Received: October 2024
DOI 10.17677/fn20714807.2025.01.01
Fluorine Notes, 2025, 158, 1-2
IONIC SERIES OF PERFLUOROCYCLOHEXANE AND PERFLUOROCYCLOHEXENES MASS SPECTRA WITH PERFLUOROALKYL SUBSTITUENTS
N. D. Kagramanov
A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences,
119991, GSP-1,
Moscow, B-334, Vavilov St. 28
e-mail: ndkagram@gmail.com
Annotation: The present report is a continuation of the ionic series studies of unsubstituted cycloalkanes, perfluorocyclohexane, and perfluoropolycycloalkanes performed earlier. It was found that, as a result of primary synchronous detachments of one, two and three fluorine atoms, perfluorocyclohexane fragments to form three major series of ions, one of which (+·M -1·F detachment) is the perfluoroallyl series: +С6F11 m/z 281, +C5F9 231, +C4F7 181, +C3F5 131, +C2F3 81. In the case of perfluorocyclohexane fragmentation, two additional series are proposed: perfluorolefins (series 4) and perfluoroalkyl (series 5), occurs as a result of decay +·M/2 (intensity of the peak +·C3F6 с m/z 150 0,5%) and, respectively, the rearrangement allyl radical ·C3F5 detachment, to form an ion +C3F7 m/z 169 (followed by intensity Tr). In contrast to perfluorocyclohexane, in the spectra of its derivatives, two of these series cannot occur as a result of the perfluoroallyl radical detachment, as well as the decay +·M/2. The aim of the study was to investigate secondary detachments in the spectra of perfluorocyclohexanes with perfluoroalkyl substituents leading to the appearance of two new series of ions: perfluoroalkyl and perfluoroolefin. Analysis of perfluorocyclohexane and eight of its derivatives ionic series was determined that two additional ionic series: perfluoroalkyl and perfluorolefins arise as a result of carbon atom rearrangement from one of perfluoroallyl series ions (+·M -1·F) and perfluoroalkenyl series (+.M -2·F).
By analyzing the fragmentations of perfluorocyclohexene and its derivatives containing two less fluorine atoms than perfluorocyclohexane, their major ionic series (+·M -1·F, +.M -2·F and +·M -3·F), their secondary ionic series, and their new ionic series have been established. In all figures, ion series are represented both graphically and digitally. When the peak intensities of an ion series are low and overlap with other ion series, in this case they are represented only digitally in the figures.
Keywords: mass spectra and ionic series of C6F12, isomers C8F16, C9F18, isomers C10F20, C11F22.
Primary detachments and ionic series mass spectra of perfluorocyclohexane and its derivatives with perfluoroalkyl substituents
Figure 1 shows the three main series (1-3) of perfluorocyclohexane mass spectra, and branching of the series (1-3), with correction of the paths presented earlier in [1], leading to the occurrence of perfluoroalkyl (+181 - c → +169.119.69), as well as to the perfluorolefins ionic series (+162 - C → +150.100.50). The conclusion about the general branching process of series 1 and 2, with a carbon atom detachment, as well as series 3 with two secondary detachments of the fluorine atom is made as a result of analyzing the branching process of series 1, 2 and 3 of perfluorocyclohexane and eight of its derivatives with perfluoroalkyl substituents.
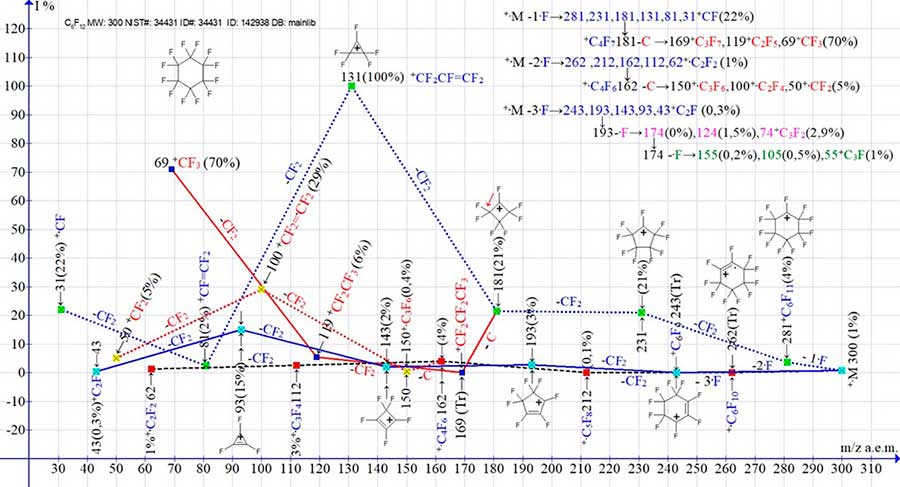
Figure 1. Three main series of fragment ion mass spectra of perfluorocyclohexane С6F12 and their four branching series. MW: 300 NIST#:34431 ID#: 142938 DB: mainlib.
The three series of ions formation as a result of primary synchronous detachments of one, two, and three fluorine atoms is a general fragmentation process of perfluorocarbon compounds due to the scattering of the kinetic energy of ionizing electrons. The primary processes depend on the structure and symmetry of the cycle substituents, their chain length and their relative position [2].
During the fragmentation of perfluorocyclohexane C6F12 [1] MW=310 molecular ions (Figure 1), there are three main series of ions with the last significant digits of the masses: 1 (M -·F) perfluoroallyl, as well as 2 (M-2·F) and 3 (M-3·F). By analyzing of the subsequent detachments it was found that in the spectrum of perfluorocyclohexane, as well as in the spectra of its derivatives with perfluoroalkyl substituents, the perfluoroalkyl and perfluoroolefin series, arise as a result of rearrangement detachments of a carbon atom from one of the perfluoroalkyl ions (+·M -·F) and, respectively, from one of the perfluoroalkenyl ions (+·M -2·F). Thus, the appearance of a series of perfluoroalkyl ions: +C3F7, +C2F5, +CF3 (169, 119, 69 with the last significant digit 9) is the result of branching of the perfluoroalkyl series with rearrangement detachment of the carbon atom (181+C4F7 -C+C3F7 m/z169). Occurrence of perfluoroolefin ionic series: +С3F6, +C2F4, +CF2 (150, 100, 50, with the last significant digit 0), the result of branching of the series (+·M -2·F) with rearrangement detachment of the carbon atom (162 +С4F6 -C+C3F6 m/z 150).
That is, the perfluoroalkyl and perfluoroolefin ion series are secondary series of the primary series (+·M -1·F) and (+·M -2·F). In the ionic series 3 (+·M -3·F), with the last significant digit of masses 3, two more secondary successive detachments of fluorine atoms occur, resulting in two additional series of ions with last significant digits of masses 4 and 5. The m/z m/z 193 -·F→174, 124, 74+C3F2 and m/z 174 -·F→155, 105, 55+C3F series in Figure 1 are represented digitally only in the +·M-3·F series fragmentation scheme.
Figure 2 shows the three ionic series mass spectral of perfluorocyclohexane with a pentafluoroethyl substituent and the four branching of these series.
