Received: October 2024
DOI 10.17677/fn20714807.2024.06.01
Fluorine Notes, 2024, 157, 1-2
REORGANIZATION OF THE STRUCTURE AND PROPERTIES OF FLUORINATED POLYURETHANE ELASTOMERS IN CONDITIONS OF VARYING TEMPERATURES
I.A. Politsimako 1, A.V. Faleva2, S.L. Shestakov2, S.V. Kudashev1, V.F. Zheltobryukhov1 and S.V. Kumbrasyeva1
1 Volgograd State Technical University, 28 Lenin Avenue, Volgograd, 400005 Russia
e-mail: kudashev-sv@yandex.ru
2 Core Facility Center «Arktika», M.V. Lomonosov Northern (Arctic) Federal University, 14 Severodvinskaya str., Arkhangelsk, 163002 Russia
Abstract: The modifying effect of polyfluorinated tetraamine synthesized by the reaction of tris-(2-aminoethylamine) and 1H,1H,9H-trihydroperfluoronan-1-ol on the structure and properties of the resulting polyurethane materials has been studied. The features of reorganization of the considered heterochain polymer structure under the influence of polyfluorinated tetraamine have been analyzed by Fourier IR and 1H NMR solid state spectroscopy methods. The stabilizing effect of the applied fluorine-containing modifier on elastomer properties under aging conditions at elevated and reduced temperatures is shown.
Keywords: fluoropolymers, polyurethane elastomers, polyfluorinated amines, modification, aging, stabilization, structure, destruction.
Introduction
Elastic polyurethanes are widely used as monolithic sports, roofing and waterproofing coatings. The operating characteristics of said coatings make it possible to carry out multi-factor degradation processes of macromolecules of the heterochain polymer under consideration [1-4].
Fluorine-containing reactive compounds (isocyanates, alcohols and thiols, carboxylic acids, amines, peroxides, polyfunctional compounds), as well as their immobilized forms on montmorillonite and graphite, which have a stabilizing effect on elastomer properties [5-9], are of special interest for chemical modification of polyurethanes. Tetraamines containing amino groups of various degrees of substitution and polyfluorinated substituents in their structure are capable of exerting a catalytic effect on the urethane formation process, facilitating urethane formation [10, 11].
In this connection, the influence of new polyfluorinated amines containing H(CF2CF2)nCH2-groups on the structure and properties of the obtained elastomeric materials requires a separate study.
The aim of the invention is to study the modifying effect of the bisalkylation product of tris-(2-aminoethylamine) 1H,1H,9H-trihydroperfluorononane-1-ol on the structure and properties of polyurethane elastomers subjected to aging under conditions of increased and low temperatures.
Experimental part
Static NMR spectra of cryogenically shredded polyurethane (particle size not exceeding 30 μm) were recorded on a Bruker AVANCE III 600 spectrometer. A standard single-pulse sequence was used to record spectra: the number of accumulations - 4096, delay - 5 s, pulse - 2.5 μs. IR spectra of polyurethane films (thickness not more than 1 μm) were recorded on FT-801 Fourier spectrometer with NPVO attachment (Simex AE). IR and NMR spectra were recorded at room temperature.
Polymer surface morphology was studied by electron microscopy on an FEI Versa 3D scanning electron microscope in low vacuum mode equipped with an EDWARDS-nEXT turbomolecular pump and an Oxford energy dispersive analysis (EDS) system with an Ultim Max 65 detector. The information depth of elemental analysis (OK line) was up to 1.5 μm.
Thermal aging of polymer samples was carried out at a temperature of 40±2 °C in a Snol drying cabinet with natural air convection, and low-temperature aging - in a laboratory freezer Pozis at minus 20±2 °C. Every day (14 days) the polyurethanes were exposed at elevated temperature for 8 h and then for 16 h at subzero temperatures.
