Received: January 2024
DOI 10.17677/fn20714807.2024.01.01
Fluorine Notes, 2024, 152, 1-2
COMPARISON OF STRUCTURE CHARACTERISTICS, PHYSICAL AND CHEMICAL PROPERTIES OF FLUORINATED POLYETHER PFPE-216 AND ITS HYDROCARBON ANALOGUES
Peganova N. V.1,2, Murav'ev A. A.1,3, Mokrushin I. G.2,4, Litvinenko E. V.2, Voronina K. А.1, Krivchun M. N. 5, Ibragimova R. I.1,6
1 St. Petersburg State University of Industrial Technologies and Design, SPbSUITD
2 Joint Stock Company "Russian Scientific Center "Applied Chemistry (GIPH)", JSC "RSC "Applied Chemistry (GIPH)"
3 Institute of Macromolecular Compounds Russian Academy of Sciences, IMC RAS
4 Federal State Autonomous Educational Institution of Higher Education "Perm State National Research University", Perm State University
5 Federal State Budgetary Educational Institution of Higher Education "State Institute of Technology Saint Petersburg Russia", SITSPR
6 Joint Stock Company "Polymetal Management", JSC "Polymetal Management"
chem_se@mail.ru, antonmuravyev@list.ru, mig@psu.ru
Abstract: The structures and some physico-chemical characteristics of fluorinated polyether PFPE-216 molecules were refined by 19F NMR and differential scanning calorimetry (DSC). Quantum chemical calculations were carried out. Proposed method for quantum chemical calculations has shown high convergence with literature data for lengths and angles of bonds, as well as on the distribution of charges of atoms of the molecule.
Key words: fluorinated ether, glass transition temperature, heat capacity, differential scanning calorimetry (DSC), dipole moment, quantum-chemical methods of calculation, polyethers, NMR.
Technologies for the synthesis of perfluorinated organic compounds are developed in JSC "RSC "Applied Chemistry (GIPH)" for several decades [1]. For the purposes of the technology and medicine, new solvents and effective surface-active substances are needed, the properties of which are established over a wide range of existence parameters and, importantly, their recycling and degradation. It is known that at a normal conditions perfluorinated compounds are highly persistent to temperature and chemical effects, therefore, to reduce their resistance and impart certain other special properties, it is practically possible to introduce heteroatoms such as oxygen, nitrogen, sulfur, phosphorus and some others into carbon skeleton. In order to create such compounds, a complex of several methods [2] has to be used, and the resulting compounds can differ in their structure [3,4]. The composition and the structure of the resulting compounds are determined by nuclear magnetic resonance spectroscopy, in particular, on fluorine nuclei, in which each signal can be identified by a wide range of chemical shifts, and the fluorine atoms in the 19F NMR have a 100% intensity [5]. The method can be used for qualitatively confirming the structure and quantifying the content of fluorine-containing compounds in any mixtures, both in the form of solutions and in emulsions.
GIPH produced polyether PFPE-216 [2], developed instead of aliphatic fluoroorganic liquids, is an electrodimerization
product according to the equation
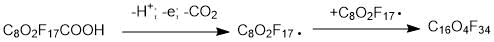
may have different structures (PFPE-216-1, PFPE-216-2 and PFPE-216-3), shown below (Figures 1-3) due to radical rearrangement. By-products are typical for this type of radical reactions.
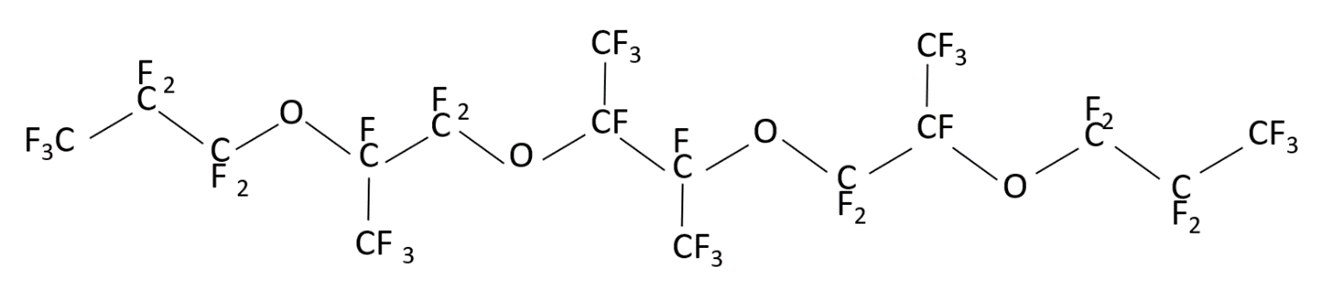
Figure 1. Structure of the possible product PFPE-216-1(1,1,1,2,2,3,3,5,6,6,8,9,11,11,12,14,14,15,16,16,16-docosafluoro-5,8,9,12-tetrakis (trifluoromethyl)-4,7,10,13-tetraoxahexadecane).