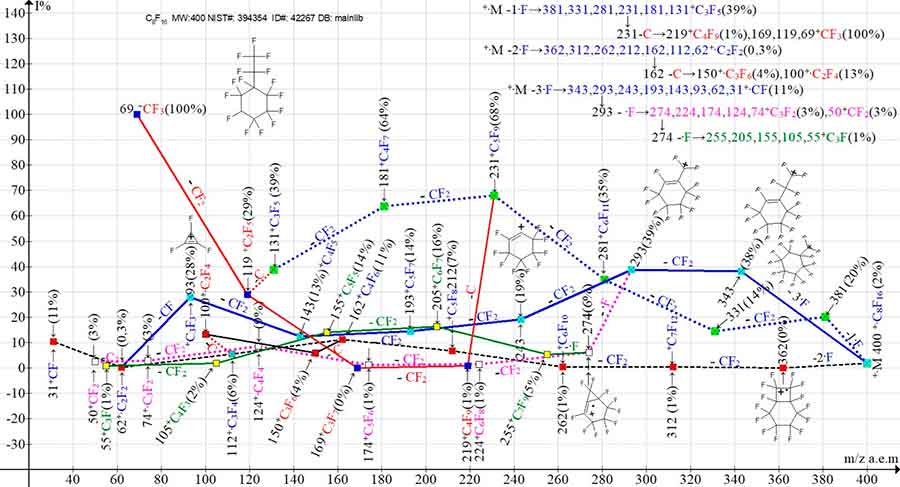
Figure 2. The three main series of primary ions mass spectra of undecafluoro(pentafluoroethyl) cyclohexane С8F16 and their four branching series.
MW: 400 NIST#:394354 ID#: 42267 DB: mainlib.
When the carbon atom is detached from the perfluoroallyl ion with m/z 231 series (+·M -1·F), a perfluoroalkyl series of ions: 219, 169, 119, 69 occurs. When the carbon atom is detached from the ion with m/z 162 series (+·M -2·F), a perfluoroalkyl series of ions: 150, 100, 50 occurs. Series +·M -3·F (Figure 2) branches twice. As a result of the secondary detachment of the fluorine atom from the ion with m/z 293 and the ion with m/z 274, two sub-series arise: namely a series of ions with the last significant digital mass (4): 293 -·F→274, 224, 174, 124, 74 and a series of ions with the last significant digital mass (5): 274-·F→255, 205, 155, 105, 55.
Primary synchronous detachments of one, two and three fluorine atoms and three branches of the three main ionic series occur both in the spectrum of undecafluoro(pentafluoroethyl)cyclohexane (Figure 2) and in the spectrum of its isomer of perfluoro-1,3-dimethylcyclohexane (Figure 3).
Figure 3 shows the three main ionic series of the perfluoro-1,3-dimethylcyclohexane mass spectrum, as well as the perfluoroalkyl ionic series arising from the detachment of the carbon atom from the perfluoroallyl ion with m/z 231. Due to the low peak intensities, the branching of the +·M -2·F series, to form the perfluoroolefin series m/z 262 -C→250, 200, 150, 100, 50, as well as the two branching of the +·M -3·F series (m/z 243 -·F and m/z 224 -·F) in the Figure 3 are presented only in digital form.
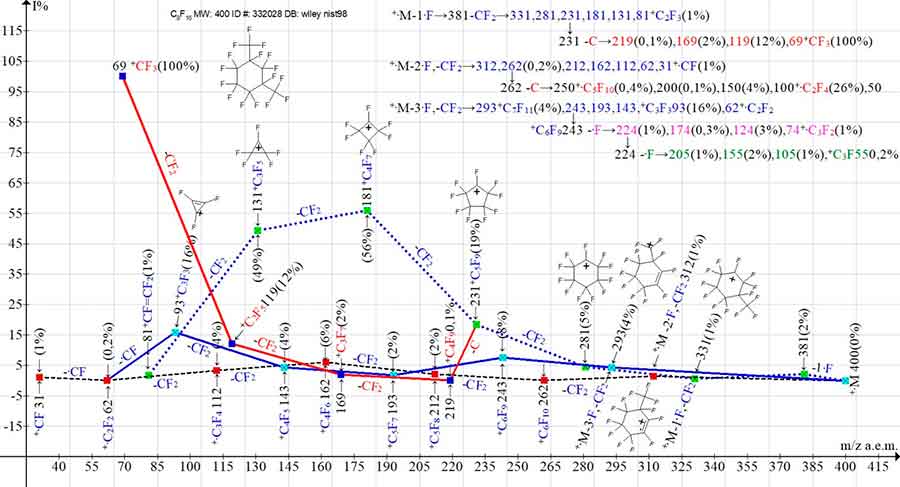
Figure 3. The three main mass spectra ion series of perfluoro-1,3-dimethylcyclohexane C8F16 and their four branching series.
MW: 400 ID#: 332028 DB:wiley_nist98.
In the spectra of the two isomers (Figures 2 and 3), branching of the perfluoroallyl series to form the perfluoroalkyl ion series occurs as a result of rearrangement detachment of a carbon atom from the same perfluoroallyl ion with m/z 231. The branching of the two main series 2 and 3 occurs by the detachment of a carbon atom and a fluorine atom (Figures 2 and 3) from two different ions.
Figure 4 shows the three main ion series (1-3) of the mass spectrum of 2,4,6-tris(trifluoromethyl) perfluorocyclohexane, as well as their four branching series.
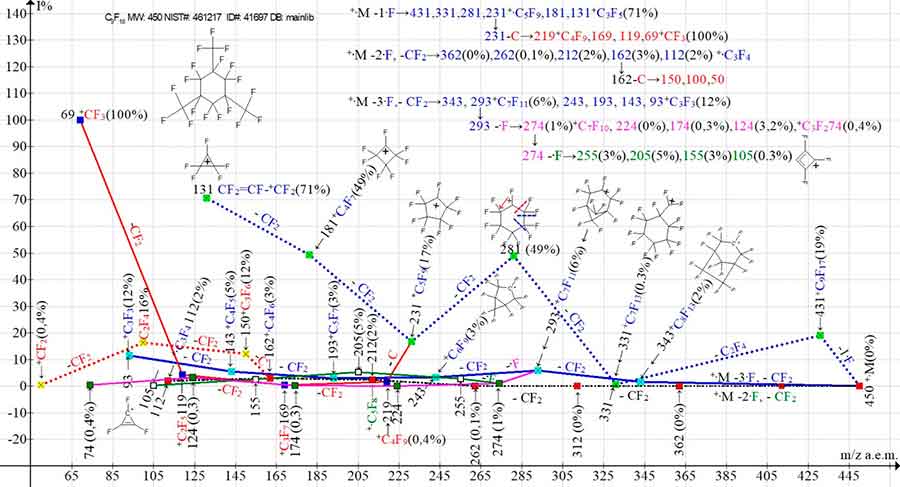
Figure 4. Three main series of mass spectral ions of 1,1,2,3,3,4,5,5,6-nanafluoro-2,4,6-tris(trifluoromethyl)cyclohexane С9F18 and their four branching series.
MW: 450 NIST#: 461217 ID#: 41697 DB: mainlib.
The ionic series and branches thereof are shown in Figure 4 in a graphical and digital form. Secondary detachments of the carbon atom in the +·M-1·F series from the ion with m/z 231 and the +·M-2·F series from the ion with m/z 162 give rise to two ionic series: perfluoroalkyl and perfluoroolefin. The ion with m/z 293 of the +·M -3·F series fragments both with CF2 detachments and the formation of the third main series 243, 193, 143, 93, and with the detachment of a fluorine atom from the ion with m/z 293 to form branching 274, 224, 174, 124, 74. The ion of this series with m/z 274 also fragments with the detachment of the fluorine atom to form another series of ions: 255, 205, 155, 105. Thus, the fragmentation of cyclohexane C9F18 (Figure 4) includes seven ionic series: three main series (1-3) and their four branching series.
Figure 5 shows the ionic mass spectrum series of 1,1,2,2,3,4,4,5,6-octafluoro-2,3,5,6-tetrakis(trifluoromethyl)cyclohexane.
