Received: February 2024
DOI 10.17677/fn20714807.2024.02.01
Fluorine Notes, 2024, 153, 1-2
SYNCHRONOUS SEPARATION OF THREE RADICALS IN IONIC SERIES OF POLYOXAPERFLUOROALKANES AND POLYOXAPERFLUOROALKYL HALIDES MASS SPECTRA
N. D. Kagramanov
A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 119991, GSP-1, Moscow, B-334, Vavilov St. 28
e-mail: ndkagram@gmail.com
Annotation: One of the ionic series of mass spectra of n-alkanes, n-perfluoroalkanes, perfluorocyclohexane begins with primary synchronous separation of three radicals. It has been previously reported about synchronous primary separation of three fluorine atoms (C2, C1 и C20F3) from two terminal groups of a perfluoroeicosane linear molecule, as well as two hydrogen atoms and a radical CH3 (C2, C1 и C20H3) in one of the six ion series of eicosane [1]. In comparison with other ion series, in which the primary separation of two radicals occurs from two terminal groups, or one radical is separated from one terminal group, the primary separation of three radicals is the result of the maximum excitation energy of the molecular ion. The article [2] shows ionic series of polyoxaperfluoroalkanes and polyoxaperfluoroalkyl halides. However, primary detachments of radicals have not been discussed, since in the spectra of some compounds there are secondary emissions of three radicals or synchronous separation of radicals occurs only from one of the terminal groups. There were several possible reasons affecting the primary or secondary of the detachments. First of all, it is a symmetry of the molecule, the mass difference of the terminal groups, as well as the presence of terminal groups with secondary or tertiary carbon atoms. Oxygen atoms are the cause of the energy heterogeneity of the polyoxaperfluoroalkane chain bonds as compared to perfluoroalkanes. In the process of fragmentation, the oxygen atoms promote both the occurrence of a stable terminal fluorocarbonyl group CF=O and the corresponding fluorocarbonyl series, and the detachment rearrangement CF2=O, as well as oxygen atom emissions to form terminal perfluoroalkyl groups, with masses greater than in the starting molecule. In order to find out possible causes of secondary emissions of three radicals and the comparison of synchronous detachments, which occur in the spectra of homologues of polyoxaperfluoroalkanes, polyoxaperfluoro halogenides and perfluoroalkanes, it is necessary to return to them again. The examples presented in the present article make it possible to match the symmetry and structures of terminal groups of compounds with the primary and secondary synchronous separations of three radicals taking place. Regrouping of polyoxaperfluoroalkanes with the separation of CF2=O, as well as the emission of an oxygen atom and the formation of perfluoroalkyl ions, with a mass greater than in the structure of their molecular ions, are discussed.
Key words: polyoxaperfluoroalkanes, polyoxaperfluoroalkyl halides, ionic series, synchronous separation of three radicals, emission of OCF2, emission of O.
Experimental part
Samples of polyoxaperfluoroalkyles with terminal halide atoms, the spectra of which are not available in NIST libraries, were provided by SIA "P&M-Invest" Ltd. Electron ionization mass spectra were recorded via Finnigan Polaris Q chromatomass spectrometer (with ion trap, range 14-1000 Da, energy 70 eV, (Rtx-5MS capillary column 5% diphenyl/95% dimethyl polysiloxane 30 m long, 0.25mm - inner diameter, initial column temperature 30℃, isotherm 10 min; heating with a speed of 10°/min up to 250°C). Since this report also contains spectra from NIST libraries obtained via magnetic or quadrupole apparatus, all presented mass spectra contain information about the type of apparatus on which this mass spectrum was recorded.
Introduction
In processes of electrosynthesis by Kolbe, the aliphatic products of radical dimerization were isolated and interpreted by NMR19F and chromatomass spectrometry methods [3].
For the first time, the homologues of a large number of polyoxaperfluoroalkanes and polyoxaperfluoroalkils with terminal halide atoms and with mass numbers from 500 to 1900 Da were synthesized and their mass spectra were recorded. It was of interest to find out how oxygen chain links change fragmentation and ionic series, in comparison with ion series of mass spectra of perfluoroalkanes and perfluoroalkyl halides.
When studying the ionic series of mass spectra of perfluoroeicosane and eicosane [1], synchronous primary separation of three fluorine atoms (C2, C1 и C20F3) in a perfluoroalkenyl series of perfluoroeicosane, as well as two hydrogen atoms and radicals ·CH3 or ·C2H5 (C2, C1 and C20H3 or C19H2СH3) in two alkenyl series of eicosane, a similar mechanism of their fragmentation is confirmed.
Three ionic series of perfluoroeicosane (perfluoroalkenyl, perfluoroalkyl and perfluoroolefinic) and six ionic series of eicosane have been established by detailed analysis of fragmentation sequences. The double increase in the number of eicosane ionic series compared to three perfluoroeicosane ionic series is the result of ·CH3 radical and the ·C2H5 radical close energies. Synchronous detachment of three fluorine atoms takes place in one of the five ionic series of perfluorocyclohexane [1] and in one of the three main ionic series of perfluoro-tert-butyl amine mass spectrum [4].
Synchronous detachments of three radicals in ionic series of oxa- and polyoxa- perfluoroalkanes
The ionic series of mass spectra of polyoxaperfluoroalkanes and polyoxaperfluoroalkyl halides, which are presented in the Figures 1-15, have been published [2]. For this reason, in this report have been examined in detail mainly ionic series, in which primary or secondary synchronous detachments of three radicals take place, since it has not been done previously.
The primary detachment of radicals takes place in mass spectra of polyoxaperfluoralanes, typically at an oxygen atom. In this case, the ejected radical may contain one or more oxygen atoms. One of the ionic series of polyoxaperfluoroalkanes spectra ends with peaks of ions with m/z 147, m/z 97, m/z 47, containing a terminal fluorocarbonyl group FC=O. The Figure 1 shows two ionic series: a fluorocarbonyl (marked blue) and a perfluoroalkyl (marked red) of C4F10O mass spectrum, containing one oxygen atom.
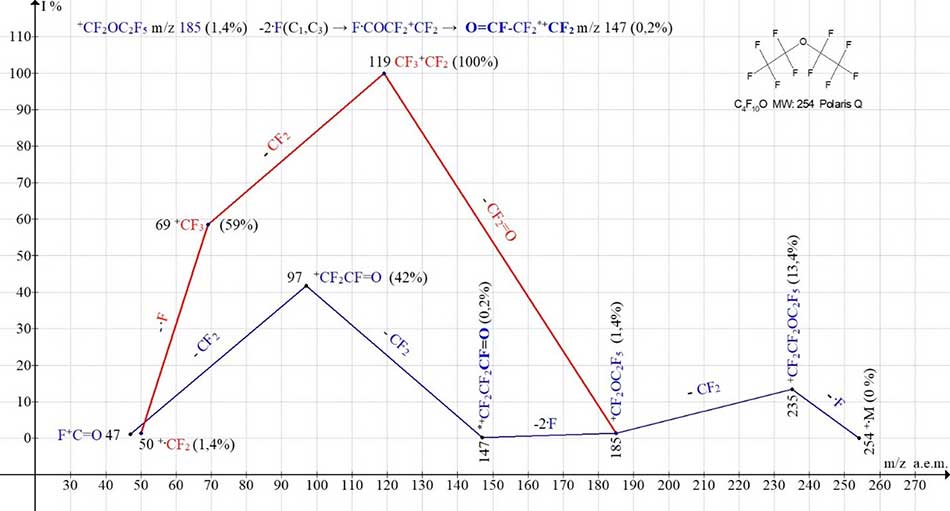
Figure 1. Two ionic series of the 1,1'oxybis(pentafluoroethane) С4F10O MW:254 Ion Trap (Polaris Q) mass spectrum.
Although the molecule С4F10O (Figure 1) is symmetrical, the primary synchronous detachment of three radicals F does not occur. Instead of the expected ion CF2=CFOCF2+CF2 with m/z 197, after successive separation of fluorine atom and difluorcarbene ion +CF2OC2F5 with m/z 185 is formed, which is fragmented in two ways. As a result of secondary synchronous detachment of two fluorine atoms from two terminal groups (C1 and C3) and rearrangement, the first fragment ion of the fluorocarbonyl series occurs (marked with blue color) +CF2CF2FC=O with m/z 147. When the ion from m/z 185 of the molecule CF2=O is separated from the ion with m/z 185, the first fragment ion of the perfluoroalkyl series occurs (marked with red color) with a mass of m/z 119.