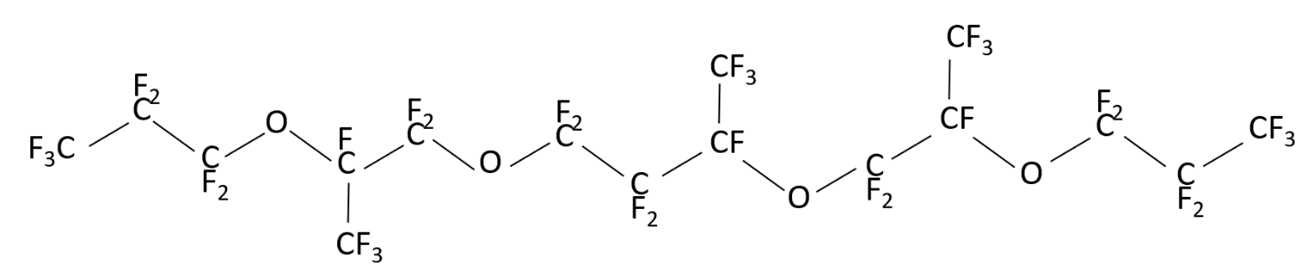
Figure 2. Structure of the possible product PFPE-216-2(1,1,1,2,2,3,3,5,6,6,8,8,9,10,12,13,15,16,17,17,17-pentacosafluoro-5,8,13-tetrakis (trifluoromethyl)-4,7,11,14-tetraoxaheptadecane).
Figure 3. Structure of the possible product PFPE-216-3 (1,1,1,2,2,3,3,5,6,6,8,8,9,10,1011,11,13,13,14,16,17,1718,18,18-octacosafluoro-5,14-bis(trifluoromethyl)-4,7,12,15-tetraoxooctadecane).
The structural formulas are shown in the Figures 1 to 3. The differences between them are concentrated in the central part of the molecule located between the second at any end of the molecule with ether oxygen groups: this is either linear structure of the central link from the CF2 groups, or one CF3 group, or two CF3 groups. The set of such isomers is typical for radical reaction products, but the ratio of a specific structure’s molecules number depending on the initiation methods, solvent and synthesis temperature, can vary significantly.
The PFPE-216 molecules structures were refined by the 19F nuclear magnetic resonance method
(Bruker Avance III HD 400 spectrometer) [6] according to the external DMSO stabilization (Figure
4).
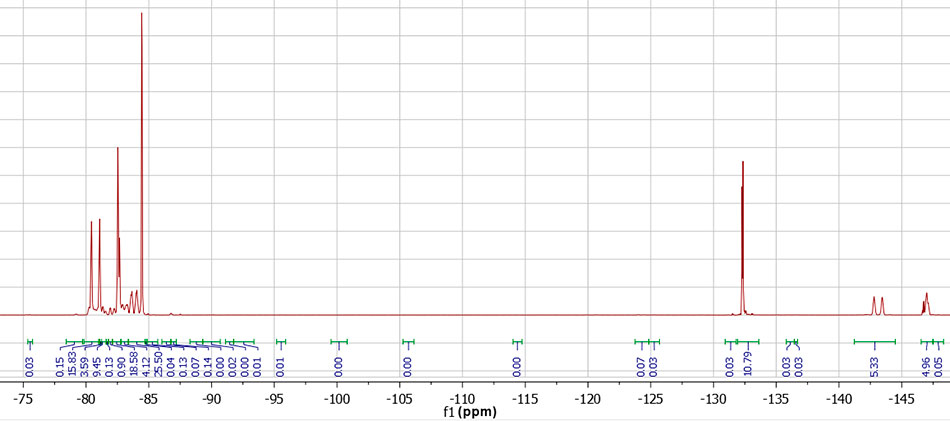
Figure 4. NMR spectrum 19F PFPE-216. NMR spectrum transcription: 19F NMR (376 MHz, DMSO-d6) δ -80.77 (d, 6F, CF3 central, J = 240 Hz), -82.47 – -82.80 (m, 6F, CF3 polyether), -80.14 – -84.21 (m), -84.40 – -84.55 (m, 6F, CF3 terminal), -132.33 (m, 4F, CF2 terminal), -143.11 (d, 2F, CF central, J = 240 Hz), -146.60 – -147.24 (m, 2F, CF polyether).
According to the NMR data, the structure PFPE-216-1 (Figure 1), PFPE-216-2 is about 1%. The PFPE-216-3 isomer is fixed in trace amounts and therefore is not discussed further.
The thermal stability of a particular molecule is largely associated with the natural ability to dissipate an external energy effect and depends on the nature thereof. One of the thermal stability components is the heat capacity – the important practical characteristic of the polyfluorinated ethers. Owing to the construction and complete replacement of hydrogen with fluorine, the heat capacity of the fluoro-substituted polyethers is small as a function of temperature, and in order to confirm this, the dependence of the heat capacity on the temperature has been studied.
