Received: December 2023
DOI 10.17677/fn20714807.2024.01.02
Fluorine Notes, 2024, 152, 3-4
METHODS OF SYNTHESIS OF PERFLUOROSULFONYL VINYL ETHERS (PSVE)
O.S. Bazanova, A.S. Odinokov, E.V. Irisova, V.G. Barabanov
JSC Russian Scientific Center “Applied Chemistry” (GIPH), 193232, Krylenko str., 26A, Saint Petersburg, Russia
Abstract: the main methods of obtaining perfluorosulfonylvinyl ethers - 1,1,2,2-tetrafluoro-2-[(trifluorovinyl)oxy]ethanesulfonyl fluoride (SSC monomer) and 1,1,2,2,3,3-hexafluoro3-[(trifluorovinyl)oxy]propanesulfonyl fluoride (PSPVE monomer), which are raw materials for the production of perfluorinated polymer ion-exchange membranes used to create fuel cells.
Keywords: perfluorovinyl ethers, monomers, synthesis, copolymerization, perfluorinated membranes, fuel cells.
Introduction
Hydrogen fuel cells (HFCs) are considered to be future world energy due to their efficiency and environmental safety. In the HFC, the energy of the chemical reaction is converted into electrical energy directly, without the need for first converting it into heat or mechanical work of the rotation of the turbines. The energy conversion efficiency of the fuel cells is substantially higher than in thermal power plants. Furthermore, the fuel used herein is hydrogen and oxygen, and the waste is water vapour.
Perfluorinated membranes, such as Nafion from Du Pont, Aquivion from Solvay Solexis Technology, Acipex from Asahi Glass Company, domestic membranes MF‑4SK, etc. are widely used in the manufacture of low temperature fuel cells. The process of producing an ion-exchange membrane for a fuel cell consists of the following steps:
- synthesis of perfluorovinyl ethers containing a sulfogroup (-SO2F)
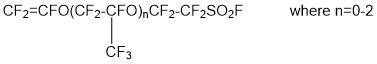
- copolymerization of synthesized perfluorovinyl ether with tetrafluoroethylene to yield copolymer:
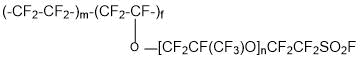
- production of ion-exchange membranes on the basis of copolymer:
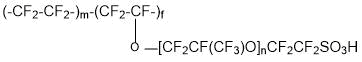
Practically the only industrial type of ion-exchange membranes has long time perfluorinated sulfocationic membranes of Nafion type. The raw material for the production of this type of membrane is tetrafluoroethylene and perfluoro (3,6-dioxa-4-methyl-7-octene) sulfonyl fluoride (PSEPVE monomer). The main advantage of said membranes is the high conductivity and chemical resistance, and the disadvantage is low working temperatures (not more than 90°C).
Membranes of Aquivion type (copolymer of tetrafluoroethylene and 1,1,2,2-tetrafluoro-2-[(trifluorovinyl)oxy]ethanesulfonyl fluoride (SSC monomer):
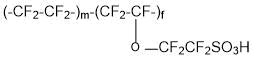
and membranes of the type Acipex (copolymer of tetrafluoroethylene and 1,1,2,2,3,3-hexafluoro-3-[(trifluorovinyl)oxy]propanesulfonyl fluoride (PSPVE monomer):
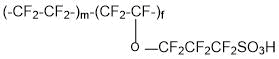
are operable at a temperature of 130°C and higher. Replacement of Nafion membranes with perfluorinated Aquivion and Acipex makes it possible to eliminate the probability of catalyst Pt poisoning in catalytic layers of fuel cells due to sorption equilibrium change of carbon monoxide (CO) and hydrogen on Pt.
Тhe establishment of ion-exchange membranes production in Russian Federation is possible only after the creation of manufacturing suitable fluorine-containing sulfomonomers (perfluorovinyl ethers). The technology of producing PSEPVE monomer (monomer for producing a Nafion type membrane), which is the most complex step for synthesis of ion-exchange membranes, is already developed in the Russian Federation [1]:
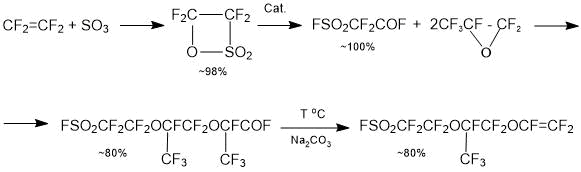
The technologies for producing fluorosulphonylperfluorovinyl ethers SSC monomer and PSPVE monomer for membranes of the Aquivion and Acipflex type are carried out in a research step, and thus the choice of optimal synthesis is an urgent problem.