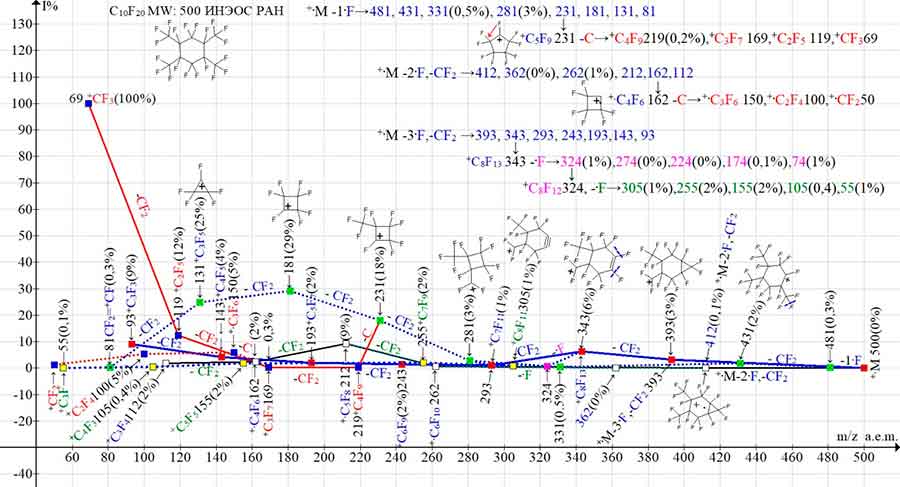
Figure 5. Three main series of fragment ions and their four branching series in the mass spectrum of 1,1,2,3,4,4,5,6-octafluoro-2,3,5,6-tetrakis(trifluoromethyl)-cyclohexane С10F20
MW: 500 INEOS RAS.
In the fragmentation of perfluorocyclohexane with four CF3 substituents (Figure 5), as in the spectra in Figures 2-4 with a smaller number of CF3 groups, primary synchronous detachments of one, two, and three fluorine atoms occur. In contrast to the perfluoroallyl series, with more intense peaks of (+·M-·F): ions: 481 (0.3%), 431 (2%), 331 (0.5%), 281 (3%), 231 (18%), 181 (29%), 131 (25%), 81 (0.3%), the peaks of the series with primary detachments (+·M -2·F) and (+·M -3·F) are less intense. Thus, the primary detachment of two fluorine atoms (+·M -2·F) and CF2 results in a series: 412 (0.1%), 362 (0%), 312 (0%), 262 (1%), 212 (9%), 162 (2%), 112 (2%). The primary detachment of three fluorine atoms and CF2 results in a third series of ions: 393 (3%), 343 (6%), 293 (1%), 243 (2%), 193 (2%), 143 (4%), 93 (9%).
It is possible that in contrast to the primary detachment (+·M-·F), several identical in energy but topologically different detachment variants of two and three fluorine atoms can partially block each other, or, on the contrary, lead to several variants of similar detachments lowering the total intensity of the ion peak. As a result, it is the peaks of the (+·M‑2·F) and (+·M-3·F) series that acquire the minimum intensities. Two additional branching series (+·M-3·F), with last significant digit 4: 324 (0.7), 274 (0%), 224 (0%), 174 (0.1%), 74 (0.7%), and also 5: 255 (1.7%), 155 (1.9%), 105 (0.4%), 55 (0.1%) arise from two consecutive detachments of a fluorine atom from an ion with m/z 343 and an ion with m/z 324. These two ionic series containing low-intensity peaks are only shown in the fragmentation scheme (Figure 5).
Figure 6 shows one branching ionic series of the undecafluoro(nonafluorobutyl)cyclohexane mass spectrum starting with the detachment of one fluorine atom.
It could be expected that during fragmentation of perfluorocyclohexane with nonafluorobutyl substituent, as in the spectrum of perfluorocyclohexane with pentafluoroethyl group C2F5 (Figure 2), similar primary synchronous detachments of one, two and three fluorine atoms would occur.
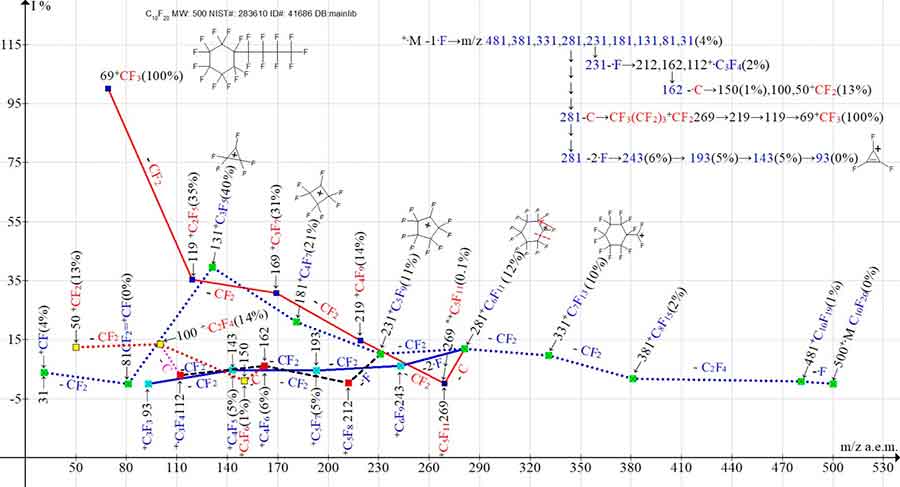
Figure 6. One primary fragment ion series and its four secondary branching series in the mass spectrum of undecafluoro-(nanafluorobutyl)-cyclohexane С10F20 MW: 500 NIST#: 283610 ID#: 41686 DB: mainlib.
However, in the spectrum (Figure 6), only the minimum in energy primary detachment of +·M -·F occurs with the formation of perfluoroallyl ionic series: 481, 431, 331, 281, 231, 181, 131, 81. The fragmentation of cyclohexane (Figure 6) with a nonafluorobutyl substituent, unlike cyclohexane with a pentafluoroethyl substituent (Figure 2), begins with a single, primary detachment of one fluorine atom, the emission of C2F4 and the subsequent two CF2 detachments. After detachment of the C4F9 substituent, the allylic series ion with m/z 281 +C6F11 fragments in three pathways. Five consecutive CF2 detachments complete the perfluoroallyl series of ions: 281, 231, 181, 131, 81, 31. In this series the ion with m/z 231 fragments with the secondary detachment of one fluorine atom and the formation of ionic series: 212, 162, 112, realizing the failed primary synchronous detachment of two fluorine atoms. The detachment of a carbon atom from the ion with m/z 162 results in a perfluoroolefin ionic series: 150, 100, 50. The detachment of carbon atom from perfluoroallyl ion with m/z 281 results in perfluoroalkyl ionic series: 269, 219, 169, 119, 69. The third way of fragmentation of perfluoroallyl ion with m/z 281 is the secondary synchronous detachment of two fluorine atoms with a new series formation of ions with the last significant digit of their masses 3: 243, 193, 143, 93. Probably, secondary detachments of two and three fluorine atoms are still realised in this way in the perfluorallyl ionic series. However, two series of seven series (the sixth M -3F, -F and the seventh series M -3F, -F, -F) with the last significant digits 4 and 5 in the spectrum (Figure 6) are not formed.