Figure 5 shows the results of measuring the dependence of the heat capacity on the temperature of the fluorine-substituted polyether PFPE-216 on a differential scanning calorimetry device (DSC) Netsch DSC Polyma 214 by comparison with a polycrystalline corundum reference. The heat capacity of the fluorine-substituted PFPE-216 has been studied in a wide range of temperatures from -135 to 120°C in a sealed crucible. Transition of vitrification at -108°C is detected. Melting under DSC analysis conditions is not found, which indicates that the oligomer is only in the amorphous phase. The values relationship analysis presented in Figure 5 shows that the heat capacity of PFPE-216 changes slightly when the temperature of the sample changes and is practically linear in the range of -95 to 120°C. Decomposition peak in sealed crucible is 254°C. This means that inclusion of oxygen bridges in the molecule structure actually noticeably reduces the degradation temperature of perfluorinated molecules – polytetrafluoroethylene is destroyed at temperatures of about 500°C. Measurement of measurement results is carried out on a CalVetC80 calorimeter of Setaram Company using " CalistoDataAcqusition " and "Calistoprocessing ". Sample evaporation start temperature in open crucible has been detected at 45°C, boiling point at 216°C.
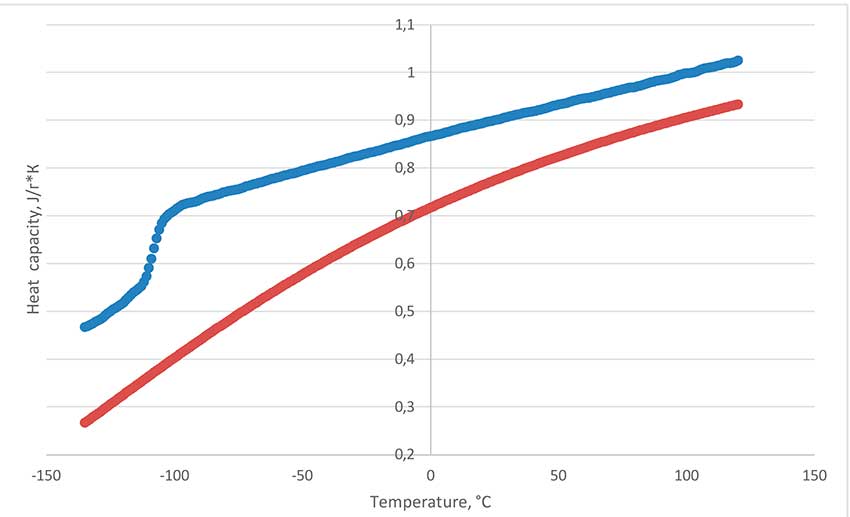
Figure 5. The heat capacity dependence of polyether (on top, blue) and corundum (from below, red) from temperature.
Based on calorimetric measurements, the equation of heat capacity dependence is obtained at constant pressure from the temperature for PFPE-216 in the range of -95 to 120°C:
Cp = 0,86323 + 0,00137 * T, R2=0.99905
Where Cp - heat capacity at constant pressure, J*g-1*K-1;
T – temperature, K;
R2 - approximation reliability value;
According to the linear appearance and coefficients of this equation, it can be concluded the low dependence of the heat capacity properties [7] on the temperature of the liquid PFPE-216 in the studied temperature range, which serves as proof of liquid composition constancy under these conditions and, apparently, absence of supramolecular formations between liquid molecules, that a priori generally can be predicted, based on the hyperconjugation between fluorine atoms within each individual PFPE molecule, which often results in a sublimation of the crystalline perfluorinated compounds due to a weak cohesive interaction between molecules of one phase. Coefficients in the heat capacity equation serve to predict the effect of temperature on the ability to absorb and transmit/dissipate energy by the mass of the substance: a positive or a negative first coefficient indicates the nature of an increase in the heat capacity with an increase in temperature. This is consistent with the fact that halogen bonds are found only in crystals with chlorine, bromine and iodine [8], halogen bonds between fluorine atoms of different molecules have not been described. The change in heat capacity can be used to judge the occurrence of partially crystalline structures or the occurrence of intermolecular associates – the subtle but important properties for certain types of electronics and technology.
To explain the thermal properties of the polyether, it is interesting to create a model of the structure of said polyether with the aid of quantum-chemical calculations.
The prediction of the polyethers properties before their synthesis is a relevant technological problem. If predictive theory and computer programs on their basis are already present for crystalline compounds (for example, an USPEX evolutionary algorithm or a CRYSTAL14 program [9]), for hydrocarbon molecules according to a chemical structure theory, for example, a CHETAH program [10], an effort [11] shows high convergence of the obtained calculated values by quantum-chemical calculation with literature data for hydrocarbon molecules, and even for predicting their biological activity [12], then such predictive theory are practically non-existent for fluoroorganic molecules.
In order to compare the existing and calculated physical and chemical properties of the previously synthesized PFPE-216, studies and quantum-chemical calculations have been conducted with the detection of clear compliance between the quantum-chemical calculated model and the properties of real molecules, in order to create a calculated model with predictive capability.
At the present time, in published sources, there is a lack of data relating to the correlation of the quantum-chemical calculations results of fluorinated organic compounds based on the quantum-chemical calculation selected by the methods, with known characteristics of the existing compounds. Obtaining such values and their comparison with real and literature data of sources is valuable information.
Quantum-chemical calculations of geometric structure optimization, as well as calculation of molecule’s energy and dipole moment are carried out with application of DFT (density functional theory) and by hybrid functional density method B3LYP, basic set 6-311G++(d, p), with established gradient tolerance value of convergence by Hartree/Bor (OPTOL) equal to 8.0×10-6.