In the present time, most of the studies known in the scientific and technical literature directed to the development of methods for producing perfluorovinyl ethers (including monomers SSC and PSPVE) are based on the use of three main methods (or combinations thereof):
Method 1. Processes based on reactions of perfluoroacyl fluorides with hexafluoropropylene oxide (hereinafter - HFPO) in the presence of catalysts (KF, CsF), followed by decomposition of addition products (hydrolysis, pyrolysis) [1–4];
Method 2. Processes based on dechlorination of 1,2-difluoro-1,2-dichloroethylene adducts with perfluorohypofluorites, which are obtained by catalytic fluorination of perfluoroacyl fluorides with elementary fluorine [5-10];
Method 3. Processes based on reactions of halogenation and dehalogenation of substrates of different nature [4, 11, 12].
For all three methods, synthesis of hard-to-reach starting materials and the need to use reagents which are dangerous in handling can be problematic. Despite extensive leading investigations, highly efficient synthesis methods have not been developed for perfluorovinyl ethers, which would make it possible to produce them using simple process schemes with high yield and low cost.
The aim of the present invention is to watch possible promising methods for synthesizing SSC and PSPVE perfluoromonomers.
SSC monomer producing methods
Method 1.1. The “standard” method, based on the addition to fluorosulphonyl difluoroacetylfluoride of one molecule of HFPO followed by pyrolysis of the addition product according to the scheme (1) for the synthesis of the SSC monomer cannot be used due to the fact that, instead of the target product, it leads to the production of cyclic sultone (I) [13, 14]:
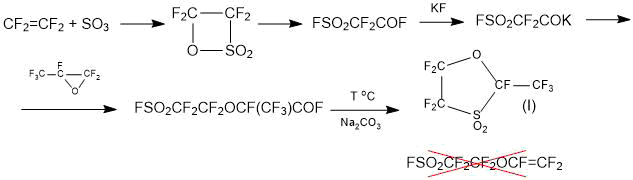
Scheme 1.
Modified variants of the Scheme 1 are developed for the synthesis of the SSC monomer.
Version 1. Monomer SSC can be obtained if it is necessary to replace HFPO with chloropentafluoropropylene oxide, Scheme 2 [15]:
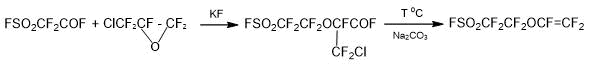
Scheme 2.
The main disadvantage of the method is the low availability of chloropentafluoropropylene oxide and the impossibility of its synthesis with high output. For this reason, the Scheme 2 cannot be considered as an effective method of producing a low-cost SSC monomer.
Version 2. Production of SSC monomer according to Scheme 3 [16, 17]:
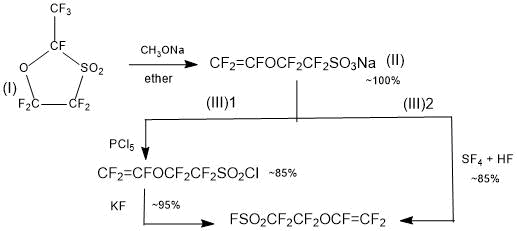
Scheme 3.
It is shown [16], that the reaction of sulfone (I) with an equimolar amount of an alkali metal alcoholate in an inert solvent results in a ring opening and, with a substantially quantitative yield, a sodium sulfonate of perfluorovinyl ether (II) is formed. The latter according to Schemes 3(1) [16] or 3(2) [17] can be converted into SSC monomer.
Method 1.2. The Solvay Solexis сompany proposed the method for producing SSC monomer, based on the reaction of perfluoro-2-fluorosulphonyl ethylhypofluorite (I) with 1,2-difluoro-1,2-dichloroethylene at low temperature, followed by dehalogenation of the formed adduct according to Scheme 4:
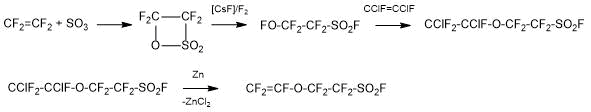
Scheme 4.
