Received: November 2024
DOI 10.17677/fn20714807.2024.06.03
Fluorine Notes, 2024, 157, 5-6
FEATURES OF SYNTHESIS OF FLUORIDE NANOCRYSTALS IN ORGANIC AND INORGANIC SOLVENTS AT HIGH PRESSURE
V.I. Sokolov, I.O. Goryachuk, S.I. Molchanova
National Research Centre "Kurchatov Institute", Moscow, Russia
Annotation: By the method of thermal decomposition of trifluoroacetates of rare-earth elements and sodium in organic and inorganic solvents (water, decanoic and oleic acids, 1-octadecene, as well as in a mixture of these solvents) at a pressure of 50-250 atm. NaYF4:Yb+3, Er+3, YF3:Yb+3,Er+3 fluoride nanocrystals with intense photoluminescence in up- and down-conversion at pumping by IR radiation with wavelength near 980 nm were synthesized. The obtained nanocrystals introduced into fluoropolymer matrix can be used to create various integrated-optical devices, in particular, waveguide amplifiers of optical radiation and waveguide lasers with distributed feedback.
Keywords: fluoride nanocrystals, rare-earth elements, photoluminescence, up- and down- conversion.
Introduction
Fluoride nanocrystals NaYF4, NaLuF4, YF3, LaF3 etc., doped with rare-earth elements Yb, Er, Tm, Gd, Ce [1-6] find wide applications in medicine [6, 7] and in engineering [8-13]. Usually, the synthesis of such nanocrystals is carried out by thermal decomposition of rare-earth elements trifluoroacetates and sodium in a mixture of oleic acid and 1-octadecene at a temperature of 300-350 °C and atmospheric pressure and is accompanied by the formation of an organic shell on the surface of nanoparticles. Since oxidation of oleic acid and 1-octadecene can occur at such temperatures, as well as the formation of a shell of nanocrystals from “cross-linked” organic material, which thickness is difficult to control, it is important to develop methods for the formation of nanoparticles with a given thickness of the organic shell or without it at all. In this report, the features of synthesis of NaYF4:Yb+3,Er+3 and YF3:Yb+3,Er+3 fluoride nanocrystals in water and organic solvents (decanoic acid, oleic acid, 1-octadecene), as well as in a mixture of water and these solvents at temperature 300-350°C and pressure 50-250 atm are investigated. The possibility of forming NaYF4:Yb+3,Er+3, YF3:Yb+3,Er+3 nanocrystals possessing intense photoluminescence (PL) in both up- and down-conversion when pumped by IR radiation with a wavelength near 980 nm has been demonstrated.
1. Experimental part
Commercial reagents were used for the synthesis of fluoride crystals NaYF4:Yb+3,Er+3, YF3:Yb+3,Er+3: yttrium, ytterbium, erbium oxides, sodium carbonate, oleic acid 90%, 1-octadecene 90% (Sigma-Aldrich) and trifluoroacetic acid 99% (PanReac). Oxides and sodium carbonate were used to prepare trifluoroacetates (TFA) Na, Y, Yb and Er according to the procedure described in studies [13, 14].
Synthesis of fluoride nanocrystals in water
The TFA mixture with the ratio of elements Na:Y:Yb:Er = 1.00:0.78:0.20:0.02 was dissolved in water and placed in a stainless steel reactor with a volume of 12 ml. The reactor was hermetically sealed with a lid with copper gaskets and heated at a rate of 15 deg/min to a temperature of 300-350 °C, while the pressure in the reactor increased to 50-250 atm (depending on the filling volume of the reactor). As the temperature of the reaction mixture reached 240-250 °C, TFA began to decompose [1, 2], which led to the formation of nuclei and the beginning of the growth of fluoride nanocrystals. The duration of the reaction after reaching the target temperature varied from 60 min to several hours. After the end of synthesis, the reactor was cooled to room temperature, the lid was opened and the solution with the formed nanoparticles was extracted.
Synthesis of fluoride nanocrystals in a mixture of oleic acid and 1-octadecene
Synthesis of fluoride nanocrystals doped with rare-earth elements in a mixture of oleic acid and 1-octadecene, taken in the ratio 1 : 1, was carried out in a similar manner at the same temperatures, but slightly lower pressure (up to 100 atm.).