It should be noted that in the fragmentation of perfluorocyclohexane with one linear perfluoroalkyl substituent C2F5 (Figure 2), the primary synchronous detachments of two and three fluorine atoms involve one and two fluorine atoms of the cycle. Unlike fragmentation of C8F16 (Figure 2) with substituent C2F5, in fragmentation C10F20 (Figure 6) with substituent C4F9, due to twice increased chain of substituent C4F9, relative to the fluorine atoms of the six-membered cycle, primary detachments of two and three fluorine atoms are not possible. However, after fragmentation of the C4F9 substituent, the ion with m/z 281 +C6F11 fragmented with a secondary synchronous detachment of two fluorine atoms.
Figure 7 shows the ionic series of the mass spectrum of perfluorocyclohexane C10F20 with substituent CF2CF(CF3)2.
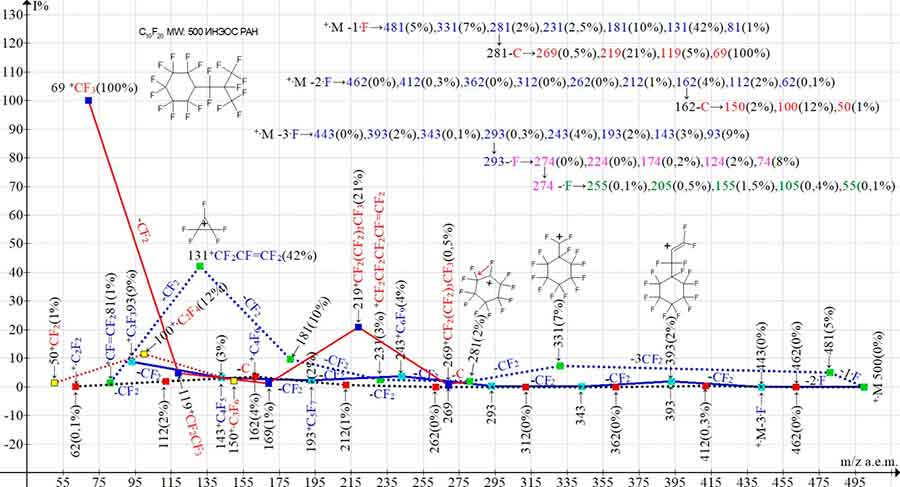
Figure 7. Three main series of ions and four their branching of the mass-spectrum of undecafluoro-[1,1,2,3,3,3-hexafluoro-2-(trifluoromethyl)propyl]cyclohexane-C10F20 MW: 500 INEOS RAS
undecafluoro[1,1,2,3,3,3-hexafluoro-2-(trifluoromethyl)propyl]cyclohexane
Perfluorocyclohexane with substituent CF2CF(CF3)2 (Figure 7) fragments to form three main and four additional series of ions.
Figure 8 shows three main ionic series and their four branching series in the mass spectrum of perfluorocyclohexane C10F20 with CF(CF3)2 and CF3 groups in the para-position. As in the fragmentation of C10F20 (Figure 7) in the spectrum of its isomer (Figure 8), the peaks of the two ionic series resulting from two branching of the +·M -3·F series have minimal intensities.
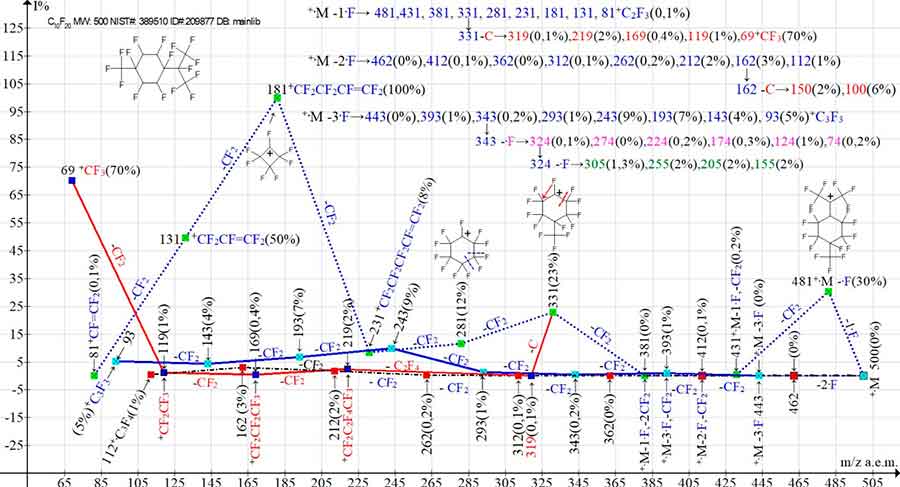
Figure 8. The three main primary ionic series and their four branching series in the 1,1,2,2,3,4,4,5,5,6-decafluoro-3-[1,2,2-tetrafluoro-1-(trifluoromethyl)ethyl]-6-(trifluoromethyl) cyclohexane С10F20 mass spectrum
MW: 500 NIST#: 389510 ID#: 209877 DB: mainlib.
According to the ratio of intensities of the base peak of the perfluoroallyl series with m/z 181 +C4F7 and a perfluoroalkyl ion with m/z 69 +CF3 mass spectrum (Figure 8) resembles the mass spectrum of perfluorocyclohexane (Figure 1), in which the base allylic peak is a peak with m/z 131. In the spectra of perfluorocyclohexanes, in contrast to the primary perfluoroalkyl ionic series, the perfluoroalkyl series is a secondary series. Depending on the structure and molecular weight of perfluorocyclohexane, the carbon atom detachment occurs from different perfluoroalkyl series ions. In the spectrum (Figure 8), the detachment of the carbon atom occurs from the ion with m/z 331. Probably, for this reason, the intensities of all perfluoroalkyl peaks of this series with m/z 319-119, except for the +CF3 peak (70%), are very low.
The intensities of the ion series peaks of the two ramifications of the third ion series (+·M-3·F) with the last significant digits 4 and 5 also have minimal intensities.
Figure 9 shows ionic series and their branching of the mass spectrum of perfluorocyclohexane C11F22, with groups C(CF3)3 and CF3 in the para-position relative to each other.
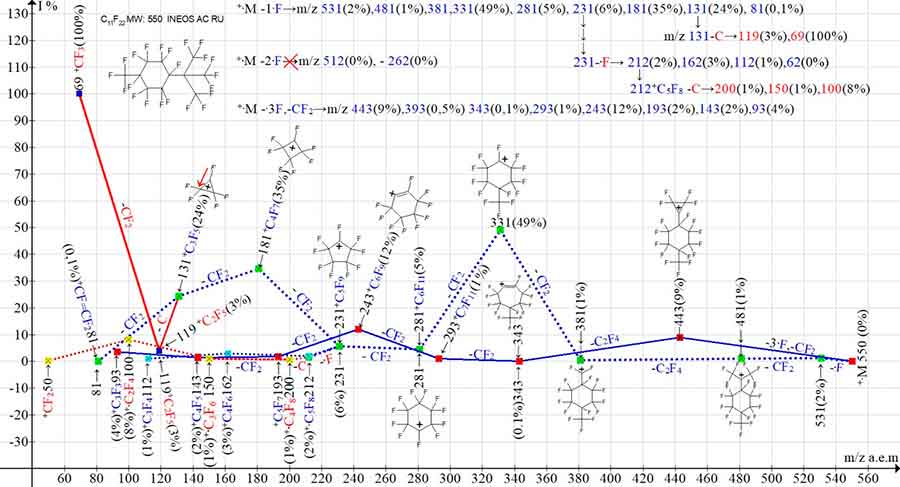
Figure 9. Two primary ion series and three branching series (+.M-1.F) in the mass spectrum 1,1,2,2,3,4,4,5,5,6-decafluoro-3-[2,2,2-trifluoro-1,1-bis(trifluoromethyl)ethyl]-6-(trifluoromethyl)cyclohexane C11F22 MW:550 INEOS RAS.