Elastomers were tested in accordance with GOST (state standard specification) 21751-76 “Sealants. Method of determination of conditional strength, relative elongation at break and relative residual deformation after break”, GOST (state standard specification) 263-75 “Rubber. Method for the determination of Shore A hardness” and GOST (state standard specification) 270-75 “Rubber. Method for the determination of elastic and tensile stress-strain properties”. Physical and mechanical properties of polymeric materials were tested on Zwick/Roell 5.0 kN and PM–3 tensile tensile tensile testing machines. Processing of the obtained results by methods of mathematical statistics was carried out by specialized software.
Preparation of elastomeric composition
The polymer compositions were prepared using a laboratory mixer by mixing (stirring speed 250 rpm-1) for 10 min 100 mass part (m. p.) of oligoetherpolyol, 1 wt. p. of branching agent, 1.5 wt. p. of plasticizer, 1.5 wt. p. of surfactant, 0.1 wt. p. of urethane catalyst and 1 wt. p. of polyfluorinated tetraamine. Next, 20 wt. p. of isocyanate was added to the reaction mixture and stirred again for 7 min. The obtained mixture was poured into molds and kept at room temperature (cold curing method) until the Shore A hardness of the elastomer reached a plateau.
Oligoesterpolyol Laprol 5003-2-B10 (Technical Specification 2226-023-10488057-95, PJSC Nizhnekamskneftekhim) was a product of polymerization of propylene oxide with glycerol followed by block copolymerization with ethylene oxide with the following characteristics: hydroxyl number 35 mg KOH/g, mass fraction of water not more than 0.1 %. The compositions were cured with toluylene diisocyanate (2,4-isomer content was 80.5 %) of Desmodur T80 brand (Wanhua, China).
Glycerol of p.a. qualification (GOST (state standard specification) 6259-75) was used as a chain branching agent. The catalyst of urethane formation was tin di-n-butyl dilaurate (in the form of 2.5 % solution in white spirit). Dioctyladipinate DOA (GOST (state standard specification) 8728-88) was used as a plasticizer. Nonionogenic surfactant was oxyethylated monoalkylphenol Neonol AF 9-12 (Technical Specification 2483-077-05766801-98, PJSC Nizhnekamskneftekhim).
Catalytic N-polyfluoroalkylation of tris-(2-aminoethylamine) with 1H,1H,9H-tri-hydroperfluoronan-1-ol was carried out according to the method [12] at the following ratio of amine and polyfluorinated alcohol (Figure 1):
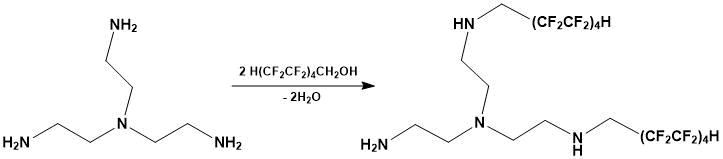
Figure 1. N-Polyfluoroalkylation of tris-(2-aminoethylamine) by 1H,1H,9H-trihydroperfluorononan-1-ol
The bisalkylation product (polyfluorinated tetraamine) was a yellow oil-like substance with a boiling point of 133-136 °C (15 mm Hg).
Results and discussion
The process of polyurethane elastomers structure formation is quite complex [1-4] and includes multiple chemical and physicochemical processes that cumulatively lead to the formation of a crosslinked polymer [13, 14]. The structural feature of the used modifier is the presence of primary and secondary amino groups, which react with NCO-groups of 2,4- and 2,6-toluylenediisocyanate to form substituted ureas under the conditions of migration polymerization of diisocyanate and polyol [10, 11]. In turn, fluorinated tetraamine substituents are able to modify the hydrogen bonding network, cumulatively changing the ratio of chemical and physical intermolecular bonds in crosslinked polyurethanes, which largely determines the properties of the obtained elastic materials [5].
Modification of the hydrogen bond mesh leads to changes in the position, width, shape, and intensity
of the absorption bands in the IR spectra (Table 1). Thus, the disappearance of the band of valence
vibrations ν N-H, not perturbed by hydrogen bonding, as well as the redistribution of the intensity
of the absorption bands of amides I-VI, characterizing the contributions of the group 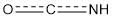 [15] constituents, is observed.
