Received: September 2024
DOI 10.17677/fn20714807.2024.05.01
Fluorine Notes, 2024, 156, 1-2
2,4,6-TRIS(1,1,3,3-TETRAFLUOROALLYL)-1,3,5-TRIAZINE –PROMISING MONOMER FOR PRODUCING FLUORINATED POLYMERS
A.V. Sinko, V.L. Don, S.M. Igumnov
a A.N. Nesmeyanov Institute of Organoelement Compounds of the Russian Academy of Sciences, 119334 Moscow, Russia, Vavilova St., 28/1
b P@M-Invest Scientific Production Association, 119119, Moscow, Russia, Leninsky ave, 42/1-2-3.
Abstract: A novel fluorine-containing triazine monomer has been synthesized.Keywords: triazine, tetrafluoroallyltriazine, fluorine-containing triazine monomer.
The unique characteristics of fluoropolymers – the chemical inertness, thermal stability, resistance to ultraviolet radiation, electrical insulating properties, the ability to repel liquids and dirt, and a low coefficient of friction – make these materials indispensable for the chemical industry, the aerospace, automotive, medical, electrical and electronics industries. Nevertheless, work is continuously underway to improve these characteristics and to search for new fluoropolymers that most fully meet the demands of modern technology [1].
Polyfluorotriazine polymers are characterized by particularly high resistance to chemical and thermal stresses [2]. They work well as seals, gaskets and molded parts in systems that are exposed to elevated temperatures and/or aggressive chemicals.
When triazine monomers are used as additives to elastomers, they are considered as modifiers that improve the properties of elastomers, in particular, triazines are used as additives to fluorinated polyesters and copolymers of polyesters with tetrafluoroethylene [3], the introduction of such additives during polymerization increases the resistance of polymers to oxidation at high temperatures, especially in the presence of metals.
Two basic strategies for crosslinking polymers with triazine moieties are known. Bifunctional nitriles, either dinitriles or unsaturated nitriles, can be used; diamidines are prepared from them and then introduced into the polymerization process, obtaining polymers pierced with triazine fragments. Another strategy is to use triazines containing polymerization-capable substituents, which also allows them to be added as additives in the polymerization of other commercially more available monomers, thus obtaining polymers with improved properties [4].
It was possible to find only one example of similar monomers in the literature. Fluorinated alkenyltriazines of formulae (I) and (II) and their use as vulcanizing additives for cross-linking fluorinated elastomers are described in application [5].
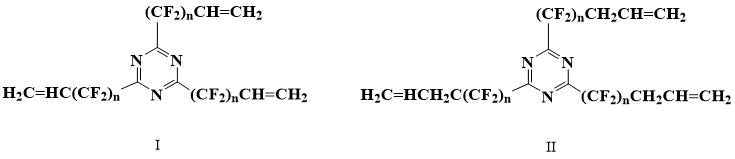
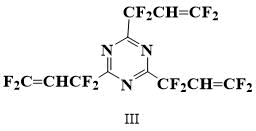
Perfluoroallyltriazine is known, but there is no evidence that it polymerizes [6].
Thus, the task was to obtain a triazine monomer with a high content of fluorine atoms in the side chains, possessing the ability to polymerize in order to further test the possibility of using it as a monomer for homopolymers and as a vulcanizing additive to elastomers. For this purpose, 2,4,6‑tris(1,1,3,3,3-tetrafluoroallyl)-1,3,5-triazine (III) was selected.
The choice of this compound as a monomer was due to the fact that, on the one hand, the fluorine content in the molecule of compound (III) is high, and on the other hand, the double bond is not completely fluorinated, which, based on the study of polymerization patterns of fluorinated monomers [7], suggested that such a compound would be capable of polymerization, which was confirmed by preliminary experiments.
2,4,6-Tris(1,1,3,3,3-tetrafluoroallyl)-1,3,5-triazine (III) was prepared from 2,4,6‑tris(iododifluoromethyl)-1,3,5-triazine, the preparation of which by interaction of iododifluoroacetic acid nitrile with ammonia was described in [8].
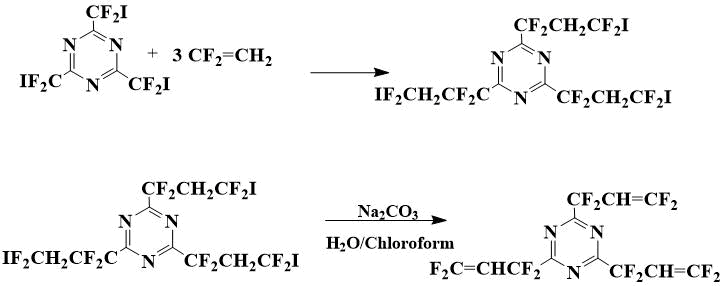
Scheme 1. Preparation of 2,4,6-tris(1,1,3,3,3-tetrafluoroallyl)-1,3,5-triazine
First, 2,4,6-tris(3-iodo-1,1,3,3-tetrafluoropropyl)-1,3,5-triazine is synthesized by interaction of 2,4,6-tris(iododifluoromethyl)-1,3,5-triazine with vinylidene fluoride at heating in autoclave. It is subjected without preliminary purification to soda solution in chloroform-water biphasic system in the presence of triethylbenzylammonium chloride (BTEAC) as an interfacial carrier, the obtained 2,4,6-tris(1,1,3,3-tetrafluoroallyl)-1,3,5-triazine is purified by distillation.
Preliminary experiments have shown that the resulting triazine is readily polymerizable, particularly using peroxide initiators.