Two ionic series of symmetric perfluorodioxaalkane C10F22O2 MW:570 are shown in Figure 2.
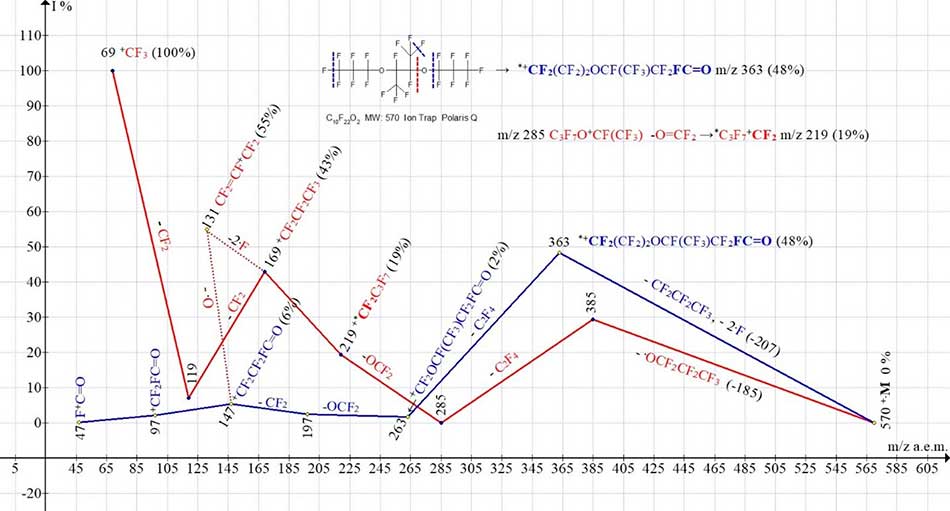
Figure 2. Two ionic series of the С10F22 O2 MW:570 Ion Trap (Polaris Q) mass spectrum.
The radicals’ detachments take place in one of the central oxygen atoms in two ionic series of the spectrum (Figure 2). Perfluoroalkyl series (marked with red color) begins with successive detachments: radical ·OC3F7 (-185), C2F4 (-100) molecule and O=CF2 (-66) carbonyl difluoride molecule. This results in a rearrangement ion +C4F9 m/z 219 having a mass greater than that of the terminal group C3F7 in the starting molecule. Marked with blue color, fluorocarbonyl ionic series begins with primary synchronous separation of three radicals: radical .C3F7 (without oxygen) and two fluorine atoms from both terminal groups (C1, C5 or C6) (Σ-207) (at Figure 2 are marked with blue dashed lines). At separation of any fluorine atom from the terminal group -CF(CF3)-O·, it is rearranged to form a terminal fluorocarbonyl group -CF2-FC=O. As a result, a rearrangement ion occurs with m/z 363. During fragmentation of the ion with m/z 363, a low-intensity series of fluorocarbonyl ions with m/z 197, 147, 97 and 47 is formed. Since there is no series of ions with a terminal perfluorovinyl group in the spectrum (Figure 2), the occurrence of an intense peak of the perfluoroallyl ion CF2=CF-+CF2 m/z 131 (55%) is likely a result of separation of two fluorine atoms from the ion with m/z 169, or an oxygen atom from an ion with m/z 147.
Two ionic series of C12F26O3 with three oxygen atoms and asymmetrical terminal groups mass spectrum are shown in Figure 3.
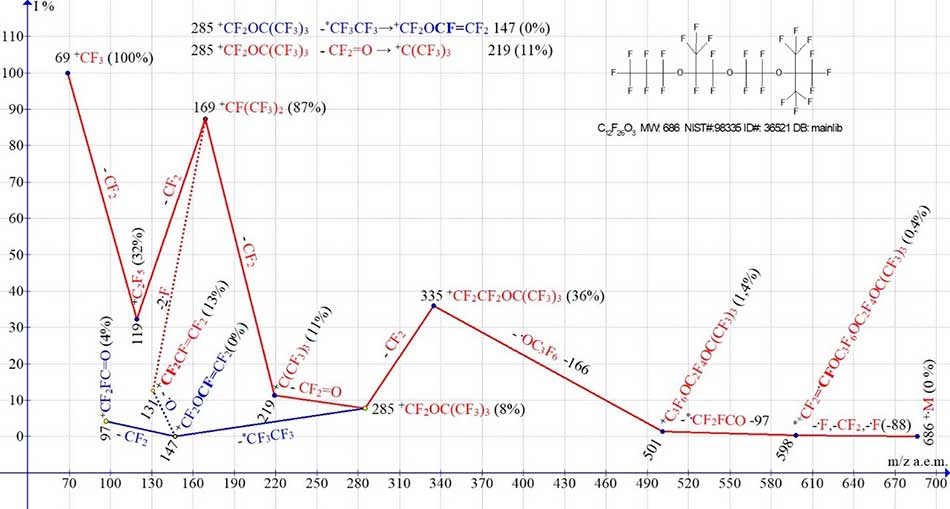
Figure 3. Two ionic series of the perfluoro-2,2,8- trimethyl-3,6,9-trioxadodecane С12F26O3 MW:686 NIST#: 98335 ID#: 36521 DB: mainlib.
From two possible variants of the fragmentation beginning [С12F26O3]+·, it starts not from the group [С12F26 O3]+.m/z = 219, with a tertiary carbon atom, but from the perfluoropropyl group ·C3F7 m/z=169, having a linear structure and a smaller mass. After synchronous detachment from one terminal group C3F7 of two fluorine atoms and -СF2, a rearrangement cation radical [CF2=CF‑OC3F6OC2F4OC4F9]+· (m/z 598) occurs, with a terminal perfluorovinyl group. It fragmented by successive detachments of radicals .CF2FC=O (-97), C3F6O (-166) and CF2 to form an ion +CF2OC(CF3)3 with m/z 285. Up to an ion with m/z 285 group C(CF3)3 with a tertiary carbon atom does not participate in fragmentation. Ion +CF2OC(CF3)3 with m/z 285 (8%) is fragmented in two ways. As with emission of CF2=O and formation of perfluoroalkyl ion +C(CF3)3 with m/z 219 (11%) (series of red color), and with hexafluoroethane molecule detachment and rearrangement ion +CF2OCF=CF2 formation with m/z 147 and its fragment ion *+CF2FC=O m/z 97 (4%) (series of blue color). The asymmetry of the terminal groups of the molecule С12F26 O3 (Figure 3), as well as the odd number of oxygen atoms, are probably the reason that the primary synchronous separation occurs at the same terminal group C3F7. Since the perfluoroalkenyl series of ions is not formed (Figure 3), the occurrence of a perfluoroallyl ion with m/z 131 is likely the result of the oxygen atom separation from the ion with m/z 147.
Two ionic series of a symmetrical molecule of polyoxaperfluoroalkane C12F26O4 MW=702 with four oxygen atoms are shown in Figure 4. Primary separation of radicals occurs at one of central oxygen atoms.
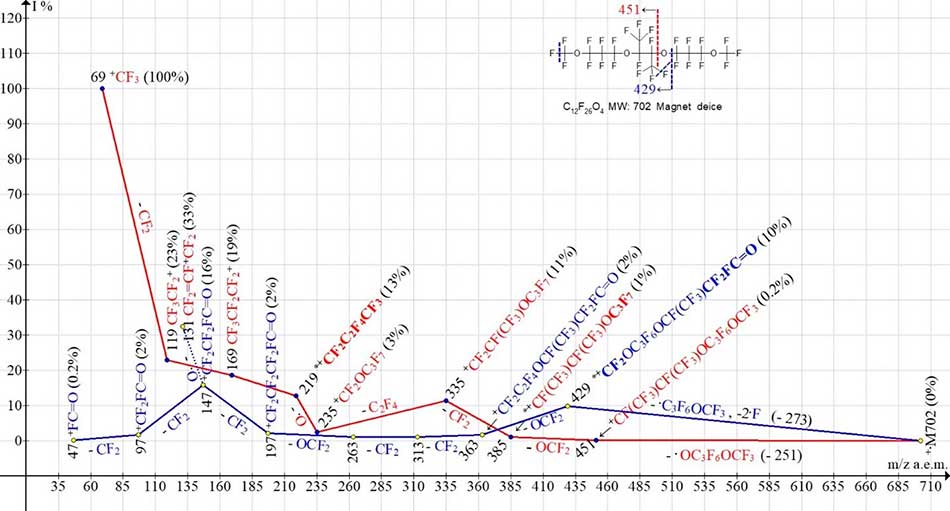
Figure 4. Two ionic series of the С12F26 O4 MW:702 mass spectrum (magnetic mass spectrometer VG-7070E).