[15] constituents, is observed.
Table 1. Data of IR Fourier spectroscopy of polyurethane elastomers
|
Polyurethane sample |
Absorption bands, cm-1 |
|
initial |
3796–3596 (ν N–H free); 3284 (ν N–H, hydrogen bonded); 3068 (amide II in Fermi resonance with N–H group); 3030–3008 (ν Сар–H); 2926 (νas C–H); 2855 (νs C–H); 1726 (amide I); 1599–1413 (ν Сар–Сар, amide II, δ СН2); 1272 (amide III); 1221 (ν С–О); 1120 (νas С–О–С); 1099 (ν С–N); 1030 (νs С–О–С); 929 (amide IV); 790 и 740 (out of plane δ Сар–H); 784 (out of plane δ С=О); 657 (amide V), 605–538 (amide VI) |
|
modified |
3270 (ν N–H, hydrogen bonded)*; 3019 (amide II in Fermi resonance with N–H group)**; 3032–3012 (ν Сар–H); 2921 (νas C–H); 2857 (νs C–H); 1712 (amide I)*; 1591–1409 (ν Сар–Сар, amide II, δ СН2); 1261 (amide III)**; 1219 (ν С–О); 1117 (νas С–О–С); 1102 (ν С–N); 1033 (νs С–О–С); 941 (amide IV)**; 819 и 747 (out of plane δ Сар–H); 791 (out of plane δ С=О); 619 (amide V)**, 610–547 (amide VI)* |
Compared to the original polyurethane:
*increasing absorption band intensity;
**absorption band splitting.
The solid-state NMR method is a valuable complement to IR spectroscopy because it allows us to study the modifying effect of additives in creating the structure of polymers [16]. For all investigated polymer samples, the 1H NMR spectrum (Figure 2) is represented by a broad line with several extrema, which is explained by the dipole-dipole interaction of a large number of different proton groupings [16, 17].
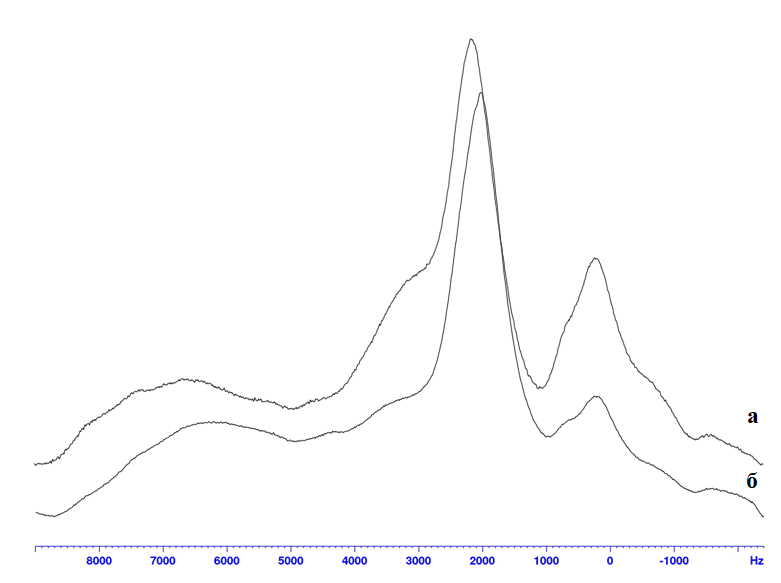
Figure 2. 1H NMR resonance lines of solid state: a - original polyurethane; b - modified polyurethane.
The analysis of the resonance line showed that it can be expressed as a sum of a number of components with different intensities, signal areas, and maxima. The most pronounced shift in position (δ, ppm) and change in half-width (ΔH, Hz) are undergone by signals with chemical shifts δ1 = 3.56 ppm (739.8 Hz), δ2 = 5.26 ppm (1454.6 Hz) and δ3 = 11.03 ppm (1824.4 Hz). For fluorinated polyurethane, these values are δ1 = 3.42 ppm (862.5 Hz), δ2 = 5.63 ppm (1394.5 Hz) and δ3 = 10.52 ppm (1762.3 Hz).
