Received: February 2024
DOI 10.17677/fn20714807.2024.02.02
Fluorine Notes, 2024, 153, 3-4
SYNTHESIS AND NMR STUDY OF THE N‑POLYFLUOROALKYLATION PRODUCT OF TRIS(2-AMINOETHYL)AMINE WITH 1H,1H,9H-TRIHYDROPERFLUORONAN-1-OL
S. V. Kudashev1, V. S. Sidelnikov2,3, I. A. Politsimako1, V. F. Zheltobryukhov1
1Volgograd State Technical University, 28 Lenin Avenue, Volgograd, 400005 Russia
e-mail: kudashev-sv@yandex.ru
2 National Research Tomsk State University, 36 Lenin Avenue, Tomsk, 634050 Russia
3 Tomsky regional Center for collective use (SECCP), Tomsk State University, 634050 Russia, Tomsk, Lenin Avenue, 36
Аbstract: By the interaction of tris(2-aminoethyl)amine with polyfluorinated alcohol in the presence of catalytic amounts of montmorillonite clay, the corresponding N-polyfluoroalkylation product was synthesized. One-dimensional (1H, 13C, 19F) and two-dimensional heteronuclear (1H-15N HMBC) methods the structure of the resulting compound was studied by NMR spectroscopy.
Keywords: polyfluorinated alcohol, polyethylene polyamines, catalysis, NMR spectroscopy.
Introduction
The synthesis of fluorinated amines [1-3] is of interest in connection with the possibility of using same as reagents for producing biologically active compounds, as well as stabilizers of the properties of polymer materials [4]. Catalytic amination of alcohols is carried out at high temperatures in the presence of catalysts based on aluminum, chromium, titanium, vanadium, iron, nickel molybdenum, magnesium oxides, as well as thorium salts and natural aluminosilicates [5].
The polyfluorinated alcohols H(CF2CF2)nCH2OH, obtained on an industrial scale by reacting tetrafluoroethylene and methanol [6], can be considered as alkylating agents of polyamines, particularly various isomeric polyalkylene polyamines, which are simultaneously capable of containing amino groups of varying degrees of substitution.
The purpose of the work - catalytic N-polyfluoroalkylation of tris(2-aminoethyl) amine with polyfluorinated alcohol (n=4) in the presence of highly dispersed montmorillonite clay and study by methods of one-dimensional and two-dimensional NMR spectroscopy of synthesized compound structure.
Results and discussion
Catalytic N-polyfluoroalkylation of tris(2-aminoethyl) amine with 1H,1H,9H-trihydroperfluoronononane-1-ol at their molar ratio 1:2 results in production of predominantly product of dialkylation:
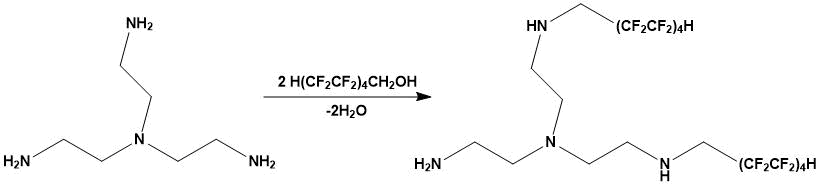
Montmorillonite clay, being a Brønsted acid [7-9], facilitates alkylation of tris(2‑aminoethyl)amine through initial stages of O- and N-protonation (only formation of monoalkylation product is shown in schemes 1 and 2). In both cases, a polyfluorinated carbocation is generated, which stability is significantly affected by fluorine atoms having a negative I-effect. The formation of intramolecular hydrogen bond in a disubstituted ammonium cation between the CF2···H2N⊕ groups favorably affects an increase in it’s stability.
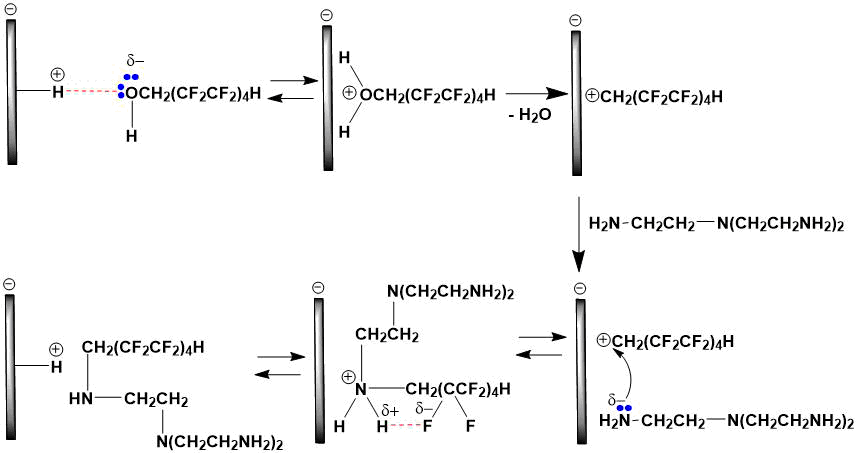
Scheme 1. The O-protonation mechanism.