In the spectrum of the symmetrical molecule С12F26 O4, two ionic series are proposed. The perfluoropolyoxaalkyl series (marked with red color) begins with the detachment of the terminal group ·OC3F6OCF3 (-251) and the formation of an ion with m/z 451. The following rearrangement separation of the molecule CF2=O takes place from the opposite terminal group CF3O. As a result, the arisen ion with m/z 385 already contains a terminal group OC3F7, which is not in M+·. Consistent detachments of CF2 and molecules C2F4 are completed with ion +CF2OC3F7 with m/z 235 (3%). It can be assumed that the +CF2OC3F7 ion will fragment with an emission of CF2=O and form an ion +C3F7 with m/z 169. However, this is not happening. The occurrence of ion +C4F9 with m/z 219 (13%) (a group that doesn’t exist in the starting M+·) confirms an emission of the oxygen atom and an energy profitability of a homogeneous perfluoroalkyl rearrangement ion +C4F9 with m/z 219 formation.
Fluorocarbonyl ionic series (marked with blue color) begins with synchronous detachment of three radicals: including a ·C3F6OCF3 radical, a fluorine atom from one of the terminal groups, as well as a fluorine atom from the opposite terminal group. In Figure 4, the fluorine atoms to be separated are marked with a dotted line blue. As a result, there is a rearrangement ion with m/z 429 with terminal fluorocarbonyl group FC=O. It fragments by successive detachments: CF2=O, CF2, CF2, CF2 = O and ends with ions with m/z 197, 147, 97 and 47 containing a fluorocarbonyl group. The occurrence of an intense perfluoroallyl ion with m/z131 (33%) is likely a result of an oxygen atom separation from an ion +CF2CF2 FC=O with m/z 147 (16%) containing a fluorocarbonyl group.
Oxa- and polyoxaperfluoroalkyl ionic series with terminal chlorine and bromine atoms
Four ionic series of asymmetric molecules ClCF2CF2O(CF2)6CF3 and BrCF2CF2O(CF2)6CF3 mass spectrum are shown in Figure 5 and Figure 6.
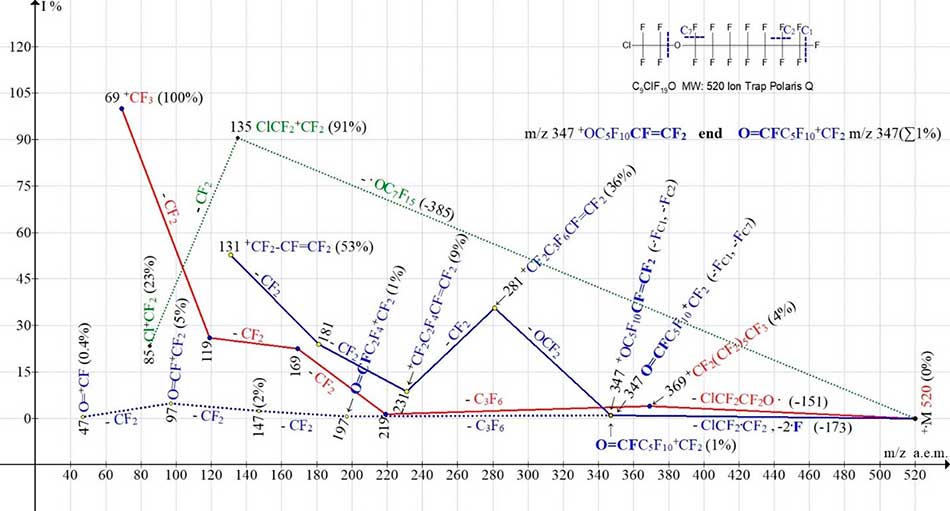
Figure 5. Four ionic series of 1-(2-chloro-1,1,2,2-tetrafluoroethoxy)pentadecafluoroheptane С9ClF19O MW:520 Ion Trap (Polaris Q) mass spectrum.
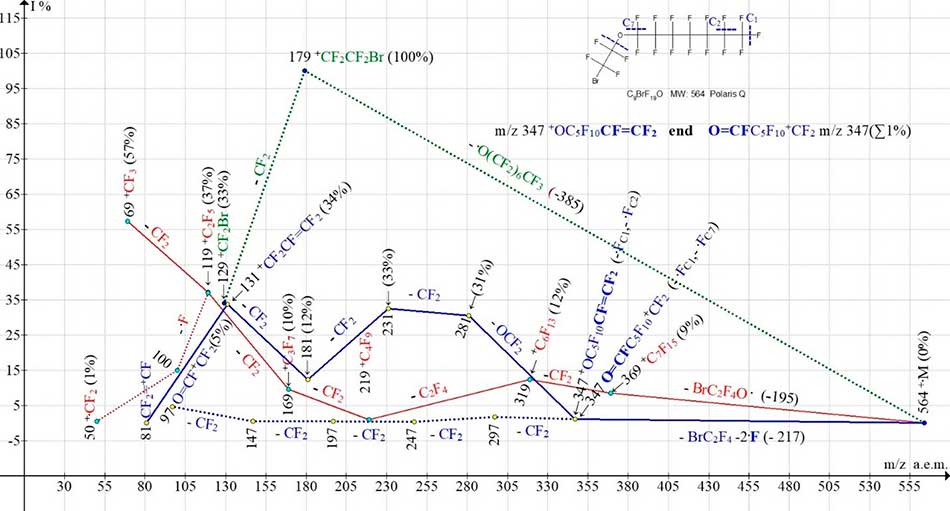
Figure 6. Four ionic series of 1-(2-bromo-1,1,2,2-tetrafluoroethoxy)pentadecafluoroheptane С9BrF19O MW:564 Ion Trap (Polaris Q) mass spectrum.
The perfluoroalkyl series of spectra (marked with red color), chloro- and bromo- perfluoroalkyl series (marked with green color), perfluoroalkenyl and fluorocarbonyl series (marked with blue color) begins at an oxygen atom of a single non-uniformity of the chain. In case of radical ·OC7F15 separation (with oxygen atom -385) there is a series of two chlorine-containing ions ClCF2+CF2 m/z 135 (91%) and Cl+CF2 m/z 85 (23%) (Figure 5), and accordingly two bromine-containing ions BrCF2+CF2 m/z 179 (100%) and Br+CF2 m/z 129 (33%) (Figure 6). The peak of the bromine-containing ion with a mass of m/z 179 (100%), unlike the peak of the chlorine-containing ion with m/z 135 (91%), has a greater intensity. When the ·OCF2CF2Cl radical is separated (with a minimum mass of 151 a.m.u.) and the radical ·OCF2CF2Br (with a maximum mass of 195 a.m.u.), a more intensive and less intensive perfluoroalkyl series of peaks with m/z 369, 219, 169, 119, 69 (Figure 5 and Figure 6) occurs. Similar to primary synchronous detachment of three fluorine atoms in spectra of perfluoroalkanes [1], in perfluoroalkylene series (Figure 5 and Figure 6) occurs an initial synchronous detachment of three radicals (HalC2F4 and 2 ·F). The carbon atoms (C2, C1 and C7, C1) marked in Figure 5 and Figure 6 blue dotted lines lose two fluorine atoms. Instead of the third fluorine atom, the radical HalCF2·CF2 (without an oxygen atom) is released. As a result of fluorine separations from carbon atoms (C2 and C7) there is a rearrangement ion with m/z 347 (1%), with two isomeric versions of the structure: structures with a terminal perfluorovinyl group +O(CF2)5CF=CF2 and structures O=CF(C5F10)+CF2 with a terminal fluorocarbonyl group. The ion with m/z 347 fragments to form two series. When the ion with m/z 347 O=CF2 is separated, a series of intense perfluoroalkyl peaks with m/z 281, 231, 181, terminating in the formation of a perfluoroallyl ion with m/z 131(53%) is formed (marked with a solid blue line). As a result of successive detachments of the difluorcarbene from an ion with m/z 347, a low-intensity series of peaks occurs (marked with a blue dotted line) with m/z 197, 147, 97 and 47 with a terminal fluorocarbonyl group (Figure 5).