Quantum-chemical optimization calculations of perfluorinated polyethers PFPE-216-1 and PFPE-216-2 structures, as well as their hydrocarbon analogues (PE) PE-1 (5,8,9,12-tetramethyl-4,7,10,13-tetraoxahexadecane) and PE-2 (5,8,13-trimethyl -4,7,11,14-tetraoxaheptadecane), are carried out using Firefly software based on QCPackage [13] and GAMESS (US) [14].
Table 1 shows the molecules energies and the dipole moment calculated values for said structures.
Hartree energy recalculation to kJ/mol, occurred according to the ratio [15] и [16]:
Ем = 2625.5*Eмх ,
where 2625500 – coefficient of energy conversion from Hartree (Hartree) (a. u. – atomic units) in J/mol;
Ем – the energy value of the molecule in J/mol;
Eмх – the energy value of the molecule in Hartree.
Table 1. Calculated values of energy characteristics of molecular structures of PFPE-216-1 and PFPE-216 -2, as well as their hydrocarbon analogues with identical structure of hydrocarbon skeleton PE-1 and PE-2, obtained by quantum-chemical method.
|
Name |
Dipole moment, Debai |
Molecule energy, Hartree |
Molecule energy 10-6, J/mol |
|
PFPE-216-1, probability of structure 99% |
0.436223 |
-4 306.483 |
-11 306.670 |
|
PFPE-216-2, probability of structure 1% |
0.961393 |
-4 306.487 |
-11 306.680 |
|
PE-1, hydrocarbon analogue PFPE-216-1 |
0.306377 |
-931.325 |
-2 445.195 |
|
PE-2, hydrocarbon analogue PFPE-216-2 |
1.556392 |
-931.324 |
-2 445.191 |
The calculated values for the isomers described in Table 1 are given with accuracy to different numbers, in this case they are thousandth of Hartree and thousandth parts of J/mol. The dipole moment values analysis (Table 1) shows a double change in dipole moment value when comparing PFPE-216-1 and PFPE-216-2, which can be explained by more symmetric structure of the PFPE‑216‑1 molecule as compared to PFPE-216-2. More asymmetric PFPE-216-2 molecule has a greater dipole moment value or a greater distance between the geometric centers of negative and positively charged atoms in the molecule. The magnitude of the dipole moment is clearly dependent on the structural variability of the molecule, but in this case it is concerned about the molecule in the state of the ideal gas and is fully optimized with the lowest possible energy according to quantum calculation data, but conclusions based on the results of calculations can be used to build a knowledge system of the composition-property of these molecules both individually and in mixtures with other compounds.
The energy values of the PFPE-216-1 and PFPE-216-2 molecules yield identical results, which testifies to the fact that both structures are energetically favorable and both structures are present as the final product in the synthesis of fluorinated polyether.
A similar trend is also observed for hydrocarbon analogues: dipole moments differ at times, wherein the energies of the molecules are almost identical.
The smaller dipole moment of the isomer predominant in the mixture can be due to some kinetic limitations in order to form an isomer with a large dipole moment, since the energy of both molecules of the isomers or the thermodynamic characteristics thereof are the same.
In this way, the predominant structure of a simple perfluorinated polyether PFPE-216-1 (Figure 1) and the predominant structure of the hydrocarbon polyether PE-1 differ structurally, but energetically not differ. This corresponds to the traditional change in the substitution of hydrogen atoms by fluorine atoms of almost the same atomic value, and take into account primarily the mutual influence of fluorine atoms in the prediction shown in physical interactions or chemical reactions of the properties of the molecule.
According to the energy characteristics of the molecules given in Table 1, it can be concluded that more negative values of the energy of the PFPE-216-1 and PFPE-216-2 molecules indicate their greater inertness, but at the same time less conformational variability of the molecules compared to the hydrocarbon analogues of PE-1 and PE -2.
Figure 6 shows the structure of the molecules and the direction of their dipole moments for PFPE-216-1 and PFPE-216-2, as well as their hydrocarbon analogues PE-1 and PE-2, indicating the direction of the dipole moment of the molecule, obtained as a result of quantum-chemical calculations of these molecules. The direction of dipole moments in the fluorosubstituted polyethers PFPE-216 passes mainly along the axis of the skeleton bent in the form of a crescent of the molecule, while in the hydrocarbon analogues PE-1 and PE-2 the direction of dipole moments is perpendicular to the axis of the skeleton of the molecule. Such differences are related to a greater degree with localization of electronegative atoms: in the case of hydrocarbon polyether, this oxygen atoms, which are relatively symmetrically located on both sides of the geometric center of the molecule, and the dipole moment occupies the position of almost the symmetry axis of the molecule.