An Ausimont Company’s patent [6] protected a single-step process for producing hypofluorite (I), based on the continuous transmission of perfluoro-β-sultone (II) in a mixture of fluorine and inert gas (nitrogen) above the fluorinated catalyst (CsF, KF), which was either fixed on a substrate with good heat exchange (copper or its alloys), or suspended in a liquid medium or an inert solvent, with continuous removal of the reaction products from the gas phase. Conversion and purity of hypofluorite are close to 100%. The process eliminates the necessity of carrying out the stage of isomerisation of perfluoro-β-sultone (II) to fluorosulphonyl perfluoroacetylfluoride.
Method 1.3. A method for producing SSC monomer according to Scheme 5 [12] is proposed by Daikin:
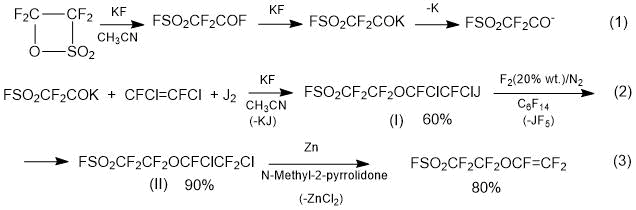
Scheme 5.
At stage 1, fluorosulphonylacetylfluoride (fluoroanhydride isomer of tetrafluoroethane-β-sultone) with 1,2-difluoro-1,2-dichloroethylene and iodine (catalyst - KF, CsF) is reacted to obtain iodine-substituted ether (I) (1,1,2,2-tetrafluoro-2-[(3,4-difluoro-3,4-dichloro-4-iodoethane)oxy]ethanesulfonylfluoride) (I); yield is 60%.
At stage 2, by treating the iododerivative (I) with a gas mixture F2/N2, an exchange reaction J/F is carried out and Cl,F-substituted ether (II) (1,1,2,2-tetrafluoro-2-[(3,4,4-trifluoro-3,4-dichloroethane)oxy]ethanesulfonyl fluoride) is obtained; the yield is ~ 90%.
At stage 3, Cl,F-substituted ether (II) with zinc in an organic solvent (N-methylpyrrolidone) are dechlorinated and perfluorovinyl ether (III) (monomer SSC) is obtained; yield is ~ 80%.
The proposed scheme makes it possible to synthesize perfluorovinyl ether with a sufficiently high yield and can be considered as a promising method for producing the SSC monomer.
PSPVE monomer producing methods
Method 2.1. It is shown [18], that in electrochemical fluorination of 1,3-propanesultone and 1,4-butanesultone in liquid HF with a sufficiently high yield, the cycles are opened and the sultones are isomerized into perfluoro-3-fluorosulphonyl propionyl fluoride of formula FSO2CF2CF2COF and perfluoro-4-fluorosulphonyl butanoyl fluoride of formula FSO2CF2CF2CF2COF, respectively.
This makes it possible to use a simple “standard” method for producing a PSPVE monomer, which is based on binding to perfluoro-3-fluorosulphonyl-propionyl fluoride of one molecule of HFPO, followed by pyrolysis of the addition product according to Scheme 6:
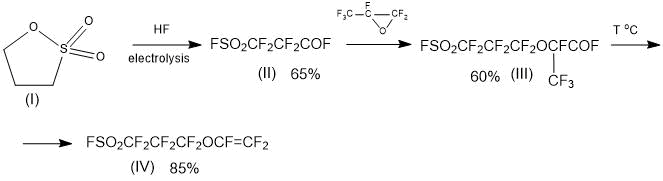
Scheme 6.
The proposed Scheme 6 can be considered as a promising method for producing the PSPVE monomer.
Method 2.1. Method of producing PSPVE monomer, based on “traditional” synthesis methods, was developed by Asahi Glass Company 40 years ago [4, 18, 19] and, to present time, with insignificant modifications remains practically significant method of its synthesis according to the following general Scheme 7 [4]:
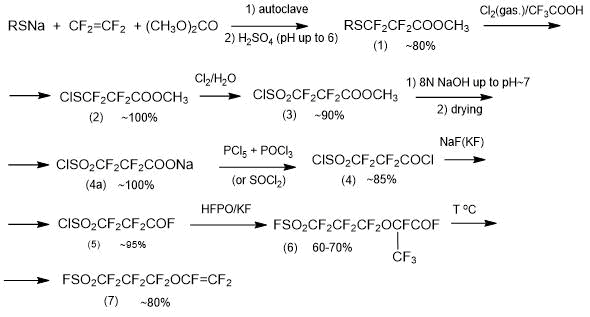
Scheme 7.