Figure 1 shows the PL spectra of nanocrystals synthesized in water as well as in a mixture of oleic acid and 1-octadecene at 330°C. The characteristic up-conversion PL peaks in Figures 1a and 1b near 405, 521, 539, and 651 nm, which appear when pumped by IR laser irradiation with a wavelength of 980±5 nm, are due to the energy structure of the rare-earth ions Er3+ in the fluoride crystals, specifically the transitions
2H9/2→4I15/2, 2H11/2 → 4I15/2, 4S3/2→4I15/2 and 4F9/2 → 4I15/2,
see inset in Figure 1a. The intense down-conversion PL band near 1530 nm in Figures 1b and 1g is due to the 4I13/2→ 4I15/2 transitions in Er3+ ions. Note that the mixture of initial rare-earth element TFAs does not luminesce in the up-conversion under IR illumination, so the appearance of PL during the reaction indicates the formation of nanocrystals.
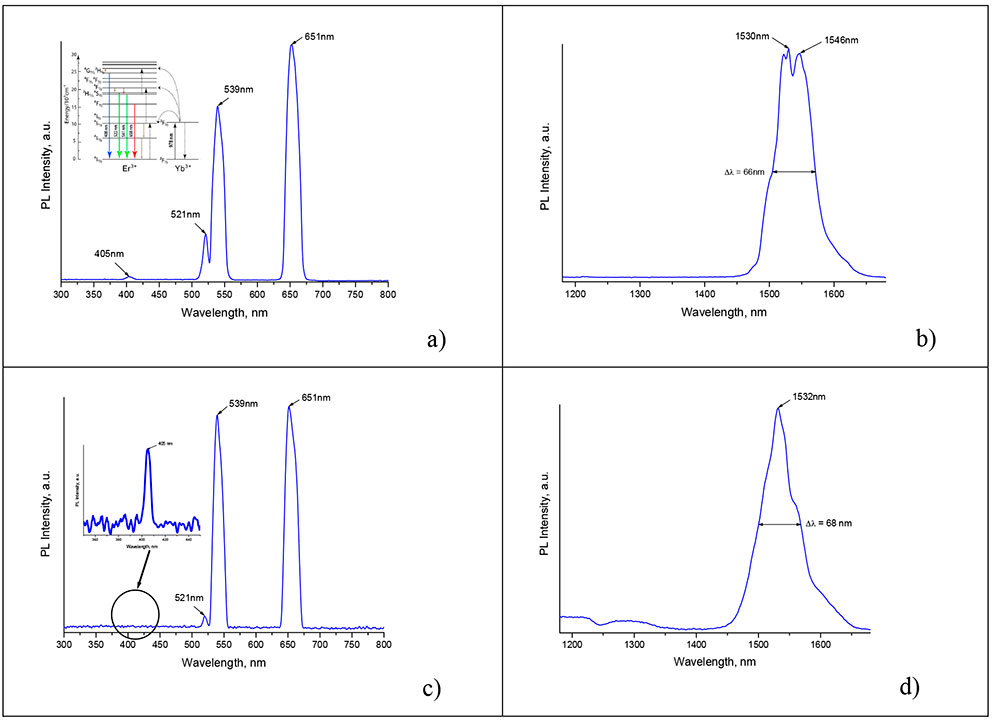
Figure 1. Up- (a, c) and down-conversion (b, d) PL spectra of fluoride nanocrystals synthesized in water (a, b) and in a mixture of oleic acid and 1-octadecene (c, d) at 330°C.
The inset to Figure 1a shows a simplified energy level system of ytterbium and erbium.Bold arrows pointing downward show the radiative transitions.
From comparing the curves presented in Figures 1a and 1b with those of Figures 1c and 1d, it can be concluded that the PL spectra of fluoride nanocrystals synthesized in water and in a mixture of oleic acid and 1-octadecene are identical.