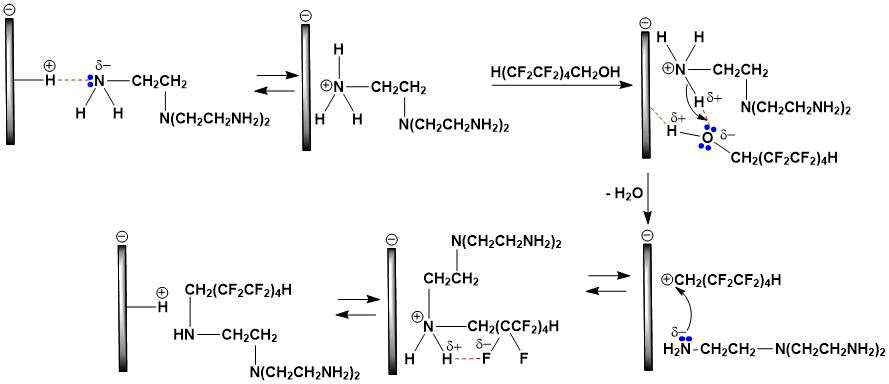
Scheme 2. The N-protonation mechanism.
The structural feature of montmorillonite clay is not only Brønsted but also Lewis acidity [7‑9], caused by the presence of vacancy-orbital centers Мn+ (Al3+, Fe3+, Fe2+, Mn2+, Ti4+, Ca2+, Mg2+, K+, Na+). Formation of donor-acceptor complexes with participation of vacancy orbitals of metal ions can be carried out as with non-divided electron pairs of nitrogen atoms of amino groups of tris(2‑aminoethyl)amine and oxygen of hydroxyl groups of 1H,1H,9H-trihydroperfluorononane-1-ol (structure I), and without participation of molecules of polyfluorinated alcohol (structure II).
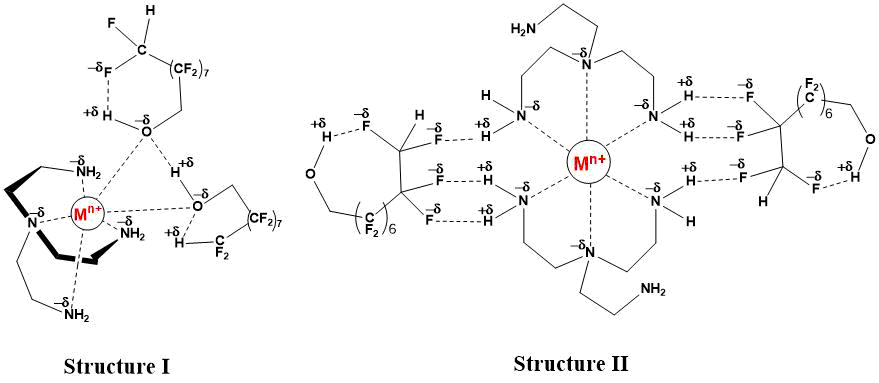
Thus, catalytic N-polyfluoroalkylation of tris(2-aminoethyl)amine with 1H,1H,9H- trihydroperfluorononane-1-ol in the presence of montmorillonite clay in a molar ratio of amine and alcohol 1:2 leads to the formation of a disubstituted product. A possible contribution of the Brønsted and Lewis acid sites to the alkylation mechanism is shown.
Experimental part
Tris(2-aminoethyl)amine (95%, Keyingchem, China) is purified by distillation and has the following characteristics: boiling point 114°C (15 mm Hg), d 0.976 g·ml-1, n20D 1.497. Polyfluorinated alcohol 1H,1H,9H-trihydroperfluorononane-1-ol (≥90%, HaloPolymer, Perm) has mp 68-70°C. Calcined montmorillonite clay (≥ 98%, JSC B-Clay, Kazakhstan) has a specific surface area of 595 m2·g-1 (in water) and 64 m2·g-1 (nitrogen), a cation exchange capacity of 100 mg-eq/100 g and is presented in the form of a mixture of three main fractions: 50-100 nm – 10 wt.%, less than 1 µm– 80 wt.%, less than 10 µm– 10 wt.%.