The transformation of the shape and position of the 1H NMR signal is caused by the involvement of more groups in multicenter proton-donor and proton-acceptor interactions in the macromolecular system (N-H···O=C, N-H···O<, N-H···F-C, C-H···F-C) including polyfluorinated tetraamine, cumulatively leading to changes in the phase composition of the amorphous structure of chemically modified polyurethane (redistribution of proton contributions in chains with maximum segmental mobility (movement of disordered sections of chains), protons in chains with difficult mobility, and protons of rigid chains).
The reorganization of the structure of modified polyurethane elastomers contributes to the increase in tensile strength, hardness and decrease in relative elongation of the polymer upon introduction of fluorine-containing modifier (Table 2).
Table 2. Physical and mechanical properties of polyurethane elastomers
|
Polyurethane elastomer |
Shore A hardness, conventional units. |
Tensile strength, MPa |
Relative elongation at break, % |
Residual strength at break *, % |
|
original |
40 |
1,6 |
200 |
51 |
|
modified |
46 |
2,0 |
170 |
602 |
*After 14 days of aging under varying temperatures.
Testing of rubbers after aging under conditions of dynamically changing (within a day) temperatures demonstrates the stabilizing effect of polyfluorinated tetramine in preserving the properties of polyurethanes. The initial polyurethane is characterized by pronounced erosive surfaces, formation of scratches, sinks and caverns, while the fluoropolymer is characterized by a decrease in the number of destructive formations (Figure 3).
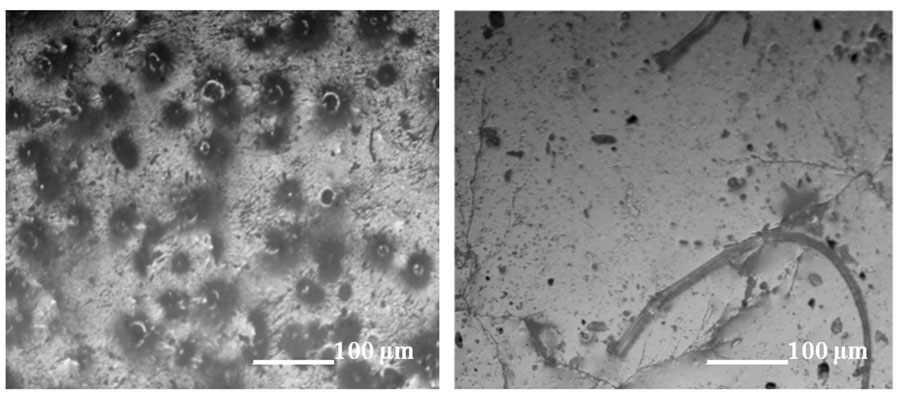
Figure 3. Microphotographs of the surface of polyurethane elastomers after aging at varying temperatures: a - original; b - modified.
Cracks, scratches, sinks and depressions of various shapes are stress concentrators, which enhance the further process of elastic polyurethane destruction. The oxygen content in the aged samples was 5.8 at.% (original polyurethane) and 3.6 at.% (modified polyurethane), respectively.
Thus, modification of polyurethane elastomers by the product of bis-alkylation of tris-(2-aminoethylamine) 1H,1H,9H-trihydroperfluorononane-1-ol leads to reorganization of the structure of the considered heterochain polymer and to the increase of its resistance to effect of increased and reduced temperatures.
Acknowledgements
NMR spectra were registered with use of the equipment of the Core Facility Center «Arktika» of the Northern (Arctic) Federal University».
References
- Thomas S., Datta J., Haponiuk J. et al., Polyurethane Polymers: Composites and Nanocomposites, Elsevier, Amsterdam, Netherlands, 2017, 634 p.