Three ionic series of the asymmetric molecule С9СlF19O2 mass spectrum are shown in Figure 7.
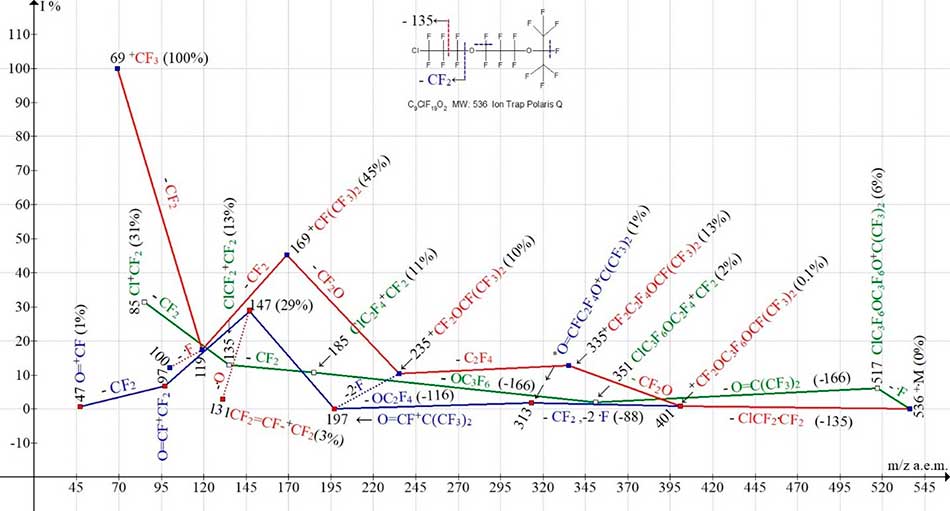
Figure 7. Three ionic series of С9ClF19O2 MW:536 Ion Trap (Polaris Q) mass spectrum.
Replacement of the terminal trifluoromethyl group С9ClF19O (Figure 6) with the heptafluoropropyl group CF(CF3)2 (Figure 7) and an increase in the number of oxygen atoms of the chain by another atom result in a significant change in the С9ClF19O2 fragmentation. In the chlorine-containing ionic series (marked with green color), the primary detachments start with the heptafluoropropyl group. It fragments with the separation of the fluorine atom, and then with the emission of hexafluoroacetone. The number of fractional chlorine-containing ions as compared to C9ClF19O spectra (Figure 5) increases from two to five. There is no primary synchronous detachment of three radicals in any of the ionic series. However, after emission of radical ·CF2CF2Cl (-135) in one of two branching series of ion with m/z 401 (marked blue color) secondary synchronous separation of three radicals - CF2, -2 ·F takes place. It is likely that this result of the asymmetric terminal group CF(CF3)2 presence in the monochloride molecule. The ion with m/z 401 fragments both with the break of the CF2=O molecule and with the synchronous emission of three radicals (two fluorine atoms and CF2). At Figure 7, the separating fluorine atoms and the ejection group CF2 are marked with blue dashed lines. The next separation from the ion with m/z 313 molecules OC2F4 occurs from the middle of the chain. It results in a fluorocarbonyl series of ions with m/z 197, 147, 97, 47. The presence of two oxygen atoms in chain increases the probability of fluorocarbonyl group containing ionic series occurrence and reduces the probability of perfluoroalkenyl series formation, ending with the appearance of CF2=CF-+CF2 m/z 131. The ion peak intensity with m/z 147, compared to the spectrum (Figure 5), increases from 2% to 29%. The ion peak intensity with m/z 131 in the С9СlF19O2 spectrum (in Figure 7 is not represented) is only 3%. Due the high ion peak intensity with m/z 147, the +C3F5 ion is possibly formed by detachment of the oxygen atom from the O=CFCF2+CF2 ion with m/z 147.
Three ionic series of the polyoxaperfluoroalkyl chloride С12ClF25O3 MW:702 non-symmetrical molecule mass spectrum are shown in Figure 8.
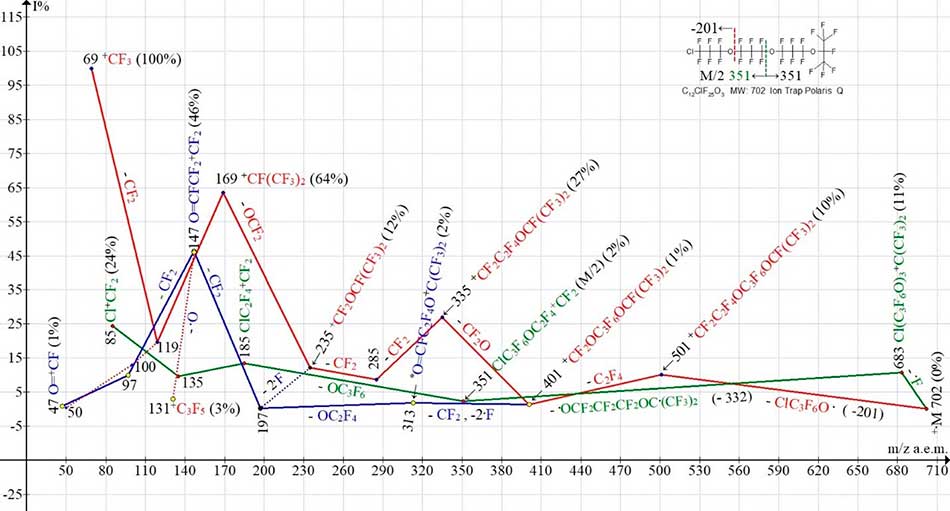
Figure 8. Three ionic series of С12ClF25O3 MW:702 Ion Trap (Polaris Q) mass spectrum.
In comparison with the spectrum of symmetrical polyoxaperfluoroalkane С12F26 O4 MW:702 (Figure 4), where the fluorocarbonyl ionic series takes place, there is primary synchronous detachment of three radicals (- ·C3F6OCF3 and -2 ·F), in spectra of non-symmetrical perfluoropolyoxyalkylchlorides (Figure 7 and Figure 8) primary separation of three radicals does not occur. The mass spectra, shown at С9ClF19O2 (Figure 7) and С12ClF25O3 (Figure 8) of perfluoropolyoxyalkylchlorides have a similar structure and differ by one OC3F6 group. Similar branching of their series and secondary synchronous detachments of three radicals (- ·CF2, -2 ·F) takes place in a fluorocarbonyl ionic series (marked with blue color) with the same mass of ion with m/z 401. One fluorine atom is separated from the OCF2 group to form a terminal fluorocarbonyl group O=CF, and the other from the opposite terminal group C(CF3)2F. In the spectrum (Figure 7), there is also an emission of the OC2F4 molecule from the middle of the ion with m/z 313 in the spectrum shown at Figure 8. With an increase in the chain of oxygen atoms, there is a marked increase in the intensities of the ion peaks containing the fluorocarbonyl group (197, 147, 97, 47). The ionic series of perfluoroallyl ions is not formed, although a weak peak of a perfluoroallyl ion with m/z 131 (3%) in the spectrum is present. It is likely to occur by separating an oxygen atom from an ion with m/z 147 (Figure 8).
Three ionic series of the symmetrical molecule dichloride С16Cl2F22O4 MW:934 with four oxygen atoms mass spectrum are shown in Figure 9.