The method of synthesis. 1 ml (6.67 mmol) of tris(2-aminoethyl)amine and 5.77 g (13.3 mmol) 1H,1H,9H-trihydroperfluorononane-1-ol in the presence of catalytic amounts of montmorillonite (0.1 mg) was dispersed at ultrasound frequency of 40 kHz in a sealed glass ampoule at 80°C for 2 hours, followed by heating to 120°C for 6 hours. The starting reagents were separated from the reaction product by extraction of the cooled i-PrOH at-6°c +1°c with further fractional vacuum distillation of the extract. Yield is 2.27 g (35%), yellow oil, Rf = 0.71, t of boiling 133 -136°C (15 mm Hg).
NMR spectrum 1Н [CDCl3], δ, ppm: 1.56 br.s (2Н, H2N), 2.43-2.90 m (12Н, СН2СН2), 4.01 t (4Н, СH2CF2, J = 14.8 Hz), 6.07 tt (2Н, HCF2, J1 = 4.8 Hz, J2 = 51.6 Hz), 7.29 s (2H, HNCH2). NMR spectrum 13С [CDCl3], δ, ppm: 38.66-39.75 (CH2NH2), 41.65 (CH2CF2), 46.01 d (CH2NHCH2CF2, J = 8.1 Hz), 49.22-59.56 (NCH2CH2NH), 60.98 (NCH2CH2NH2), 104.67-114.00 (CF2). NMR spectrum 19F [CDCl3], δ, ppm: -121.83 – -122.40 m (CF2), -123.70 – -129.76 m (CF2CН2), -137.21 d (CF2Н, J = 48.9 Hz. NMR spectrum 15N (1H-15N HMBC) [CDCl3], δ, ppm: 18 (NH2), 30 (NH), 43 (NCH2). Found, %: С 29.41; Н 2.26; N 5.50. C24H22F32N4. Calculated, %: С 29.58; Н 2.28; N 5.57. М 974.41.
NMR spectra were recorded at room temperature on a Bruker AVANCE III HD (400 MHz) device in СDCl3 (1H – 400.17 MHz, 13С – 161.99 MHz, 19F – 376 MHz), using tetramethylsilane as an internal standard. The chemical shifts of the 19F cores are defined relative to CFCl3 and the 15N nuclei relative to liquid ammonia as an external standard. Elemental analysis was carried out on a CHNS/O Euro EA3100 analyzer. TLC was carried out on Sorbfil plates (Russia), eluent is chloroform-acetone (1:1).
References
1. Yang Y., Taponard A., Vantourout J. C., Tlili A., Synthesis of Fluorinated Amines: A Personal Account, ACS Org. Inorg. Au., 2023, 3(6), 364-370.
2. Yang L., Fan W.-X., Lin E., Tan D.-H., Li Q., Wang H., Synthesis of α-CF3 and α-CF2H amines via the aminofluorination of fluorinated alkenes, Chem. Commun., 2018,54, 5907-5910.
3. Baasner B., Klauke E., Hagemann H., Amines containing fluoroalkyl groups: Methods of synthesis and properties, Journal of Fluorine Chemistry, 1983, 23(5), 478.
4. S. V. Kudashev, Methods of introducing poly- and perfluorinated fragments in to a macromolecular system (Review), Fluorine notes, 2020, 3(130), 3-4.
5. Kluev M.B., Khidekel M.L., Catalytic amination of alcohols, aldehydes and ketones, Uspekhi Khimii, 1980, XLIX (1), 28-53. (in Russian)
6. Ebnesajjad S., Fluoropolymer Additives: S. Ebnesajjad, R. A. Morgan, Elsevier, William Andrew, 2019, 304 p.
7. Physical and chemical features of natural clays, L. G. Gilinskaya, T. N. Grigor’eva, L. I. Razvorotneva, L.B. Trofimova, Zhurnal neorganicheskoj khimii, 2005, 50(4), 689-698. (in Russian)
8. S. V. Kudashev Modification of some heterochain polymers with compositions based on polyfluorinated alcohols and montmorillonite, Doctor of Chemical Sciences Dissertation Abstract (speciality 02.00.06), Volgograd State Technical University, Volgograd, 2020, 48 p. (in Russian)
9. S. V. Kudashev, Structure of composite material based on polyfluorinated alcohol and montmorillonite, Zhurnal fizicheskoi khimii, 2018, 92(10), 1582-1587. (in Russian)
ARTICLE INFO
Received 21 February 2024
Accepted 09 April 2024
Available online April 2024
Recommended for publication by PhD V.L. Don
eLIBRARY Document Number (EDN) PUWKYI

Fluorine Notes, 2024, 153, 3-4