In the case of a perfluorinated polyether, fluorine atoms are more electronegative than the oxygen atoms and are significantly larger in the molecule, and the location and effect are more diverse. Respectively, the negative charges center of the structure is far from the symmetry center of the molecule, creating a more complex-directed magnetic field of its own
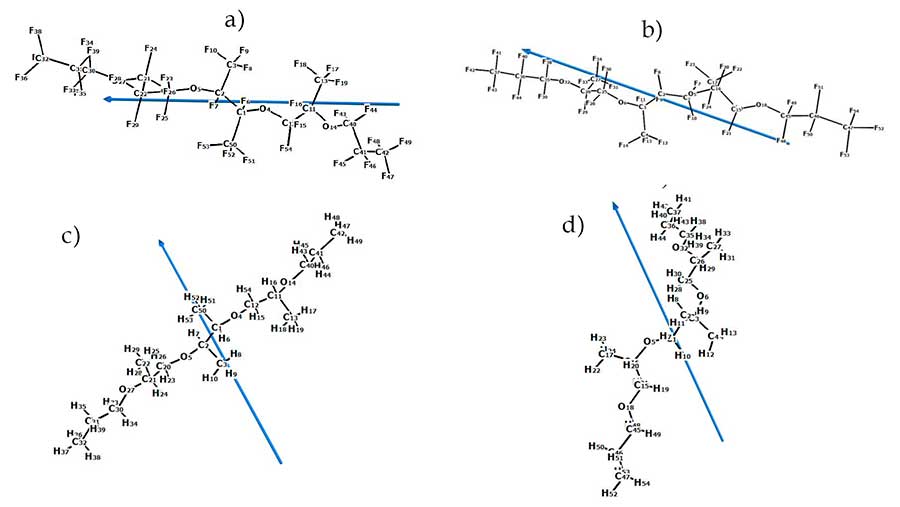
Figure 6. Calculated dipole moments of polyethers:
a) an optimized structure of PFPE-216 -1, 99% according to the NMR spectra of 19F;
b) an optimized structure of PFPE-216 -2, 1% according to the NMR spectra of 19F;
c) PE-1; d) PE-2.
Quantum-chemical calculation of the bond lengths, the location angles of the atoms and the charge values of each atom for PFPE-216-1 and PFPE-216-2, as well as their hydrocarbon analogues PE-1 and PE-2, was carried out.
Table 2. The value of bond lengths, the angle of arrangement of atoms relative to each other and the charge value of each atom of the PFPE-216-1 molecule.
|
PFPE-216-1, probability of structure 99% |
|||||
|---|---|---|---|---|---|
|
Atom type and number |
Atom charge, Mulleken |
Designation of bond atoms |
Bond length value, Å |
Designation of angle atoms |
Bond angles value, degrees |
|
C1 |
-1.309749 |
R(1-2) |
1.586 |
A(2-1-4) |
104.4 |
|
C2 |
-0.813605 |
R(1-4) |
1.397 |
A(2-1-6) |
108.3 |
|
C3 |
0.799327 |
R(1-6) |
1.359 |
A(2-1-50) |
113.1 |
|
O4 |
0.33864 |
R(1-50) |
1.578 |
A(1-2-3) |
113.4 |
|
O5 |
0.431444 |
R(2-3) |
1.579 |
A(1-2-5) |
106.9 |
|
F6 |
0.068969 |
R(2-5) |
1.402 |
A(1-2-7) |
108.7 |
|
F7 |
0.057866 |
R(2-7) |
1.356 |
A(4-1-6) |
111.0 |
|
F8 |
-0.024253 |
R(3-8) |
1.333 |
A(4-1-50) |
113.9 |
|
F9 |
0.000545 |
R(3-9) |
1.336 |
A(1-4-12) |
125.6 |
|
F10 |
-0.056933 |
R(3-10) |
1.336 |
A(6-1-50) |
106.0 |
|
C11 |
-1.643975 |
R(4-12) |
1.382 |
A(1-50-51) |
109.4 |
|
C12 |
0.252222 |
R(5-20) |
1.378 |
A(1-50-52) |
111.3 |
|
C13 |
0.954032 |
R(11-12) |
1.579 |
A(1-50-53) |
110.5 |
|
O14 |
0.086152 |
R(11-13) |
1.572 |
A(3-2-5) |
108.0 |
|
F15 |
-0.009412 |
R(11-14) |
1.394 |
A(3-2-7) |
107.3 |
|
F16 |
0.039637 |
R(11-16) |
1.357 |
A(2-3-8) |
111.2 |
|
F17 |
-0.018644 |
R(12-15) |
1.344 |
A(2-3-9) |
110.4 |
|
F18 |
-0.068201 |
R(12-54) |
1.344 |
A(2-3-10) |
109.3 |
|
F19 |
-0.005167 |
R(13-17) |
1.338 |
A(5-2-7) |
112.5 |
|
C20 |
-0.15421 |
R(13-18) |
1.336 |
A(2-5-20) |
123.7 |
|
C21 |
-1.148324 |
R(13-19) |
1.335 |
A(8-3-9) |
109.5 |
|
C22 |
0.882427 |
R(14-40) |
1.379 |
A(8-3-10) |
108.0 |
|
F23 |
-0.00332 |
R(20-21) |
1.576 |
A(9-3-10) |
108.3 |
|
F24 |
0.036055 |
R(20-23) |
1.344 |
A(4-12-11) |
107.7 |
|
F25 |
0.001865 |
R(20-25) |
1.347 |
A(4-12-15) |
112.5 |
|
F26 |
-0.035878 |
R(21-22) |
1.570 |
A(4-12-54) |
110.6 |
|
O27 |
0.10858 |
R(21-24) |
1.360 |
A(5-20-21) |
107.4 |
|
F28 |
-0.040098 |
R(21-27) |
1.394 |
A(5-20-23) |
112.5 |
|
F29 |
-0.016583 |
R(22-26) |
1.334 |
A(5-20-25) |
111.7 |
|
C30 |
0.208552 |
R(22-28) |
1.339 |
A(12-11-13) |
113.7 |
|
C31 |
-0.265798 |
R(22-29) |
1.336 |
A(12-11-14) |
103.3 |
|
C32 |
0.632992 |
R(27-30) |
1.379 |
A(12-11-16) |
107.8 |
|
F33 |
-0.034372 |
R(30-31) |
1.566 |
A(11-12-15) |
109.7 |
|
F34 |
-0.038449 |