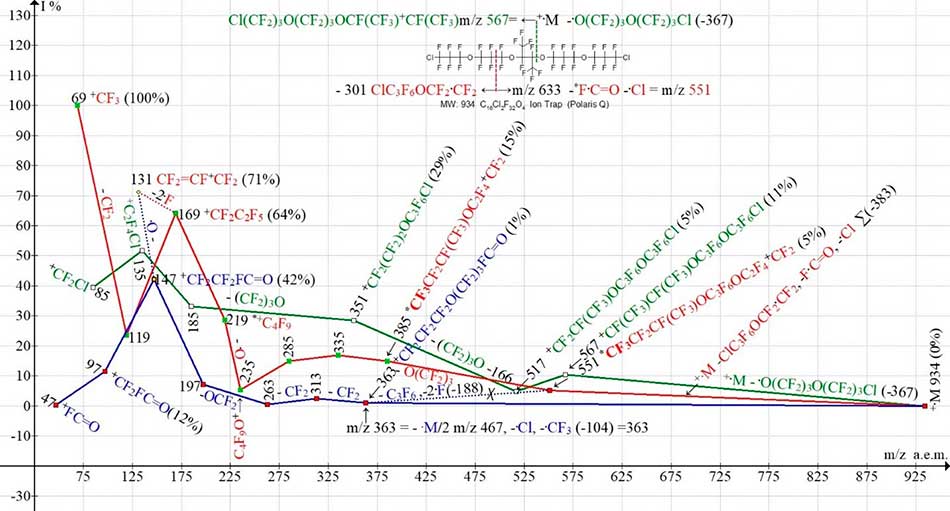
Figure 9. Three ionic series of С16Cl2F32O4 MW:934 Ion Trap (Polaris Q) mass spectrum.
The first rearrangement ion *CF3CF2CF(CF3)OC3F6OC2F4+CF2 with m/z 551 occurs in a polyoxoperfluoroalkyl ion series (marked with red color) as a result of the primary synchronous separations of three radicals: ClС3F6OCF2·CF2 (- 301), F·C=O (-47) and the second chlorine atom (‑35) Σ-383. The ion with m/z 551 contains a terminal group C4F9, which is not in the starting molecule. Fragmentation of this series results in perfluoroalkyl ions with m/z 219, 169, 119 and 69. The article [2] has been mistakenly reported that a secondary synchronous separation of three radicals also occurs in the same ionic series. The ion with m/z 551 fragmented with a secondary synchronous emission of the C3F6 molecule and two fluorine atoms forming a series of ions with a fluorocarbonyl terminal group (marked blue) with m/z 363, 313, 263, 197, 147, 97 and 47. The error in establishing the sequence of the fluorocarbonyl series occurrence was that two synchronous detachments of three radicals can not occur in the same ionic series since the maximum excitation energy of the molecular ion is necessary for one such separation. At Figure 9, the dotted line connecting an ion with m/z 551 and an ion with m/z 363 in the circuit, is crossed out. The most probable way of a fluorocarbonyl ionic series occurrence is the synchronous primary decomposition +·M/2 and separation of radicals ·Cl and ·CF3.
The intense peak (71%) of the perfluoroallyl ion CF2=CF-+CF2 with m/z 131 is not a peak of the corresponding allyl series. Optionally, it is formed from an ion +СF2CF2FC=O with m/z 147 when the oxygen atom is separated therefrom. Given the asymmetric ion structure with m/z 169 +CF2CF2CF3 (Figure 9), unlike the symmetrical ion structures +CF(CF3)2 in the spectra (Figure 7 and Figure 8), the intense perfluoroallyl ion 71% (Figure 9) may also occur by the detachments of two fluorine atoms from the ion with m/z 169.
Four ionic series of С7BrF15O2 mass spectrum are shown in Figure 10. Two bromine-containing series are marked with green color. One series of perfluoroalkyl is marked with red color and one series of ions containing fluorocarbonyl group is marked with blue color.
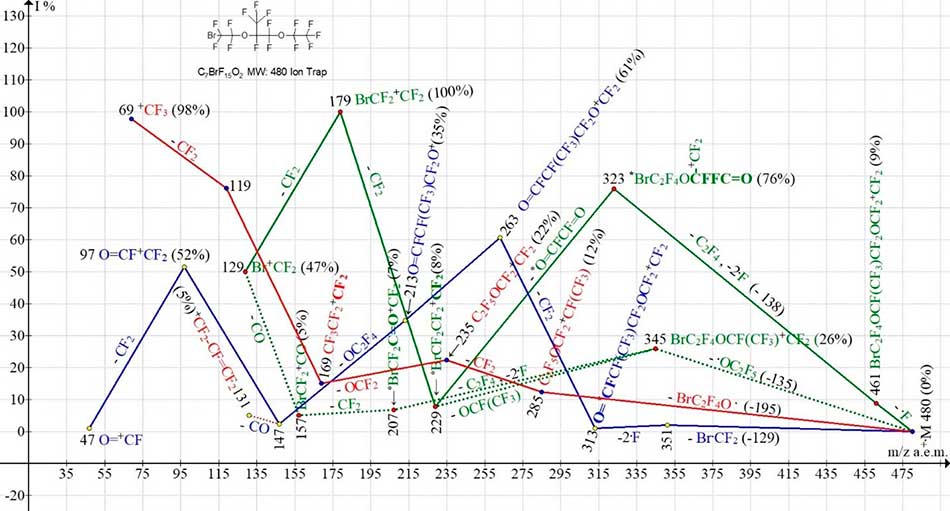
Figure 10. Four ionic series of С7BrF15O2 MW:480 Ion Trap (Polaris Q) mass spectrum.
The molecule is asymmetrical; therefore, it is possible that for this reason neither in one of the four ion series of the spectrum the primary synchronous separation of three radicals does not occur. However, "secondary" synchronous emissions of three radicals occur in two bromine-containing series after the ejection of an atom ·F or the radical ·OC2F5. In bromine-containing series of ions, with more intense ion peaks, after primary detachment of fluorine atom, synchronous separation of C2F4 molecule and two fluorine atoms takes place with formation of rearrangement ion BrC2F4O+CFCF2FC=O (m/z 323). The ion with m/z 323 ejects the dicarbonyl difluoroethane molecule O=CF-CF=O, turning into the BrC2F4+CF2 (m/z 229) ion. In less intensive bromine-containing series, after separation of ·OC2F5 with formation of ion BrC2F4OCF(CF3)+CF2 (m/z 345) and secondary synchronous release C2F4 and two fluorine atoms there is a rearrangement ion BrCF2C=O+CF2 with m/z 207. Subsequent detachment of CF2 to form ion BrCF2+C=O m/z 157 (5%) and release of CO (-28) complete ion Br+CF2 m/z 129 (47%). Series marked with blue color begins with successive separation of Br.CF2 and then two fluorine atoms. It is coming to an end by ions with a fluorocarbonyl group with m/z 197, 147, 97 and 47.
Marked with red color, dioxaperfluoroalkyl series starts with detachment of maximum by weight radical BrC2F4O. (-195 а.е.м.). Perfluoroalkyl ions +C3F7 m/z169, +C2F5 m/z 119 and +CF3 m/z 69 are formed therein after detachment of CF2 and ejection from the middle of the OCF2 chain.
Three ionic series of С11BrF23O3 mass spectrum are shown in Figure 11.
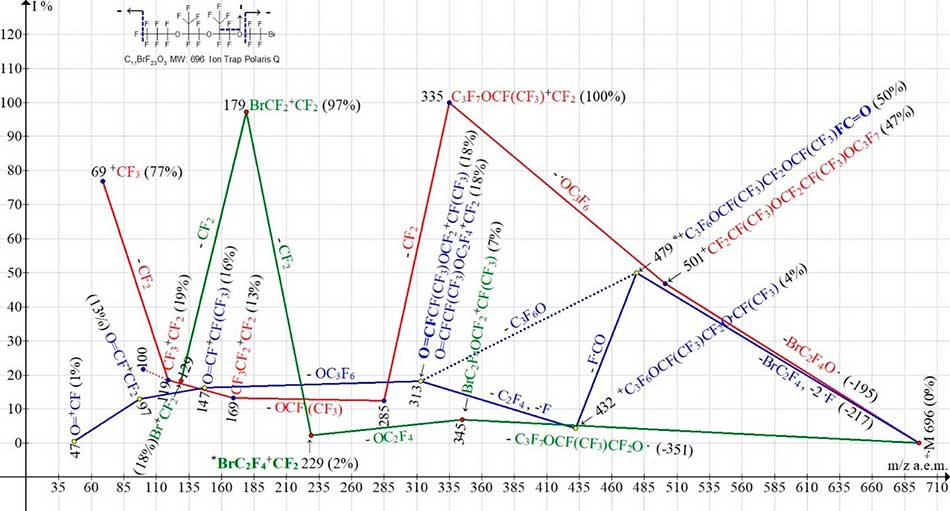
Figure 11. Three ionic series of С11BrF23O3 MW:696 Ion Trap (Polaris Q) mass spectrum.