In the spectrum of C11F22 (Figure 9), primary synchronous detachments of only one and three fluorine atoms +·M-1·F and +·M-3·F occur. The primary detachment of two +·M- 2·F fluorine atoms from the substituent C(СF3)3 does not occur. The reason for this is probably the energetic impossibility and inefficiency of the two fluorine atoms detachment from the substituent with three CF3 groups compared to the three fluorine atoms detachment. The peaks that can be attributed to the +·M- 2·F series are only three peaks: 212(2%),162(3%) and 112(1%). The absence of the preceding peaks (512-262) allows us to conclude that an independent full-fledged +·M-2·F ionic series does not form in the spectrum C11F22 (Figure 9).
One of the two branching of the perfluoroalkenyl ionic series +·M-1·F (231-·F) results in the perfluoroalkenyl ionic series: 212, 162, 112, 62. Secondary detachment from the ion with m/z 212 carbon atom leads to the formation of perfluoroolefin ionic series: m/z 212-C→200, 150, 50. Another detachment of a carbon atom from a perfluoroalkyl ion with m/z 131 results in a perfluoroalkyl series of ions: +C2F5 m/z 119 and +CF3 m/z 69. Thus, the second series of peaks: +·M-2·F (512, 462, 412, 362, 312, 262, 212, 162, 112) and its branching appear to replace the more energetically favorable secondary and tertiary detachments occurring in the first series of ions. The appearance of three ions with m/z 212, 162, and 112 is the result of a fluorine atom secondary detachment from the ion with m/z 231 of the first +·M -·F series. A similar fragmentation with very weak peaks of the +·M-2·F series occurs in the spectrum of С10F20 with the substituent CF2CF(CF3)2 (Figure 7) MW500.
Primary detachments and ionic series mass spectra of perfluorocyclohexene and its derivatives with perfluoroalkyl substituents
The most simple and interesting example illustrating the changes in the ionic series compared to the perfluorocyclohexane series are the perfluorocyclohexene series. These changes are due to the absence of two fluorine atoms in the cyclohexene molecule and the presence of a double bond.
Figure 10 shows the eight ion series of the mass spectrum of perfluoro-cyclohexene C6F10.
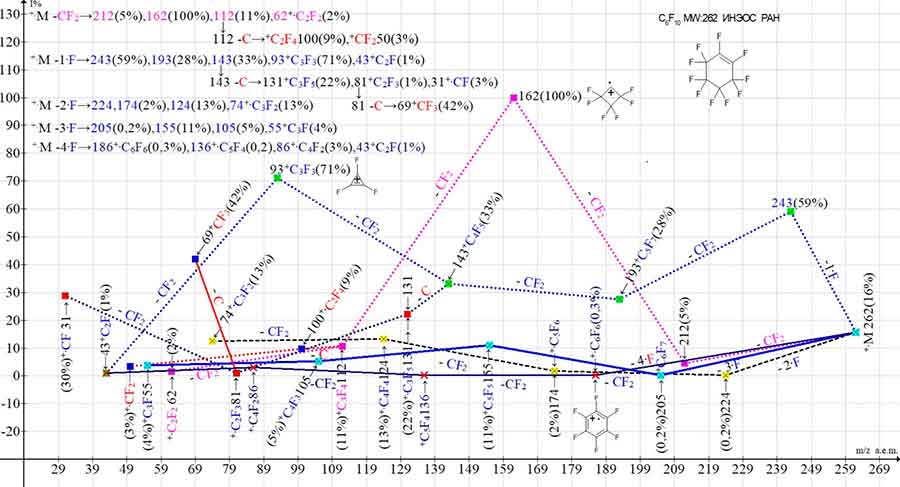
Figure 10. Five primary ionic series and their three branching series in the mass spectrum of perfluorocyclohexene С6F10 MW: 262 INEOS RAS.
Compared to perfluorocyclohexane, the presence of a single, stronger double bond in the perfluorocyclohexene molecule leads to the fact that along with the primary synchronous detachments of one, two, and three fluorine atoms, a less energy-consuming sequential detachment of four CF2 groups occurs. The CF2 detachments result in a series of ions: 262, 212, 162, 112, 112, 62 with the last significant digit 2 and the +·C2F2 m/z 62, with a triple bond. When a carbon atom is detached from the +C3F4 m/z 112, the series branches to form the olefin ion +·C2F4 m/z 100.
The detachment of one fluorine atom from +·M and successive emissions of CF2 gives rise to a series of ions: 243, 193, 143, 93, 43 with the last significant digit of mass 3. This series is branched by the detachment of a carbon atom from an ion with m/z 143, to form the perfluorallyl series: 131, 81, 31. Another detachment of a carbon atom from the allylic ion +·С2F3 m/z 81 leads to the formation of a single perfluoroalkyl ion +CF3 with an intensity of 42%. As a result of initial synchronous detachments of two and three fluorine atoms, two non-branching series of ions with the last significant digits of masses 4 and 5 occurs. Another series of ions, shown in Figure 10, begins with the synchronous detachment of four fluorine atoms and the formation of the hexafluorobenzene ion +C6F6 with m/z 186.
Two subsequent CF2 emissions are completed by ions with m/z 136 +·C5F4 and m/z 86 +·C4F2 and probably the +С2F ion with m/z 43.
Figure 11 shows the ionic series of the mass spectrum of perfluorocyclohexene C10F18 with CF(CF3)2 and CF3 groups in the para-position.
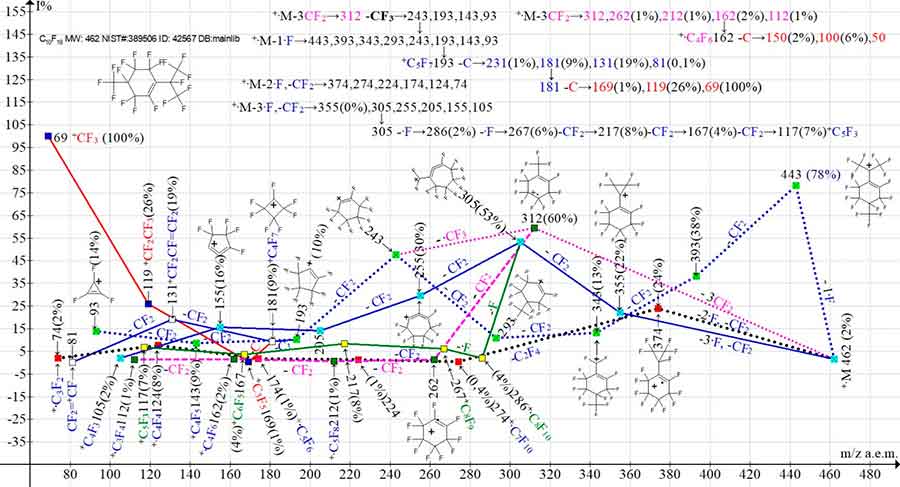
Figure 11. Four primary ionic series and their four branching series in the mass spectrum of 1,3,3,4,4,5,6,6-octafluoro-2-[1,2,2,2-tetrafluoro-1-(trifluoromethyl)ethyl]-5-cyclohexene С10F18 MW: 462 NIST#: 389506 ID#: 42567 DB: mainlib.
In the spectrum of perfluorocyclohexene (Figure 11) with two branched substituents, along with the primary synchronous detachments of one, two, and three fluorine and CF2 atoms from two substituents, synchronous separation of three CF2 also takes place. After the synchronous detachment of three CF2, four more successive detachments of CF2 are completed with ion + · C3F4 with m/z 112. A series of ions occurs: 312, 262, 212, 162, 112. At separation of radical ·CF3 from ion with m/z 312 series branches with formation of new ionic series: 243, 193, 143, 93.