R(30-33) |
1.349 |
A(11-12-54) |
108.3 |
|
F35 |
-0.039028 |
R(30-34) |
1.343 |
A(13-11-14) |
113.0 |
|
F36 |
-0.076908 |
R(31-32) |
1.563 |
A(13-11-16) |
107.5 |
|
F37 |
-0.087581 |
R(31-35) |
1.348 |
A(11-13-17) |
109.4 |
|
F38 |
-0.079782 |
R(31-39) |
1.349 |
A(11-13-18) |
110.7 |
|
F39 |
-0.043116 |
R(32-36) |
1.336 |
A(11-13-19) |
110.8 |
|
C40 |
0.245408 |
R(32-37) |
1.337 |
A(14-11-16) |
111.6 |
|
C41 |
-0.226797 |
R(32-38) |
1.337 |
A(11-14-40) |
125.7 |
|
C42 |
0.59073 |
R(40-41) |
1.565 |
A(15-12-54) |
108.0 |
|
F43 |
-0.038198 |
R(40-43) |
1.343 |
A(17-13-18) |
108.3 |
|
F44 |
-0.030768 |
R(40-44) |
1.349 |
A(17-13-19) |
108.9 |
|
F45 |
-0.04374 |
R(41-42) |
1.563 |
A(18-13-19) |
108.7 |
|
F46 |
-0.037226 |
R(41-45) |
1.349 |
A(14-40-41) |
105.7 |
|
F47 |
-0.088184 |
R(41-46) |
1.348 |
A(14-40-43) |
112.5 |
|
F48 |
-0.080065 |
R(42-47) |
1.336 |
A(14-40-44) |
111.4 |
|
F49 |
-0.075932 |
R(42-48) |
1.337 |
A(21-20-23) |
108.1 |
|
C50 |
0.98814 |
R(42-49) |
1.336 |
A(21-20-25) |
110.0 |
|
F51 |
-0.027068 |
R(50-51) |
1.338 |
A(20-21-22) |
114.7 |
|
F52 |
0.010519 |
R(50-52) |
1.334 |
A(20-21-24) |
107.3 |
|
F53 |
-0.039886 |
R(50-53) |
1.336 |
A(20-21-27) |
102.5 |
|
F54 |
-0.032851 |
- |
- |
A(23-20-25) |
107.2 |
|
- |
- |
- |
- |
A(22-21-24) |
107.0 |
|
- |
- |
- |
- |
A(22-21-27) |
113.4 |
|
- |
- |
- |
- |
A(21-22-26) |
111.0 |
|
- |
- |
- |
- |
A(21-22-28) |
109.4 |
|
- |
- |
- |
- |
A(21-22-29) |
110.5 |
|
- |
- |
- |
- |
A(24-21-27) |
111.7 |
|
- |
- |
- |
- |
A(21-27-30) |
126.4 |
|
- |
- |
- |
- |
A(26-22-28) |
108.0 |
|
- |
- |
- |
- |
A(26-22-29) |
109.0 |
|
- |
- |
- |
- |
A(28-22-29) |
108.8 |
|
- |
- |
- |
- |
A(27-30-31) |
105.8 |
|
- |
- |
- |
- |
A(27-30-33) |
111.0 |
|
- |
- |
- |
- |
A(27-30-34) |
112.7 |
|
- |
- |
- |
- |
A(31-30-33) |
109.4 |
|
- |
- |
- |
- |
A(31-30-34) |
109.8 |
|
- |
- |
- |
- |
A(30-31-32) |
115.5 |
|
- |
- |
- |
- |
A(30-31-35) |
108.5 |
|
- |
- |
- |
- |
A(30-31-39) |
108.1 |
|
- |
- |
- |
- |
A(33-30-34) |
108.0 |
|
- |
- |
- |
- |
A(32-31-35) |
107.7 |
|
- |
- |
- |
- |
A(32-31-39) |
107.8 |
|
- |
- |
- |
- |
A(31-32-36) |
110.9 |
|
- |
- |
- |
- |
A(31-32-37) |
108.6 |
|
- |
- |
- |
- |
A(31-32-38) |
110.9 |
|
- |
- |
- |
- |
A(35-31-39) |
109.1 |
|
- |
- |
- |
- |
A(36-32-37) |
108.7 |
|
- |
- |
- |
- |
A(36-32-38) |
109.0 |
|
- |
- |
- |
- |
A(37-32-38) |
108.7 |
|
- |
- |
- |
- |
A(41-40-43) |
109.9 |
|
- |
- |
- |
- |
A(41-40-44) |
109.5 |
|
- |
- |
- |
- |
A(40-41-42) |
115.5 |
|
- |
- |
- |
- |
A(40-41-45) |
108.1 |
|
- |
- |
- |
- |
A(40-41-46) |
108.5 |
|
- |
- |
- |
- |
A(43-40-44) |
107.8 |
|
- |
- |
- |
- |
A(42-41-45) |
107.8 |
|
- |
- |
- |
- |
A(42-41-46) |
107.7 |
|
- |
- |
- |
- |
A(41-42-47) |
108.6 |
|
- |
- |
- |
- |
A(41-42-48) |
110.9 |
|
- |
- |
- |
- |
A(41-42-49) |
110.9 |
|
- |
- |
- |
- |
A(45-41-46) |
109.1 |
|
- |
- |
- |
- |
A(47-42-48) |
108.7 |
|
- |
- |
- |
- |
A(47-42-49) |
108.7 |
|
- |
- |
- |
- |
A(48-42-49) |
109.0 |
|
- |
- |
- |
- |
A(51-50-52) |
109.0 |
|
- |
- |
- |
- |
A(51-50-53) |
107.7 |
|
- |
- |
- |
- |
A(52-50-53) |
108.9 |