Although the molecule is asymmetric, however, its fluorocarbonyl ion series (Figure 11) (marked with blue color) begins with the primary synchronous ejection of three radicals: BrCF2.CF2 and two fluorine atoms (C7, C1). Fragmentation of С11BrF23O3 (Figure 11) along with fragments of two other non-symmetrical perfluorooxahalides HalC2F4O(CF2)6CF3 (Figure 5, Figure 6) is the third exception when the asymmetric molecule with three oxygen atoms is fragmented with the primary synchronous separation of three radicals. The synchronous detachment of three radicals, which is marked on the structure of the molecule (Figure 11) by dotted lines, takes place from two terminal groups. This results in ion with m/z 479 with terminal fluorocarbonyl group. It is likely that it fragments in two ways with the formation of two ions with m/z 313, differing only by linear or nonlinear construction of its terminal group C3F6.
As a result of detachment from an ion with m/z 479 molecules -C3F6O, or three consecutive breaks (-FCO and -C2F4, -F), an ion with m/z 313 is formed, which forms a series of ions with a terminal fluorocarbonyl group: 147, 97 and 47. The first fragment ion +С3F6OC2F4Br with a mass of m/z 345 (7%) fragmented with the emission of OC2F4 and the formation of a rearrangement ion *BrC2F4+CF2 with an m/z 229 - group, which is not present in the initial molecule. The Figure 11 does not indicate a perfluoroallyl ion, which intensity does not exceed 6%.
Three ionic series of С16BrF33O5 mass spectrum are shown in Figure 12.
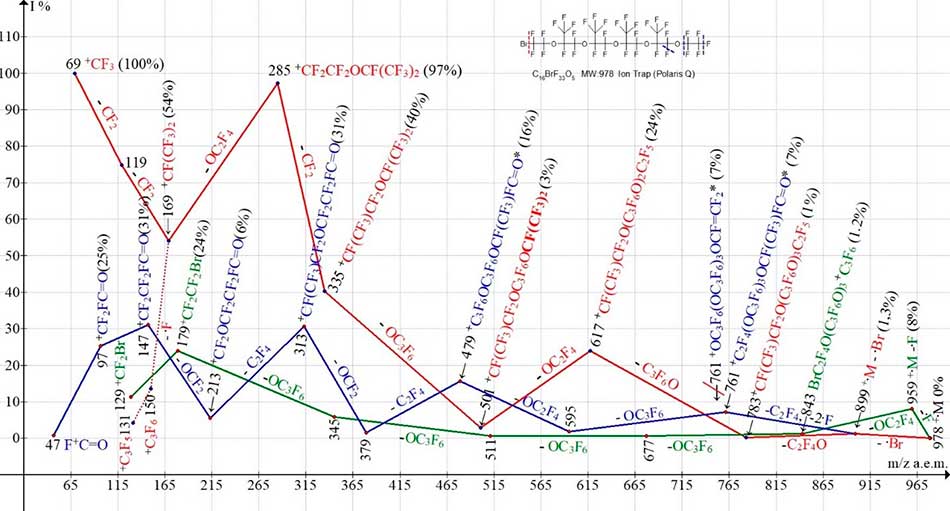
Figure 12. Three ionic series of С16BrF33O5 MW:978 Ion Trap (Polaris Q) mass spectrum.
Since the molecule C16BrF33O5 is asymmetrical, none of its ionic series of the primary synchronous separation of three radicals occurs. Primary detachments leading to the occurrence of two ionic series (Figure 12) are: the detachment of fluorine radical in the bromine-containing series (marked with green color) and the bromine radical in the oxaperfluoroalkyl series (marked with red color). After separation of bromine atom, oxфperfluoroalkyl series branches to form fluorocarbonyl series (marked with blue color). It should be noted that after the ejection of the ·Br atom, the secondary separation of two fluorine atoms and C2F4 occurs from the opposite terminal group. Otherwise, the resulting ion with m/z 761 would have a terminal group OCF=CF2, which contradicts further fragmentation detachments.
Four ionic series of a symmetrical molecule С6Br2F12O3 mass spectrum are shown in Figure 13.
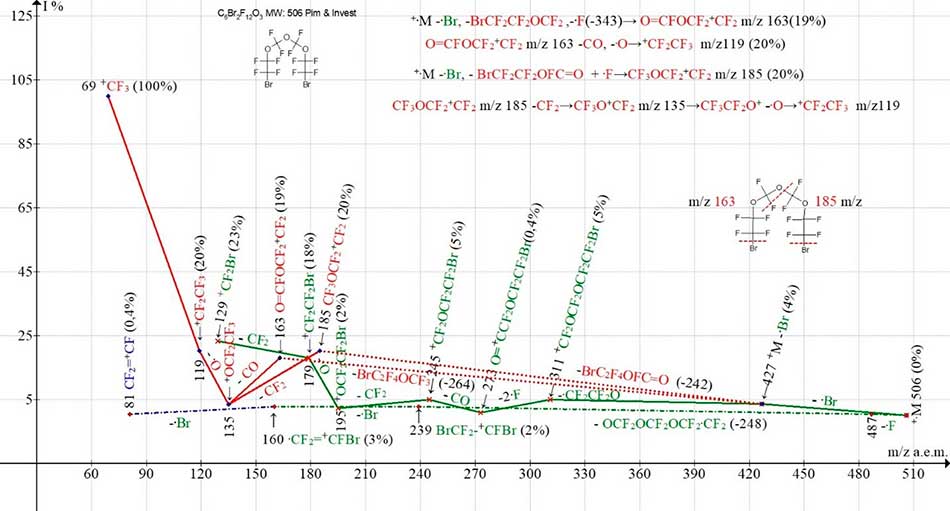
Figure 13. Four ionic series of С6Br2F12O3 MW:506 Ion Trap (Polaris Q) mass spectrum.
Despite the fact that the molecule С6Br2F12O3 is symmetrical, neither primary nor secondary synchronous detachments of the three radicals in any of its four ionic series does not occur. The presence of O-CF2-O-CF2-O central group in molecule results in asymmetric section of three oxygen atoms and one fluorine atom in two perfluorooxaalkyl series (marked with red color). After primary detachment of bromine atom, massive bromine-containing molecules are separated in these two ionic series: BrC2F4OFC=O (-242) and -BrC2F4OCF3 (-264). Both series ends with the formation of perfluoroalkyl ions. Two bromine-containing series marked with green color: more intense mono-bromine-containing series and less intensive dibromine-containing series, arise as a result of detachment of bromine atom and, respectively, emission of central group (OCF2)3CF2 with formation of ion BrCF2-+CFBr with m/z 239. In contrast to the ionic series of other compounds represented in the present report, no fluorocarbonyl series is formed in the С6Br2F12O3 spectrum (Figure 13). Instead, during separation from ion +CF2=CFBr c m/z 160 of bromine atom there is a single ion with perfluorovinyl group CF2=+CF m/z 81.
Four ionic series of a symmetrical molecule dibromide C14Br2F28O2 mass spectrum are shown in Figure 14.
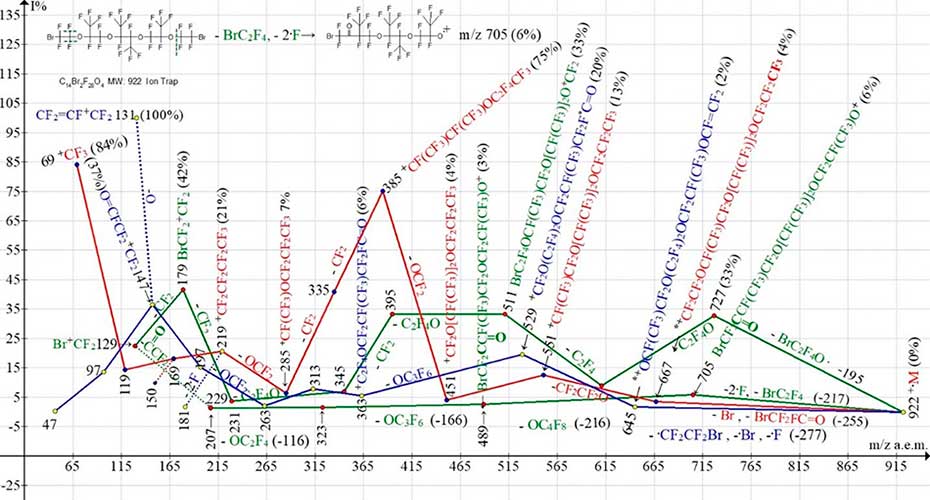
Figure 14. Four ionic series of С14Br2F28O4 MW:922 Ion Trap (Polaris Q) mass spectrum.