2. Structural diagnostics of synthesized crystals
Structural diagnostics of the obtained fluoride nanocrystals was carried out on a Rigaku Miniflex600 X-ray diffractometer (Cu, λ = 1.54184 Å) in the range of incidence angles 2 = 5-70 deg. The diffractograms of the particle powders are presented in Figure 2. From the analysis of the diffractogram shown in Figure 2a, it follows that the particles synthesized in water contain nanocrystals of β-NaYF4:Yb,Er, which are in the hexagonal β-phase (sharp diffraction peaks at 2θ = 17.0, 29.8, 30.7, 34.6, 39.5, 43.4, 46.3, 53.0 and 53.6 deg [13, 4] correspond to this phase). In addition, YF3:Yb,Er crystals of orthorhombic structure are present in the powder, see the peaks at 2θ = 23.9, 24.5, 25.9, 27.8, 31.0, 36.0, 36.0, 37.2, 38.6, 40.9, 43.9, 45.5, 47.0, 47.6 and 49.1 deg [5, 15].
The particles synthesized in the mixture of oleic acid and 1-octadecene (Figure 2b) are mainly represented by α-NaYF4:Yb,Er nanocrystals (cubic α phase of the crystal lattice), which is characterized by diffraction peaks at angles 2θ = 27. 9, 32.3, 32.3, 46.5, 55.1, 57.8, and 67.8 deg, with a small inclusion of β-NaYF4:Yb,Er particles (hexagonal β-phase). The nanocrystals β-NaYF4:Yb,Er correspond to diffraction peaks at 2θ = 17.0, 29.8, 30.7, 39.5, 43.4, 53.1 and 53.6 deg [13, 14].
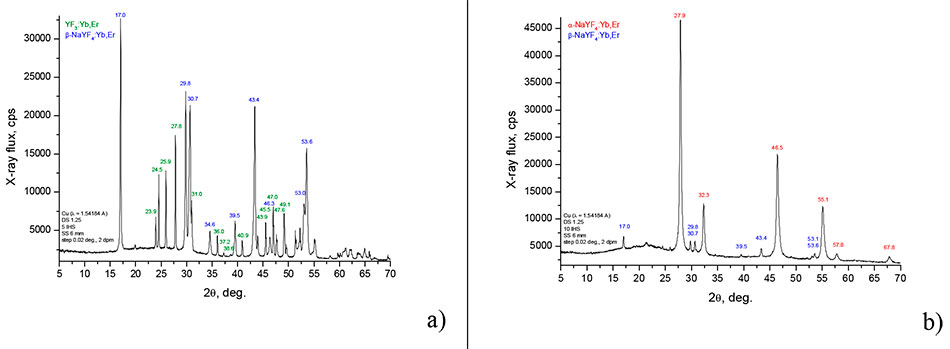
Figure 2. Diffractogram of nanoparticle powders synthesized at 330°C in water (a) and in a mixture of oleic acid and 1-octadecene (b).
θ is the angle of incidence of the X-ray beam on the sample.
3. β-NaYF4:Yb+3,Er+3 particle size measurement
The average diameter <D> of the synthesized particles was measured using UV optical microscopy [14]. Figure 3 shows photographs of the nanocrystals synthesized in oleic acid and 1-octadecene solution, obtained with a LUMAM IUUV-1 optical luminescence microscope in transmitted light at 405 nm, as well as in their up-conversion PL at the same wavelength. As can be seen in Figure 3a, four distinct objects with diameters D ranging from 400 to 600 nm are observed in the field of view. These objects luminesce in the up-conversion at a wavelength of 405 nm when pumped with 980 ± 5 nm IR light (see Figure 3b). This confirms that the observed particles are NaYF4:Yb+3,Er+3 nanocrystals.