The detachment of one fluorine atom from +·M and the subsequent emission of CF2 leads to a series of ions: 443, 393, 343, 293, 243, 193, 143, 93, to which the branching of the +-M series +·M ‑3CF2, -·CF3→243 is connected. This series is branched twice with the detachment of a carbon atom and the formation of two series perfluoroallyl and perfluoroalkyl. Upon detachment of the carbon atom from the ion with m/z 193, an allylic series of ions arises: +С5F7 -C→181, 131, 81. The detachment of a carbon atom from an allylic ion with m/z 181 gives rise to a perfluoroalkyl series of ions: 181 +С4F7 -C→169, 119, 69.
The primary, synchronous detachment of two fluorine atoms and CF2 results in a non-branching series of ions: + M -2·F, -CF2→374, 274, 224, 124, 74. The synchronous detachment of three fluorine atoms and subsequent CF2 emissions results in a series of ions: 355, 305, 255, 205, 155, 105. This series branches off as a result of successive secondary detachments of two fluorine atoms from the ion with m/z 305 +C8F11 -F→286 -F→267, forming another series of ions: 267, 217, 167, 117. In the mass spectrum of perfluorocyclohexene (Figure 11), the total number of ionic series is eight: four main series and their four branching series.
Figure 12 shows the ionic series of the mass spectrum of perfluorocyclohexene C12F22 with two C3F7 substituents at the double bond.
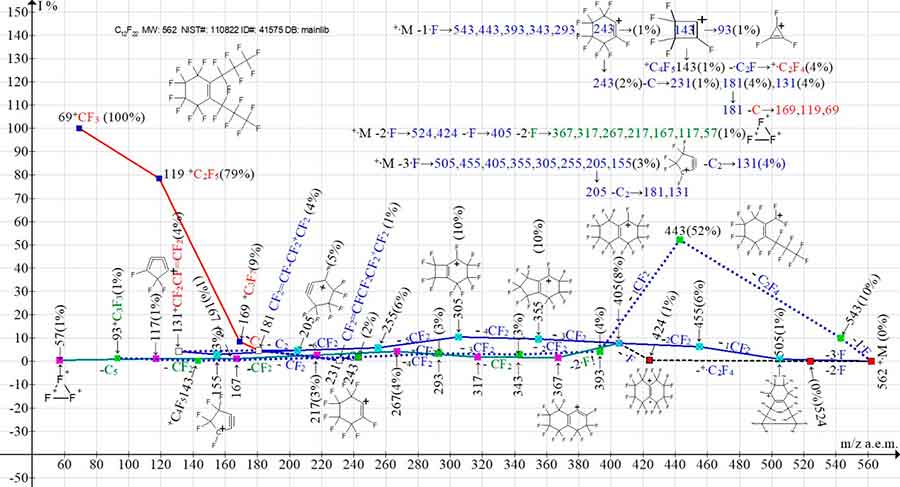
Figure 12. Three primary ionic series and their four branching series in the mass spectrum of perfluoro(1,2-dipropylcyclohexene) C12F22 MW: 562 NIST#: 110822 ID#: 41575 DB: mainlib
In the spectrum of perfluorocyclohexene with two linear substituents at the double bond (Figure 12), in contrast to unsubstituted perfluorocyclohexene and to perfluorocyclohexene with two substituents but only one of which is located at the double bond (Figure 11), there is no primary detachment of the three CF2. Fragmentation begins with initial synchronous detachments of one, two, and three fluorine atoms from the two substituents of the cycle. The detachment of one fluorine atom and C2F4 followed by CF2 emission gives rise to a fragmented ionic series with the last significant digit of mass 3: 543, 443, 393, 343, 293, 243, 143, 93. This series branches three times. The detachment of a C atom from the +C6F9 ion m/z 243 results in a perfluoroallyl ionic series: 231, 181, 131. The detachment of a C atom from an allylic ion with m/z 181 results in a perfluoroalkyl series of ions: 169, 119, 69. The detachment of the -·C2F radical from the +C4F5 ion with m/z 143 gives rise to the perfluoroolefin ion +·C2F4.
The +·M -2·F series and the more intense +·M -3·F series combine to form an ion with m/z 405 and a series of fragment ions with the last significant digit 5: 405, 355, 255, 205, 155. Since ions with m/z 105 and 55 in this series are absent, it is likely that when C2 detaches from the ion +C5F5 with m/z 155, the second pathway of the perfluoroallyl ion with m/z 131 is realized. The ion with m/z 405, resulting from the fusion of two series, also fragments with a secondary synchronous detachment of two fluorine atoms, forming a series of ions with the last significant digit 7: 367, 317, 267, 217, 167, 117, 57.
Figure 13 shows the ionic series of the mass spectrum of perfluorocyclohexene C12F22 with C2F5 and C3F9 substituents at the double bond.
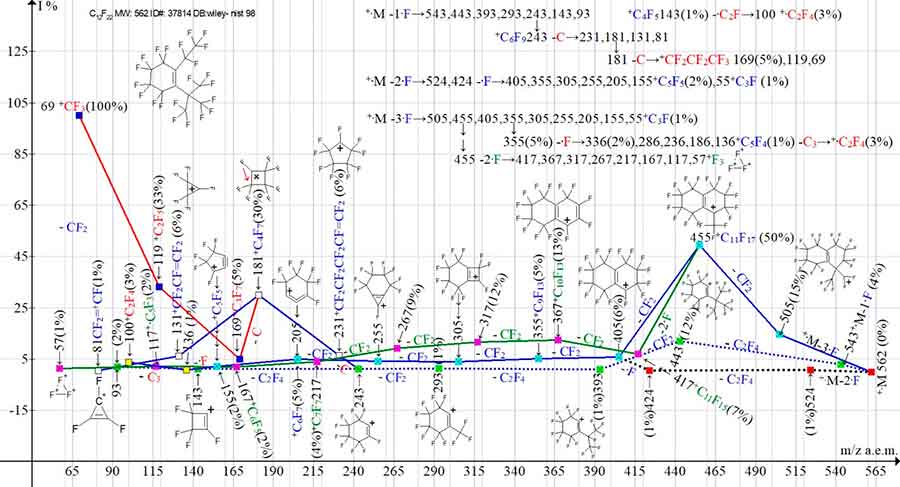
Figure 13. Three primary ionic series and their four branching series in the mass spectrum of зerfluoro[1-(1-ethyl-1-methylpropyl)] cyclohexene C12F22 MW: 562
NIST#: ID#:378104 DB: wiley-nist98.
In the mass spectrum of perfluorocyclohexene with two different substituents at the double bond, one linear and the second branched (Figure 13), as well as in the spectrum with two identical linear substituents at the double bond (Figure 12), primary detachment of the three CF2 groups does not occur.
The +·M -·F series: 543, 443, 393, 293, 243 branched off the carbon atom from the ion with m/z 243 to form the perfluoroallyl ion series: 231, 181, 131, 81. One of the possible processes leading to the formation of a single perfluoroolefin ion +·C2F4 c m/z 100(3%) in the spectrum in Figure 13, as well as in the spectrum of the isomer in Figure 12, is probably the detachment of the .C2F radical from the +C4F5 ion.
