygen in the presence of 3-7 molar percent of tetrafluoroethylene dibromide or hexafluoropropylene dibromide. This method highlights the reaction behavior of perfluorinated allylic esters when interacting with this oxidizer at various temperatures, both in the presence of inert solvents and without them. The yield of the target product FSOAF after rectification exceeds 90%, with a purity of no less than 99.5% as determined by GC. The structure of the target compound is confirmed by ¹⁹F NMR, ¹³C NMR, and IR spectroscopy.
FSOAF is of particular interest due to its dual functionality ‑ containing both fluorosulfate and fluorinated anhydride groups. The sequential or simultaneous utilization of their reactive properties offers promising synthetic opportunities for developing fluoroorganic products, including monomers and polymers designed for operation at elevated temperatures and under harsh conditions (aggressive chemical environments, processes involved in fuel cells, and other hydrogen energy applications).
An effective method for the high-yield production of fluorosulfonyloxadifluoroacetyl fluoride, suitable for industrial implementation, has been proposed.
References
- England D.C. Perfluoroallyl fluorosulfate and its sultone polymers: Patent USA 4206138, DuPont; applied 1980.
- Krespan C.G., England D.C. Perfluoroallyl fluorosulfate, a reactive new perfluoroallylating agent . J. Am. Chem. Soc., 1981, Vol. 103, pp. 5598–5599.
- Banks R.E. et al. Perfluoroallyl fluorosulphonate—Preliminary note . J. Fluorine Chem., 1982, Vol. 20, pp. 133–134.
- Rondarev D.S. et al. Method for producing perfluoroallyl fluorosulfate: Patent USSR 1766912, MKI C07C 317/60; filed December 27, 1988; published June 23, 1993, Bulletin No. 23.
- Wlassics I. et al. Perfluoro Allyl Fluorosulfate (PAFS): A Versatile Building Block for New Fluoroallylic Compounds. Molecules, 2011, Vol. 16, Issue 8, pp. 6512–6540. DOI: 10.3390/molecules16086512.
- Method for producing perfluoroallyl fluorosulfate: Patent Russia 2443719, MKI C08F 214/00; filed August 10, 2010; published February 27, 2012, Bulletin No. 6.
- Patent of Japan JPH01149749A, 1989.
- Patent of Japan JPH01163173A, 1987.
- Lebedev N.V. et al. Journal of Applied Chemistry (Russia), 2009, Vol. 82, Issue 3, p. 455.
- Method for producing perfluoroallyl fluorosulfate: Patent Russia 2484081; published December 27, 2012, Bulletin No. 36.
ARTICLE INFO
Received 24 December 2024
Accepted 06 February 2025
Available online February 2025
Recommended for publication by PhD O.V. Bryzgalova
eLIBRARY Document Number (EDN) NJBHUW

Fluorine Notes, 2025, 158, 3-4