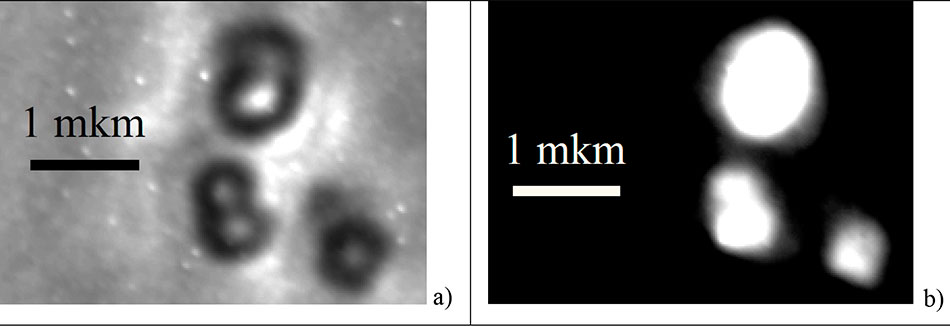
Figure 3. Photographs of NaYF4:Yb+3,Er+3 nanocrystals synthesized in oleic acid and 1-octadecene solution at 330 ºC. Photographs were obtained on a LUMAM IUUV-1 optical microscope at 405 nm in transmitted light (a) and in photoluminescence light (b) when pumped by IR radiation at 980 ± 5 nm.
Figure 4 shows photographs of nanoparticles synthesized in water, obtained in the same modes on a LUMAM IUUV-1 microscope. It can be seen that synthesis in water leads to the formation of both individual nanoparticles with diameter D = 400 - 600 nm and their agglomerates.
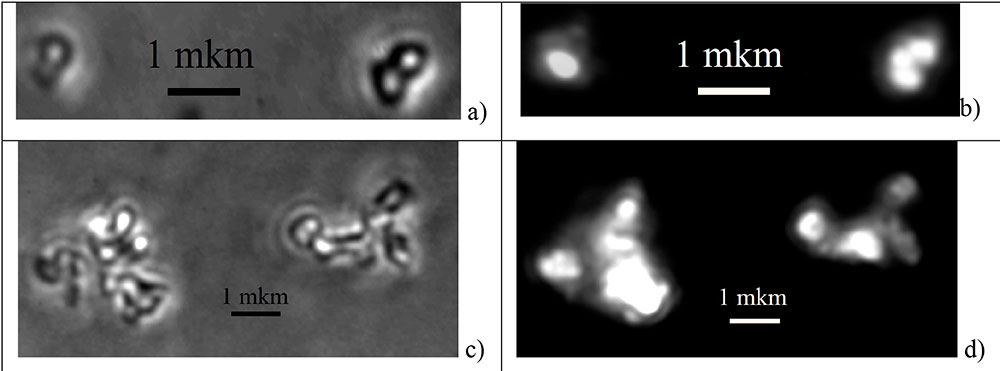
Figure 4. Photographs of NaYF4:Yb,Er, YF3:Yb,Er, fluoride crystals synthesized in water at 330°C and 150 atm pressure. Photographs were obtained on the optical microscope LUMAM IUUV-1 at a wavelength of 405 nm in transmitted light (a, c) and in photoluminescence in up-conversion (b, d) under pumping IR radiation of 980 ± 5 nm.
4. Results and discussion
The synthesis of fluoride nanocrystals from trifluoroacetates of rare-earth elements and sodium in decanoic acid and in a mixture of water with oleic acid and 1-octadecene was also carried out. The synthesis temperature was varied between 300-350 °C and the maximum pressure was 150 atm. In all cases, the formation of nanoparticles exhibiting photoluminescence in both up- and down-conversion was observed. We believe that using solutions with different molar ratio of water and organic solvents for synthesis, it is possible to form fluoride nanocrystals possessing different thickness of organic shell.
Conclusion
The possibility of synthesis of fluoride nanocrystals NaYF4:Yb,Er, YF3:Yb,Er from trifluoroacetates of rare-earth elements and sodium in water at temperature 300-350 °C and pressure 50-250° atm has been demonstrated. This approach allows to obtain nanoparticles without organic shell. Synthesis of fluoride nanocrystals in a mixture of water with oleic acid and 1-octadecene opens the possibility to form nanocrystals with different thickness of the organic shell. The produced nanocrystals have intense photoluminescence in up-and down-conversion during pumping with infrared radiation of 980 ± 5 nm and can be used for creating various active devices for integrated optics, for example waveguide amplifiers and lasers with distributed feedback.