When considering the molecule structure, it can be seen that fluorine atoms with a reduced electron density are linked to the “skeletal” carbon atom of the molecule, which, in turn, is connected to an ether oxygen bridge and a CF3 group, which is the main electronic density acquirer. It is calculated that in the structure of PFPE-216-1 all oxygen atoms have a positive charge, since fluorine atoms are more electronegative, but somewhat unexpected that part of the fluorine atoms (F6, F7, F9, F16, F24, F25, F52) also have a positive charge. The general structural formula of PFPE-216 has the form of C16O4F34. A total of 34 fluorine atoms are present in the electrically neutral structure of PFPE‑216, respectively, the percentage of positively charged fluorine atoms from the total number of fluorine atoms in the structure is about 21%.
The length of the carbon bond with these fluorine atoms (1.358 ± 0.002) Å, which is in accordance with reference data for the lengths of bonds with fluorine atoms in ionic tetrahedral structures [17], while the lengths of C-F bonds in the CF3 fragment (1.336 ± 0.003) Å are typical for these groups [18], both in perfluorinated molecules and as a substituent in organic molecules.
The lengths of carbon-carbon bonds in the structures of perfluorinated ethers are (1.570 ± 0.014) Å, which is somewhat greater than the lengths of bonds in organic molecules and approaches the length of the bonds between the carbon atoms in the stressed cycles with carbon substituents [17], while in hydrocarbon analogues the PE bonds between the carbon atoms (1.528 ± 0.011) Å (Tables 4, 5) are less. However, the length of the carbon-oxygen bonds (1.388 ± 0.014) Å is only slightly less than in simple organic ethers (1.426 ± 0.005) Å (Tables 4, 5), which is somewhat surprising in view of the completely different distribution of charges across the molecule, and should result in the compression of the structure of the molecule seemingly.
If the fraction of all positively charged atoms in PFPE-216-2 is to be read: carbon, oxygen and fluorine from the total number of atoms of the molecule, then we will get the content of positively charged centers in the molecule about 37%, which is almost twice as large as that of the examined above PFPE-216-1 molecule. Different-charged centers testify to presence of dipole moment characteristic of fluorinated polymers of partially crystalline structure [19], while at the first gaze the polyether structure does not indicate a similar property. The difference in the percentage of positively charged atoms between PFPE-216-1 and PFPE-216-2 is linked to the number of groups CF3 in the molecule: more groups-more fluorine atoms which lose their electron cloud in front of these substituents in the main chain of the fluoropolyether molecule.
Trifluoromethyl CF3 groups introduce a strong polarizing effect, but one such group in the molecule makes a greater contribution to the asymmetry of the charges and creates a greater dipole moment than two groups CF3, which appear to compensate in part the polarizing influence of each other.
Table 3. The value of bond lengths, the angle atoms arrangement relative to each other and each atom charge value of the PFPE-216-2 molecule.