In the fragmentation of symmetric dibromide C14Br2F28O4 (Figure 14), four ion series occur. Two parallel bromine-containing series (marked with green color), one perfluorooxaalkyl series (marked with red color) and one perfluorocarbonyl, branching with formation of intense perfluoroallyl ion m/z 131 (marked with blue color). The synchronous primary detachments of three radicals take place in two ionic series. Namely, in a less intensive bromine-containing series and in a perfluorocarbonyl series (marked with blue color). Primary detachments in two bromine-containing series differs by the fact that in a series with more intense peaks BrC2F4O· takes place (-195), and in a fewer intensive series, primary synchronous separation of three radicals: -BrC2F4 and two fluorine atoms (Σ-217), from the opposite terminal group BrCF2CF2O. In less intensive bromine-containing series (ion with m/z 705) terminal group becomes group BrCF2C=O.
The next, the out-of-order emission of -OС4F8 in this series occurs from the middle of the chain. Presence of carbonyl group in ions with m/z 705, 489 and 207 confirms detachment from ion with m/z 229 of molecule O=CCF2 (-78), with formation of ion Br+CF2. Two different fragmentation paths +·M (C14Br2F28O4) to form two series of bromine-containing ions is a result of two different excitation energies +·M. The blue colored fluorocarbonyl series also begins with primary synchronous detachment of three radicals. So, as a result of separation from one of the terminal groups of the radical ·CF2CF2Br, and from the other terminal group ·Br and ·F there is a rearrangement ion with m/z 645 (2%), with a terminal group CF2=FC. This ionic series is completed by the formation of ions with a fluorocarbonyl group: 147, 97 and 47, as well as an intense perfluoroallyl ion m/z 131, which is likely to occur when the oxygen atom is separated from the ion with m/z 147. The red-marked series of polyoxaperfluoroalkyl ions begins with ejection of the bromine atom and separation of the rearrangement molecule BrCF2FC=O. As a result, a rearrangement ion with a terminal group CF3 (m/z 667) occurs. After a series of separations of oxygen-containing fragments, an ion with m/z 219 occurs, which fragments to form a series of perfluoroalkyl ions. Primary and secondary synchronous separations of three radicals, that occurs in ionic series of mass spectra of polyoxaperfluoroalkanes and polyoxaperfluoroalkyl halides (Figures 1-14), are presented in Table 1.
Table 1. Primary and secondary synchronous separations of three radicals (Figures 1-14).
|
MW |
Formula |
Figure number |
Primary and secondary synchronous separations of three radicals |
|
254 |
CF3CF2OCF2CF3 |
1 |
no |
|
570 |
CF3CF2CF2OCF(CF3)CF(CF3)OCF2CF2CF3 |
2 |
primary - CF3CF2·CF2, - 2 ·F |
|
686 |
CF3CF2CF2OCF(CF3)CF2OCF2CF2-C(CF3)3 |
3 |
primary - CF2, - 2 ·F occurred from one terminal group CF3CF2CF2 |
|
702 |
CF3O(CF2)3OCF(CF3)CF(CF3)O(CF2)3OCF3 |
4 |
primary - CF3OC2F4·CF2, - 2 ·F |
|
520 |
CF3(CF2)6OCF2CF2Cl |
5 |
primary - ClCF2·CF2, - 2 ·F (C2, C1) and (C7, C1) |
|
564 |
CF3(CF2)6OCF2CF2Br |
6 |
primary - BrCF2·CF2, - 2 ·F (C2, C1) and (C7, C1) |
|
536 |
ClCF2CF2CF2O(CF2)3OC(CF3)2F |
7 |
secondary - CF2 и - 2 ·F |
|
702 |
ClCF2CF2CF2O(CF2)3O(CF2)3OC(CF3)2F |
8 |
secondary - CF2 и - 2 ·F |
|
934 |
Cl(CF2)3O(CF2)3OCF(CF3)CF(CF3)O(CF2)3O(CF2)3Cl |
9 |
two primary - ·Cl, - F·C=O, - Cl(CF2)3OCF2·CF2 and - ·M/2, - ·Cl, - ·CF3 |
|
480 |
BrCF2CF2OCF(CF3)CF2OCF2CF3 |
10 |
secondary - C2F4 and - 2 ·F in two series |
|
696 |
BrCF2CF2O[CF2CF(CF3)O]2CF2CF2CF3 |
11 |
primary - BrCF2·CF2, - 2 ·F (C7, C1) |
|
978 |
BrCF2CF2O[CF(CF3)CF2O]4CF2CF3 |
12 |
secondary - C2F4 and - 2 ·F |
|
506 |
Br(CF2)2OCF2OCF2O(CF2)2Br |
13 |
no |
|
922 |
Br(CF2)2OCF(CF3)CF2OCF(CF3)CF(CF3)OCF2CF(CF3)O(CF2)2Br |
14 |
two primary - BrC2F4, - 2 ·F and - BrC2F4, - ·Br, - ·F |
In column 4 of Table 1, the color of separating radicals (red, blue or green) corresponds to the perfluoroalkyl, perfluoroalkenyl and perfluorohalogenated ionic series, in which these detachments take place. From the fourteen compounds presented in (Table 1), in the mass spectra of seven compounds (Figures 2, 4, 5, 6, 9, 11 and 14), primary synchronous detachments of three radicals occur. They occur both in symmetrical compounds (Figures 2, 4, 9, 14) and in asymmetric compounds (Figures 5, 6) with one oxygen atom, as well as with three oxygen atoms (Figure 11). Primary synchronous detachments of three radicals do not occur in a symmetrical connection with one oxygen atom (Figure 1) and in a symmetrical compound (Figure 13) with a central group of OCF2OCF2O. In an asymmetric connection with the terminal group OC(CF3)3 (Figure 3), the primary synchronous detachment takes place only from one, terminal CF3CF2CF2O group. In four asymmetrical compounds: (Figure 7 and Figure 8) with a terminal group O(CF3)2F, as well as (Figure 10) and (Figure 12) instead of primary separations of three radicals, secondary separations of radicals occurs. Due to the maximum excitation energy +·M, the detachment of three radicals can be secondary. So, depending on the structure of the molecular ion, and the distribution of energy on the bonds, it is likely that in some cases the primary separation of three radicals may also include less energy-consuming, primary separation of one or more radicals of another ionic series.
The article [1], devoted to the primary synchronous separation of three radicals in the mass spectra of symmetrical linear molecules of perfluoroeicosane and eicosane, was represented by a spectrum of asymmetrical non-linear perfluoro-2,4-dimethyl-3-ethylpentane (Figure 1) C9F20 molecula. The primary detachment of three fluorine atoms and the subsequent emission of CF2 in the C9F20 spectrum was also interpreted as for a linear perfluoroeicosane molecule. This was not right, since in the C9F20 spectrum, the first fragment ion was ion +·M-F with m/z 469 (0.4%) and, consequently, synchronous detachment of three fluorine atoms could only be secondary. Unlike ionic series of linear molecules, namely, the non-linear structure of C9F20 is likely to cause the coincidence of the match of the primary detachment of the fluorine atom in the perfluoroalkyl ionic series to form an ion with m/z 469 and a secondary synchronous ejection of three radicals (-CF2,-2 ·F) in a perfluoroalkenyl series. Ionic series of non-symmetrical non-linear perfluoro-2,4-dimethyl-3-ethylpentane molecule are presented at (Figure 15).