- Galimberti M, Rubber-Clay Nanocomposites, Science, Technology, and Applications, John Wiley & Sons Limited, 2011, 627 p.
- Clemitson I. R., Castable Polyurethane Elastomers, CRC Press (Taylor & Francis Group), 2015, 272 p.
- Nistratov, A. V., Fiziko-himicheskie principy razrabotki receptur i tekhnologii kompozicij na osnove oligotiolov, oligodienov i oligoefirov, ispol'zuemyh dlya polucheniya polimernyh materialov s uluchshennymi tekhniko-ekspluatacionnymi harakteristikami: Dis. ... doktora tekhn. nauk: 02.00.06, Volgograd, 2014, 448 с. (in Russian)
- Kudashev S. V., Methods of introducing poly- and perfluorinated fragments in to a macromolecular system (Review), Fluorine notes, 2020, 3 (130), 3-4; Kim, H. A, Study for mechanical and platelet adhesion properties of fluorinated polyurethanes, Polymer Korea, 2001, 25(3), 343–348.
- Smirnova O., Glazkov A., Yarosh A., Sakharov A., Fluorinated Polyurethanes, Synthesis and Properties, Molecules, 2016, 21(7), 1–10.
- Wu Z., Tang L., Dai J., Qu J., Synthesis and properties of fluorinated non-isocyanate polyurethanes coatings with good hydrophobic and oleophobic properties, Journal of Coatings Technology and Research, 2019, 16, 1233–1241.
- V.A. Ponomarenko, S.P. Krukovskij, A.Yu. Alybina. Ftorsoderzhashchie geterocepnye polimery. M. Nauka, 1973, 304 p (in Russian).
- B. F. Malichenko, Ftorsoderzhashchie poliamidy i poliuretany, Kiev: Naukova Dumka, 1977, 231 p.
- S.V. Kudashev, I.A. Politsimako, V.F. Zheltobryukhov, Investigation by rotational viscometry methods, NMR 119Sn spectroscopy and quantum chemistry of the effect of a catalytic system based on tin di-n-butyldilaurate and polyfluorinated tetraamine on the curing process of elastic polyurethanes, Fluorine notes, 2024, 4(155), 1-2.
- S.V. Kudashev, A.V. Faleva, I.A. Politsimako, V.F. Zheltobryukhov, Microcalorimetric study of the interaction of toluene diisocyanates with polyfluorinated tetraamines, Fluorine notes, 2024, 4(155), 3-4.
- S. V. Kudashev, V. S. Sidelnikov, I. A. Politsimako, V. F. Zheltobryukhov, Synthesis and NMR study of the N-polyfluoroalkylation product of tris(2-aminoethyl)amine with 1H,1H,9H-trihydroperfluoronan-1-ol, Fluorine notes, 2024, 2(153), 3-4.
- Lipatov Yu.S., Kercha Yu.Yu., Sergeeva L.M. - Struktura i svoistva poliuritanov. AN USSR, Institut Khimii Vysokomolekuliarnykh Soedinenii, Kiev: Naukova Dumka, 1970, 280 p. (in Russian)
- J. H. Saunders and K. C. Frisch, Polyurethanes: chemistry and technology, M.: Khimiya, 1968, 470 p. (in Russian)
- L. J. Bellamy, The infra-red spectra of complex molecules, Moscow: Publishing house of foreign lit., 1963, 590 p. (in Russian)
- Mathias L. J. (Ed.), Solid State NMR of Polymers, Springer, 2013, 408 p.
- Kudashev S. V., Modifikaciya ryada geterocepnyh polimerov kompoziciyami na osnove poliftorirovannyh spirtov i montmorillonita: Dis. ... doktora khimicheskih nauk: 02.00.06, Volgograd, 2020, 283 с. (in Russian)
ARTICLE INFO
Received 30 October 2024
Accepted 15 November 2024
Available online December 2024
Recommended for publication by PhD M.A. Manaenkova
eLIBRARY Document Number (EDN) PFTEQY

Fluorine Notes, 2024, 157, 1-2