In the first step, by reacting tetrafluoroethylene with a carboxylic acid ether (preferably with dimethyl carbonate) and mercaptide of formula RSM (R is alkyl (Me, Et, Bu, Am), M is an alkali metal (Na, K)) in an inert solvent (tetrahydrofuran, ether, etc.), an ether of formula RSCF2CF2COOCH3 is obtained with yield of ~80%. Tetrafluoroethylene is used in gaseous state. It can be introduced into the reaction system at any pressure-reduced (preferably) or increased. The process is carried out until the tetrafluoroethylene pressure is constant under the reaction conditions. The permissible temperature range is 0-80°C. The reaction, if possible, is carried out at a reduced temperature to prevent the formation of a ketone of formula (RSCF2CF2)2CO (by-product of synthesis).
All semi-products and the end product of synthesis are obtained with high output, which enables, despite the multi-step process, to consider the possibility of its successful use for preparative production of monomer PSPVE.
Conclusion
Based on the literature data, the most promising methods for producing fluorosulphonyl perfluorovinyl ethers are:
- for monomer SSC of formula FSO2CF2CF2OCF=CF2 method 1.1 (version 2), which enables to obtain SSC monomer with yield of not less than 85%;
- for monomer PSPVE of formula FSO2CF2CF2CF2OCF=CF2 method 2.1, which enables to obtain PSPVE monomer with yield of not less than 80%.
The proposed methods can be used to develop large-scale methods for producing SSC and PSPVE monomers based thereon.
References
- Fluorine-containing membrane-catalytic polymer materials and systems (Collection of scientific papers), Edited by Barabanov V.G., Kornilov V.V., Maksimov B.N., St. Petersburg, Silver Age, 124 p., 2010, ISBN 978-5-902238-70-6.
- Arcella V., Ghielmi A., Tommasi G., Ann. N. Y. Acad. Sci., 2003, 984, 226-44.
- Process for producing fluorinated vinyl ether, US 7196235 B2, 2007.
- Fluorierte vinylaetherverbindungen, ihre herstellung und verwendung, DE 3047439 A1, 1981.
- Method of obtaining chlorofluoroalkyl esters, SU 1533624 A3, 1989.
- Process for preparing Hypofluorites and bis-hypofluorites, EP 0259817 A2, 1987.
- Method and image processing system for reconstruction of an image, EP 0269993 A2, 1991 (see also Process for producing 2-bromo-perfluoroethyl-hypofluorite, SU 1784040 A3, 1992).
- Process for the preparation of fluorooxy-fluorosulfonyl compounds by direct fluorination of fluoro-beta-sultones, EP 0384261 A1, 1990.
- Fluorinated derivatives of bis-vinyloximemetane (versions), and based on them polymers and copolymers, RU 2144044 C1, 1995.
- Process for the production of ethers, typically thf, US 6936727 B2, 2005.
- Production of perfluoro(alkyl vinyl ethers), US 5350497 A, 1994.
- Pat. US5616813, Vinyl ether compound, process for producing the same and copolymer containing the same, 1997.
- 1) Sulphonic acid derivatives of fluorocarbon vinyl ethers, and polymers thereof, GB 1034197 A, 1966; 2) Novel Fluorinated Compounds, GB 2053902 A, 1980.
- Fluorocarbon ethers containing sulfonyl groups, US 3301893 A, 1967.
- Trichlorotrifluoropropylene oxide and process for producing the same, Pat. JP4666107, 2011.
- Preparation of sulfoncacid containing fluorocarbon vinyl ethers, US 3560568A, 1971.
- Process for producing fluorinated fluorosulfonylalkyl vinil ether, US 7005545 B2, 2006.
- Process for the preparation of (omega-fluorosulfonyl) haloaliphatic carboxylic acid fluorides, EP 0062430 A1, 1982.
- Fluorinated copolymers and cation exchange membrane and process for producing the same, GB 2051831 A, 1981.
ARTICLE INFO
Received 07 December 2023
Accepted 18 January 2024
Available online February 2024
Recommended for publication by PhD M.A. Manaenkova
eLIBRARY Document Number (EDN) UENIJD

Fluorine Notes, 2024, 152, 3-4