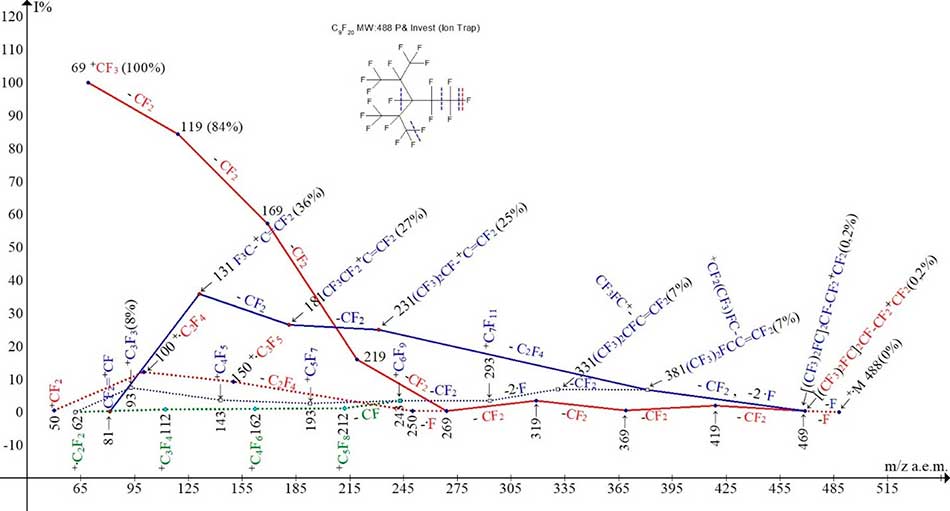
Figure 15. Ionic series of perfluoro-2,4-dimethyl-3-ethylpentane C9F20 MW:488 mass spectrum.
In contrast to linear perfluoroeicosane, that fragments by the primary synchronous discharge of three fluorine atoms, and then CF2 detachment, there is another sequence of separations in the C9F20 (Figure 15) spectrum: -·F, (-CF2, -2 ·F). Branching of two ionic series takes place immediately after detachment of fluorine atom. Ions with m/z 469 (Figure 15) have the same mass, but different excitation energies, since they fragments to form two ionic series: perfluoroalkenyl (last significant digit of mass -1, marked with blue color) and perfluoroalkyl (last significant digit of mass -9, marked with red color). In spectrum C9F20 (Figure 15) as a result of secondary synchronous detachment of three radicals CF2 and two atoms ·F a first fragment ion of perfluoroalkenyl series with m/z 381 occurs. Eight separations of CF2 from ion with m/z 469 completes the basic peak +CF3 less energy-consuming, but more intensive perfluoroalkyl series. So, in the alkenyl ionic series of the non-symmetric C9F20 molecule as in asymmetric molecules of polyoxaperfluoroalkanes and polyoxaperfluoroalkyl halides, secondary detachments of three radicals (Figure 15) occurs. The primary or secondary of the maximum excitation energy of the synchronous separations of three radicals depends on the symmetry of the molecule, its terminal groups, and the number and frequency of the oxygen links.
It should be noted that the synchronous separation of three fluorine atoms in one of the five ionic series of the perfluorocyclohexane mass spectrum is also secondary separation since it precedes the cycle break [1].
Conclusions
In the case of fragmentation of perfluoropolyoxaalkanes and perfluoropolyoxaalkyl halides, depending on the symmetry of the terminal groups, the difference in their masses and the number of oxygen atoms, primary or secondary separations of three radicals takes place. In the spectrum of symmetric compound CF3C2F4O(CFCF3)2OC2F4CF3 MW:570 (Figure 2), containing two oxygen atoms, synchronous primary detachment of three radicals (-·CF2CF2CF3 and -2 ·F) takes place from two terminal groups. After an emission of radical -·CF2CF2CF3, the detachment of two fluorine atoms takes place from two terminal groups (one fluorine atom from the new terminal group arising after ejection of -.CF2CF2CF3, the other from the opposite terminal group). In the spectrum of the non-symmetrical CF3C2F4O(CFCF3)CF2OC2F4OC(CF3)3 MW:686 (Figure 3) molecule, with the terminal group C(CF3)3, the three radicals (-·F, -CF2, -·F) occurs only from one opposite terminal group CF2CF2CF3. In the spectrum of symmetric compound C12F26O4 MW:702 (Figure 4), with terminal groups OCF3, synchronous primary detachment of three radicals (-·C3F6OCF3, -2 ·F) takes place from two terminal groups.
In the spectra of two asymmetric C9HalF19O compounds, where Hal = Cl, Br MW:520 and MW:564 (Figures 5 and 6) with one oxygen atom, the primary synchronous separation (‑·C2F4Hal, -2 ·F) occurs from two terminal groups.
In the spectrum of symmetric compound C16Cl2F32O4 MW:934 (Figure 9), with terminal groups OC3F6Cl, synchronous primary separations of three radicals occur in two ionic series: (‑·C2F4OC3F6Cl, -F.C=O, -·Cl) in perfluoroalkyl and (- M/2, -·Cl, -·CF3) in the fluorocarbonyl series. In the spectrum of symmetric compound C14Br2F28O4 MW:922 (Figure 14), with terminal groups OC2F4Br synchronous primary separation of three radicals also occur in two ionic series: (- C2F4Br, - ·Br, - ·F) in the fluorocarbonyl series and (-C2F4Br, -2 ·F) in bromoperfluoroalkyl.
Four asymmetric monohalides ionic series are exceptions, in which instead of primary three radicals synchronous detachments occur secondary synchronous detachments.
In the spectra of two asymmetric compounds: ClC3F6OC3F6OCF(CF3)2 MW:536 (Figure 7) and ClC3F6(OC3F6)2OCF(CF3)2 MW:702 (Figure 8), with a terminal group CF(CF3)2, after primary detachments: (-·C2F4Cl in Figure 7) and (-·OC3F6Cl, then -C2F4 in Figure 8) occurs an ion +CF2OC3F6OCF(CF3)2 with m/z 401, that fragments with a secondary emission of three radicals (-CF2, -2 ·F Figures 7, 8). It is possible that the presence of massive terminal groups CF(CF3)2 with m/z 169 or C(CF3)3 with m/z 219 (Figure 3) in asymmetric molecules (Figures 7, 8) causes a change in the order of radicals’ detachment. The secondary synchronous breaks of three radicals also occur in the ionic series of two non-symmetrical, mono-bromine-containing compounds: BrC2F4OCF(CF3)OC2F5 MW:480 (Figure 10) and BrC2F4(OCFCF3)4OC2F5 MW:978 (Figure 12). In the spectrum (Figure 10), the secondary emission of three radicals takes place in two bromine-containing ionic series, and in the spectrum (Figure 12), the secondary detachment of three radicals occurs after the bromine atom ejection, with the formation of a fluorocarbonyl ionic series.
Common fragmentation patterns for representing mass spectra usually comprise only the most informative and intensive ions of the spectrum, without detailed sequences establishment of their formation and decay. Ionic series analysis of mass-spectrum makes it possible to include practically all ions in fragments series and to avoid errors in considerable degree when determining their formation and decomposition sequence.
The mass spectrum of the compound with the sequences of fragmentation-ion series established is more informative than the traditional scheme of occurrence possible in terms of the structure and chemistry of the pathways for the most intense ions. The primary detachments taking place in the ionic series of the spectrum make it possible to compare their relative energy.
Acknowledgements
This work was supported by the Ministry of Science and Higher Education of the Russian Federation and was performed employing the equipment of Center for Molecular Composition Studies of INEOS RAS.
References
- N.D. Kagramanov, Ratios of primary separations in ionic series of mass-spectra of perfluoroalkanes, perfluorocyclohexane, eicosane, cyclotriacontane, containing regular fragment groups (C2H4 or CF2), Fluorine notes, 2023, 6(151), 1-2.
- N.D. Kagramanov, S.R. Sterlin, A.A. Tyutyunov, Decomposition sequences - Ion series of mass spectra of polyoxaperfluoroalkanes and polyoxaperfluoroalkyls with terminal halide atoms, Fluorine notes, 2023, 2(147), 1-2.
- V.A. Grinberg, A.A. Tyutyunov, N.D. Kagramanov, N.A. Mayorova, E.I. Maevskii, S.R. Sterlin, The peculiarities of electrochemical behavior of ω-bromopolyoxaperfluorocarboxylic acids, Fluorine notes, 2017, 2(111), 1-2.
- 2. N.D. Kagramanov, Three series ions of perfluorotributylamine mass-spectrum (PFTBA), Fluorine notes, 2020, 3(130), 1-2.
ARTICLE INFO
Received 15 February 2024
Accepted 20 March 2024
Available online April 2024
Recommended for publication by PhD M.A. Manaenkova
eLIBRARY Document Number (EDN) KOHFTE

Fluorine Notes, 2024, 153, 1-2