Acknowledgments
The work was carried out within the state assignment of NRC "Kurchatov Institute"
References
- H.-X. Mai, Y.-W. Zhang, L.-D. Sun, C.-H. Yan, Size- and phase-controlled synthesis of monodisperse NaYF4:Yb,Er nanocrystals from a unique delayed nucleation pathway monitored with upconversion spectroscopy, J. Phys. Chem. C, 2007, 111, 13730.
- X. Ye, J.E. Collins, Y. Kang, J. Chen, D.T.N. Chen, A.G. Yodh, C.B., Murray, Morphologically controlled synthesis of colloidal upconversion nanophosphors and their shape-directed self-assembly, Proc. Nat. Acad. Sci. USA, 2010, 107(52), 22430.
- X. Liu, X. Zhang, G. Tian, W. Yin, L. Yan, L. Ruan, Z. Yang, D. Xiao, Z. Gu, A simple and efficient synthetic route for preparation of NaYF4 upconversion nanoparticles by thermo-decomposition of rare-earth oleates, CrystEngComm, 2014, 16, 5650.
- K. Zheng, W. Qin, Ch. Cao, D. Zhao, L. Wang, NIR to VUV: Seven-Photon Upconversion Emissions from Gd3+ Ions in Fluoride Nanocrystals, Journal of Physical Chemistry Letters, 2015, 6, 556.
- J. Wang, S. Bo, L. Song, J. Hu, X. Liu and Z. Zhen, One-step synthesis of highly water-soluble LaF3:Ln3+ nanocrystals in methanol without using any ligands, Nanotechnology, 2007, 18, 465606.
- F. Wang and X. Liu, Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals, Chemical Society Reviews, 2009, 38, 976.
- S. Alyatkin, I. Asharchuk, K. Khaydukov, A. Nechaev, O. Lebedev, Y. Vainer, V. Semchishen, E. Khaydukov, The influence of energy migration on luminescence kinetics parameters in upconversion nanoparticles, Nanotechnology, 2017, 28, 035401.
- A. Rapaport, J. Milliez, M. Bass, A. Cassanho, H. Jenssen, Review of the properties of up-conversion phosphors for new emissive displays, Journal of display technology, 2006, 2(1), 68.
- H.A. Hoeppe, Recent developments in the field of inorganic phosphors, Angew. Chem., Int. Ed., 2009, 48, 3572.
- H. Zhu, X. Chen, L.M. Jin, Q.J. Wang, F. Wang, S.F. Yu, Amplified spontaneous emission and lasing from lanthanide-doped up-conversion nanocrystals, ACSNano, 2013, 7(12), 11420.
- X. Zhai, J. Li, Sh. Liu, X. Liu, D. Zhao, F. Wang, D. Zhang, G. Qin, W. Qin, Enhancement of 1.53 μm emission band in NaYF4:Er3+,Yb3+,Ce3+ nanocrystals for polymer-based optical waveguide amplifiers, Optical Materials Express, 2013, 3(2), 270.
- G.F.R. Chen, X. Zhao, Y. Sun, Ch. He, M.Ch. Tan, D.T.H. Tan., Low loss nanostructured polymers for chip-scale waveguide amplifiers, Scientific Reports, 2017, 7, 3366.
- V.I. Sokolov, I.M. Asharchuk, E.N. Glazunova, I.O. Goryachuk, A.V. Lyubeshkin. Synthesis of β-NaYF4/Yb+3/Er+3 fluoride nanocrystals at high pressure, Fluorine Notes, 2021, 1(134), 1-2.
- I.O. Goryachuk, E.N. Glazunova, S.I. Molchanova, V.I. Sokolov, Synthesis and study of fluoride nanocrystals β-NaYF4:Yb3+,Tm3+ using luminescent UV microscopy, Fluorine Notes, 2023, 6(151), 5-6.
- M. Runowski, S. Lis. Nanocrystalline rare earth fluorides doped with Pr3+ ions, Journal of Rare Earths, 2016, 34, 802.
ARTICLE INFO
Received 26 November 2024
Accepted 11 December 2024
Available online December 2024
Recommended for publication by PhD M.A. Manaenkova
eLIBRARY Document Number (EDN) WZBJTH

Fluorine Notes, 2024, 157, 5-6