Experimental part
1. 2,4,6-tris(3-iodo-1,1,3,3-tetrafluoropropyl)-1,3,5-triazine
120 g (0.197 mol) of 2,4,6-tris(iododifluoromethyl)-1,3,5-triazine are charged into a 0.2 liter steel autoclave with a needle valve. The autoclave is hermetically sealed, evacuated, cooled with liquid nitrogen, and 60 g (0.937 mol) of vinylidene fluoride is loaded from the chamber through a needle valve. The autoclave is then placed in a rocking furnace and held at 200°C for 20 hours.
The autoclave is cooled to room temperature and the excess pressure is released via a valve. 147 g of liquid is discharged, which is 85% of crude 2,4,6-tris(3-iodo-1,1,3,3-tetrafluoroallyl)-1,3,5-triazine, which contains an admixture of 2,4-bis(3-iodo-1,1,3,3-tetrafluoropropyl)-6-iododifluoromethyl-1,3,5-triazine. The raw material is used in the next stage without purification.
1H NMR spectrum (300 MHz, CDCl3) 3.87 ppm (m, 1H).
13C-NMR spectrum (100 MHz, CDCl3) 171.94 ppm (m, C=N, 3C), 114.43 ppm (t, JCF=240 Hz, N=C-CF2, 3C), 89.57 ppm (JCF=311 Hz, N=C-CF2-CH2, 3C), 52.66 ppm (m, CH2, 3C).
19F NMR spectrum (282 MHz, CFCl3, CDCl3) -37.52 ppm (m, 2F, CF2I), -99.90 ppm (m, 1F, CF2‑C=N).
2. 2,4,6-Tris(1,1,3,3-tetrafluoroallyl)-1,3,5-triazine
In a four-necked flask of 1 liter capacity, equipped with a mechanical stirrer, a thermometer, a dropping funnel and a reflux condenser connected at the outlet with a Tishchenko vial with water, 147 g of raw material obtained at the previous stage, 200 ml of chloroform, 2 g of BTEAC are charged, and while stirring, a solution of 58 g (0.55 mol) Na2CO3 in 150 ml of water is added dropwise for 30 minutes. When added, the reaction mass is foamed slightly and heated to 35°C, and carbon dioxide is released.
The reaction mass is refluxed until a gas emission ceases to about 6 hours, then cooled, filtered, the filtrate is transferred to a separatory funnel, the lower layer is separated, placed in a distillation flask, and chloroform is distilled off in a water bath, heating it to refluxing. Then, the receiving flask is replaced and the chloroform residue is distilled off with water in a vacuum of the water-jet pump. The remaining raw material is distilled in an oil pump vacuum of 0.7-1 Torr, heating the cube to 120°C. A 40 g crude 2,4,6-tris(1,1,3,3,3-tetrafluoroallyl)-1,3,5-triazine of about 80% purity containing an admixture of 2,4-bis(1,1,3,3,3-tetrafluoroallyl)-6-iododifluoromethyl-1,3,5-triazine derived from the corresponding admixture contained in the raw material, obtained at the first step. The crude product is re-distilled in a vacuum of 1 Torr, selecting a pre-distillate boiling to 71°C (65-71°C) and a main fraction boiling at 71-72°C, thus obtaining 31 g of 2,4,6-tris(1,1,3,3-tetrafluoroallyl)-1,3,5-triazine.
The yield is 38% for the parent 2,4,6-tris(iododifluoromethyl)-1,3,5-triazine.
1H NMR spectrum (300 MHz, CDCl3) 5.23 ppm (m, 1H).
13C NMR spectrum (100 MHz, CDCl3) 172.97 ppm (m, C=N, 3C),159.00 ppm (JCF=301 Hz, CF2=CH, 3C), 113.72 ppm (t, JCF=257 Hz, N=C-CF2, 3C), 77.18 ppm (m, CH, 3C).
19F NMR spectrum (282 MHz, CFCl3, st. CDCl3) -73.93 ppm (m, 1F, CF2=CH), -77.01 ppm (m, 1F, CF2=CH), -96.73 ppm (d. JFH=11Hz, 2F, CF2-CH).
Acknowledgments
This work was supported by the Ministry of Science and Higher Education of the Russian Federation (Contract/agreement No. 075-00277-24-00 and was performed employing the equipment of «Center for molecular composition studies» of INEOS RAS.
References
- Polyaddition of Fluorinated Vinyl Monomers, p.72, T. Narita in Fluorinated polymers v.2, 2017, RSC, edited by B. Ameduri and H. Sawada, The Royal Society of Chemistry, 2017.
- US 4434106 А (1984).
- US 5942598 А (1999).
- Shuaihua Zhang, Qian Yang, Chun Wang, Xiliang Luo, Jeonghun Kim, Zhi Wang, Yusuke Yamauchi, Adv. Sci., 2018, 5, 1801116.
- WO 9705122 (1997).
- Brown, Henry C.; Cheng, Ming T., Journal of Chemical and Engineering Data, 1968, 13(4), 560.
- L.A. Wall, (Ed.), Fluoropolymers, Wiley, New York, 1972; Narita, Tadashi, Macromolecular Rapid Communications, 2000, 21(10), 613-627.
- Ming-H. Hung, Lu Long, and Zhen-Yu Yang, J. Org. Chem., 2004, 69, 198-201.
ARTICLE INFO
Received 17 September 2024
Accepted 18 October 2024
Available online August 2024
Recommended for publication by PhD M.A. Manaenkova
eLIBRARY Document Number (EDN) MKBUCY

Fluorine Notes, 2024, 156, 1-2