The perfluoroallyl ion with m/z 181 ejects a carbon atom to form the perfluoroalkyl ion series: 169, 119, 69. The +·M -2·F series: 524, 424 has another fluorine atom detachment and it joins the +·M -3·F series. The +·M -3·F series branches off two times. First, there is a synchronous detachment of two fluorine atoms from the ion with m/z 455 to form a non-branched series of ions: 417, 367, 317, 267, 217, 167, 117, 57. Then, the detachment of one fluorine atom from the ion with m/z 355 results in a series of ions : 336, 286, 236, 186, 136 +C5F4 (1%). Since in this series the final ion with m/z 86 is absent, it is reasonable to assume that the ion with m/z 136 possibly fragments with the detachment of C3 molecules, realizing another pathway of occurrence of a single perfluoroolefin ion +·C2F4. Thus, the number of ionic series of the C12F22 spectrum is eight.
Figure 14 shows the ionic series of the mass spectrum of perfluorocyclohexene C11F20, with CF3 and C(CF3)3 substituents.
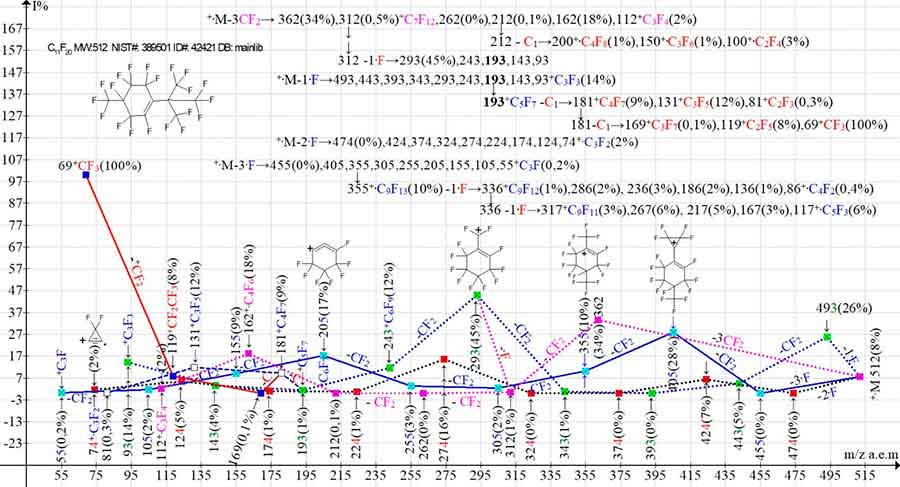
Figure 14. Four primary ionic series and their five branching series in the mass spectrum of 1,3,3,4,4,5,6,6-Octafluoro-2-[2,2,2-trifluoro-1,1-bis(trifluoromethyl)ethyl]-5-(trifluoromethyl)cyclohexene C11F20 MW: 512 NIST#: 389501 ID#: 42421 DB: mainlib.
In the spectrum of perfluorocyclohexene C11F20 with one branched substituent at the CF3-group double bond in the para-position to this substituent (Figure 14), as in the spectrum of C10F18 (Figure 11), the detachments of the three CF2 groups occur from two substituents. Unlike the C10F18 isomer (Figure 11), which then fragments with the ·CF3 emission, in the spectrum of C11F20 (Figure 14), instead of the ·CF3 radical, there is first the detachment of another CF2 group and then the detachment of a fluorine atom to form the +C7F11 ion with m/z 293, the +·M -1·F series. That is, the +·M -4СF2, -·F series is joined to the +·M -1·F series. The +·M -1F series: 493, 443, 393, 343, 293, 243, 193, 143, 93 fragments with the detachment of a carbon atom from the ion with m/z 193 +C5F7 to form the perfluoroallyl ionic series: 181, 131, 81. Upon detachment of the carbon atom from the ion with m/z 181, a perfluoroalkyl series of ions: 169, 119, 69 occurs.
The +·M -4CF2 series fragments also without ejection of the fluorine atom by successive CF2 detachments to form the series: 312, 262, 212, 162, 112. The detachment of the carbon atom from the ion with m/z 212 leads to branching of the series and formation of perfluoroolefin ion series: 200, 150, 100. The +·M -2·F series, with the last significant digit of ion mass 7 does not branch. The +·M -3·F series, with the last significant digit of ion masses 5 is branched twice as a result of two consecutive detachments of the fluorine atom. Thus, four primary ion series and six branching series occur in the spectrum in Figure 14: two branching +·M -3CF2 series (m/z 212), two branching +·M -1F series (m/z 193 and m/z 181), and two branching +.M ‑3·F series (m/z 355 and m/z 336). Because of the low peak intensities, the perfluoroolefin series 200, 150, 100, occuring from the detachment of the carbon atom from the perfluoroallyl ion with m/z 212, are only represented in the fragmentation scheme.
Figure 15 shows the ionic series mass spectrum of perfluorocyclohexene C12F22, with a C(C2F5)2(CF3) substituent at the double bond.
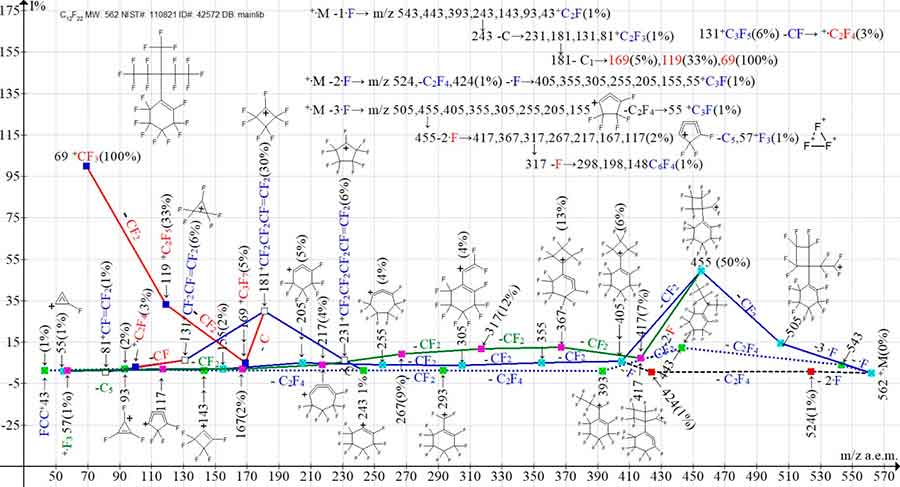
Figure 15. Three primary ionic series and their five branching series in the mass spectrum of perfluoro[1-(1-ethyl-1-methylpropyl)]cyclohexene C12F22 MW: 562
NIST#: 110821 ID#: 42572 DB: mainlib.
In the spectrum of perfluorocyclohexene with one branched substituent at the double bond (Figure 15), as well as in the spectra with two substituents at the double bond, the primary detachment of +·M -3CF2 does not occur. Fragmentation begins with synchronized detachments of one, two, and three fluorine atoms from the C3F13 substituent. Three series of ions occurs:
+·M -1·F→543, 443, 393, 293, 243, 143, 93, 43;
+·M -2·F→524(1%), -С2F4 424, - F 405, 355, 305, 255, 205, 155, 55;
+·M -3·F→505, 455, 405, 355, 305, 255, 205, 155, 55;
The secondary detachment of a fluorine atom in the +·M -2·F→524, 424, -·F→405 series leads to its unification with the +·M -3·F series. The +·M -3·F series is branched twice.
As a result of the secondary synchronous detachment of two fluorine atoms from the ion with m/z 455 455 -·2F→417, a new series of ions with the last significant digit 7 appears: 417, 367, 317, 267, 217, 167, 117, 57. Thus, in the mass spectrum of substituted perfluorocyclohexene shown in Figure 15, the total number of ionic series is eight: three main series and five branching series +·M ‑1·F and +·M -3·F.
Conclusions
The mass spectrum typically includes molecular cation-radicals of a compound with different excitation energies and their ionic series, which can branch and join.
The ionic series mass spectra of unsubstituted perfluorocyclohexane and perfluorocyclohexene are the central objects of the present report. Comparison of these spectra allows us to visualize their similarities and differences, as well as general patterns of ionic series formation (Table 1).
Table 1. Ionic series of perfluorocyclohexane and perfluorocyclohexene.
|
Perfluorocyclohexane - 7 series |
Perfluorocyclohexene - 8 series |
|||
|
C6F12 |
MW=300 |
C6F10 |
MW=262 |
|
|
+·M -1·F 300-19= 281(4%) |
281, 231, 181, 131, 81, 31 +C4F7 181-C→+C3F7 169 →+С2F5 119, +CF3 69 |
+·M -1·F 262-19= 243(5%)
|
243, 193, 143, 93, 43 +C2F 143 -C→131 +C3F5 (22%), 81 + C2F3 (1%), 31 +·CF(3%) 81 +C2F3 -C→+CF3 69 (42%) |
|
|
+·M -2·F= 262(0%) |
262 (0%), 212, 162, 112, 62 +·C4F6 162 -C→150 +·C3F6, →100+·C2F4, +·CF2 50 |
+·M -2·F= 224 (0,7%) |
224, 174, 124, 74 +·C3F2 (13%) |
|
|
+·M -3·F= 243(0%) |
243 (0%), 193, 143, 93, 43 193 -·F→174 (0%), 124 (1,5%), +C3F2 74 (2,9%) 174 -·F→155 (0,2%), 105 (0,5%), +C3F 55 (1%) |
+·M -3·F= 205 (0,2%) |
205, 155, 105, 55 +C3F (3%) |
|
|
+·M -4·F= |
186, 136 +·C5F4, 86 +·C4F2(3%), 43 +C2F(1%) |
|||
|
+·M -4 CF2= |
212, 162, 112, 62 F+CC·F(2%)
112 - C→ +C2F4 100 (9%) |
|||
In the mass spectra of perfluorocyclohexane, after synchronous detachments of one, two, and three fluorine atoms and successive CF2 emissions, three main series of ions are formed, with the last significant digits of the masses: 1, 2 и 3. As a result of a carbon atom detachment, the first and second series branch to form, respectively, perfluoroalkyl ionic series, with the last significant digit 9 and perfluoroolefin ionic series, with the last significant digit 0. If in the first and second main ionic series of perfluorocyclohexane +·M-·F and +·M‑2·F their branching is the result of a carbon atom detachment, the two branchings of the ionic series +·M-3·F are the result of secondary detachments of one and then the second fluorine atom, with the formation of two subseries, with the last significant digit 4 and respectively 5.
In the mass spectrum of perfluorocyclohexene after synchronous detachments of one, two, and three fluorine atoms and consecutive CF2 emissions, three main ionic series with the last significant digit of masses 3, 4, and 5 occurs. As a result of the detachment of the carbon atom, the first ionic series branches off to form the perfluoroallyl ionic series, with the last significant digit 1. As a result of a carbon atom detachment from the allyl ion with m/z 81, the perfluoroalkyl ion +СF3 (42%), with the last significant digit 9, is formed. The other two main ionic series: the second ionic series +·M-2·F (last significant digit of mass 4) and the third ion series +·M-3·F (last significant digit of mass 5) do not branch. In the perfluorocyclohexene mass spectrum, two additional ionic series appear: +·M -4·F with the last significant digit of mass 6 and +·M -4CF2 with the last significant digit of mass 2. Their occurrence is the result of a double bond presence in the perfluorocyclohexene molecule. Four fluorine atoms synchronous detachment is the result of energetically favorable transformation of perfluorocyclohexene molecular ion into perfluorobenzene molecular ion +·С6F6 m/z 186 (0.3%). Consecutive detachment of the four CF2 groups is completed by the formation of the difluoroacetylene cation radical +·C2F2 with m/z 62 (2%). Upon a carbon atom detachment from the +·C3F4 ion with m/z 112 (11%), the series branched to form the perfluoroolefin ion +·C2F4 with m/z 100 (9%). In the mass spectra of perfluorocyclohexanes and perfluorocyclohexenes with perfluoroalkyl substituents, depending on the number of substituents of the cycle, their structure (branched or linear), and the number of carbon atoms of the linear substituent, the primary synchronous detachments can occur separately, breaking the synchrony of the three primary detachments. The reason for the violation of the three primary detachments is the structural impossibility of synchronous detachments of two and three fluorine atoms. An example of such structural impossibility is the mass spectrum of undecafluoro(nonafluorobutyl)cyclohexane C10F20 (Figure 6). Its fragmentation starts from the linear substituent C4F9, namely by detaching one fluorine atom and discarding four CF2 groups. Then the series branches as a secondary synchronous detachment of two fluorine atoms from the +С6F11 cycle becomes possible.
This example is not the only one. The primary as well as secondary synchronous three-radical detachments occurring in the ionic series mass spectra of polyoxaperfluoroalkanes and polyoxaperfluoroalkyl halides (Figures 1-14) are presented in Table 1 [2]. Of the fourteen compounds, primary synchronous detachments of three radicals occur in the mass spectra of seven compounds (Figures 2, 4, 5, 6, 9, 11 and 14). In four unsymmetrical compounds (with terminal group O(CF3)2F and OCF2CF3) (Figures 7, 8, 10, 12), secondary three radical detachments occur instead of primary detachments [2]. In the mass spectrum of 1,2,4,5-tetrafluoro-3,6-bis(pentafluoroethyl)benzene C10F14 MW: 386, synchronous detachment of two and three fluorine atoms does not occur [3] because the linear substituents are isolated from each other since they are in the para-position. In contrast to the mass spectrum of hexakis(trifluoromethyl)-benzene C12F18, synchronous +·M -3·F and +·M -2·F detachments also do not occur in the mass spectra of hexakis(trifluoromethylthio)benzene C12S6F18 and hexakis(trifluoromethylseleno)benzene C12Sе6F18 [4]. Apparently, sulfur and selenium atoms, more precisely their six bonds with CF3 groups and their six bonds with the C6 cycle, take away that part of the excitation energy that is necessary for the synchronous detachments of two, as well as three fluorine atoms.
Acknowledgements
This work was supported by the Ministry of Science and Higher Education of the Russian Federation and was performed employing the equipment of Center for Molecular Composition Studies of INEOS RAS.
References
- N.D. Kagramanov, A Series of Fragment Ions of Cycloalkanes, Perfluorocyclohexane, Perfluoropolycycloalkanes, Fluorine notes, 2021, 3(136), 3-4.
- N.D. Kagramanov, Synchronous separation of three radicals in ionic series of polyoxaperfluoroalkanes and polyoxaperfluoroalkyl halides mass spectra, Fluorine notes, 2024, 2(153), 1-2.
- N.D. Kagramanov, Decay sequences - ion series of mass spectra hexamethylbenzene and hexakis(trifluoromethyl)benzene, Fluorine notes, 2022, 5(144), 1-2.
- N.D. Kagramanov, E.I. Mysov, Ionic series of hexakis (trifluoromethylthio)- and hexakis (trifluoromethylseleno) benzenes and fluorobenzenes with perfluoroalkyl substituents, Fluorine notes, 2024, 3(154), 1-2.
ARTICLE INFO
Received 11 October 2024
Accepted 15 November 2024
Available
online February 2025
Recommended for publication by PhD M.A. Manaenkova
eLIBRARY Document Number (EDN) AAGCSQ

Fluorine Notes, 2025, 158, 1-2