|
PFPE-216-2, probability of structure 1% |
|||||
|---|---|---|---|---|---|
|
Atom type and number |
Atom charge, Mulleken |
Designation of bond atoms |
Bond length value, Å |
Designation of angle atoms |
Bond angles value, degrees |
|
C1 |
-0.054924 |
R(1-2) |
1.568 |
A(2-1-5) |
107.7 |
|
C2 |
0.375702 |
R(1-5) |
1.377 |
A(2-1-7) |
107.8 |
|
C3 |
-1.743212 |
R(1-7) |
1.343 |
A(2-1-10) |
109.3 |
|
C4 |
1.082164 |
R(1-10) |
1.348 |
A(1-2-3) |
119.2 |
|
O5 |
0.405634 |
R(2-3) |
1.579 |
A(1-2-8) |
107.2 |
|
O6 |
0.33351 |
R(2-8) |
1.351 |
A(1-2-9) |
106.8 |
|
F7 |
-0.016021 |
R(2-9) |
1.348 |
A(5-1-7) |
112.6 |
|
F8 |
0.009384 |
R(3-4) |
1.566 |
A(5-1-10) |
111.9 |
|
F9 |
0.014333 |
R(3-6) |
1.407 |
A(1-5-16) |
126.1 |
|
F10 |
0.004965 |
R(3-11) |
1.354 |
A(7-1-10) |
107.4 |
|
F11 |
0.039476 |
R(4-12) |
1.338 |
A(3-2-8) |
106.1 |
|
F12 |
-0.02287 |
R(4-13) |
1.335 |
A(3-2-9) |
108.7 |
|
F13 |
-0.001654 |
R(4-14) |
1.335 |
A(2-3-4) |
116.7 |
|
F14 |
-0.046151 |
R(5-16) |
1.396 |
A(2-3-6) |
105.1 |
|
C15 |
0.271916 |
R(6-25) |
1.374 |
A(2-3-11) |
108.5 |
|
C16 |
-1.585437 |
R(15-16) |
1.573 |
A(8-2-9) |
108.6 |
|
C17 |
0.888419 |
R(15-18) |
1.379 |
A(4-3-6) |
106.9 |
|
O18 |
0.040516 |
R(15-19) |
1.348 |
A(4-3-11) |
107.3 |
|
F19 |
-0.038344 |
R(15-21) |
1.344 |
A(3-4-12) |
110.2 |
|
F20 |
0.063785 |
R(16-17) |
1.573 |
A(3-4-13) |
110.9 |
|
F21 |
0.012383 |
R(16-20) |
1.358 |
A(3-4-14) |
109.2 |
|
F22 |
-0.03529 |
R(17-22) |
1.333 |
A(6-3-11) |
112.5 |
|
F23 |
-0.057756 |
R(17-23) |
1.335 |
A(3-6-25) |
123.0 |
|
F24 |
-0.005835 |
R(17-24) |
1.337 |
A(12-4-13) |
109.2 |
|
C25 |
0.019802 |
R(18-45) |
1.385 |
A(12-4-14) |
108.4 |
|
C26 |
-1.263178 |
R(25-26) |
1.576 |
A(13-4-14) |
108.9 |
|
C27 |
0.954253 |
R(25-28) |
1.346 |
A(5-16-15) |
112.9 |
|
F28 |
-0.029069 |
R(25-30) |
1.347 |
A(5-16-17) |
103.5 |
|
F29 |
0.034601 |
R(26-27) |
1.571 |
A(5-16-20) |
111.6 |
|
F30 |
-0.0019 |
R(26-29) |
1.359 |
A(6-25-26) |
107.4 |
|
F31 |
-0.040466 |
R(26-32) |
1.394 |
A(6-25-28) |
111.7 |
|
O32 |
0.104271 |
R(27-31) |
1.334 |
A(6-25-30) |
112.1 |
|
F33 |
-0.041718 |
R(27-33) |
1.339 |
A(16-15-18) |
107.8 |
|
F34 |
-0.02015 |
R(27-34) |
1.336 |
A(16-15-19) |
108.9 |
|
C35 |
0.18297 |
R(32-35) |
1.378 |
A(16-15-21) |
109.8 |
|
C36 |
-0.236858 |
R(35-36) |
1.566 |
A(15-16-17) |
114.2 |
|
C37 |
0.622581 |
R(35-38) |
1.349 |
A(15-16-20) |
107.3 |
|
F38 |
-0.035027 |
R(35-39) |
1.343 |
A(18-15-19) |
111.5 |
|
F39 |
-0.038839 |
R(36-37) |
1.563 |
A(18-15-21) |
111.1 |
|
F40 |
-0.038774 |
R(36-40) |
1.348 |
A(15-18-45) |
121.5 |
|
F41 |
-0.077175 |
R(36-44) |
1.349 |
A(19-15-21) |
107.8 |
|
F42 |
-0.088057 |
R(37-41) |
1.336 |
A(17-16-20) |
107.2 |
|
F43 |
-0.079762 |
R(37-42) |
1.337 |
A(16-17-22) |
110.6 |
|
F44 |
-0.044023 |
R(37-43) |
1.337 |
A(16-17-23) |
109.2 |
|
C45 |
0.195155 |
R(45-46) |
1.564 |
A(16-17-24) |
110.7 |
|
C46 |
-0.1607 |
R(45-48) |
1.345 |
A(22-17-23) |
108.3 |
|
C47 |
0.58055 |
R(45-49) |
1.345 |
A(22-17-24) |
109.1 |
|
F48 |
-0.06278 |
R(46-47) |
1.563 |
A(23-17-24) |
108.9 |
|
F49 |
-0.042783 |
R(46-50) |
1.349 |
A(18-45-46) |
106.2 |
|
F50 |
-0.047666 |
R(46-51) |
1.348 |
A(18-45-48) |
112.0 |
|
F51 |
-0.034781 |
R(47-52) |
1.336 |
A(18-45-49) |
110.7 |
|
F52 |
-0.087055 |
R(47-53) |
1.337 |
A(26-25-28) |
108.2 |
|
F53 |
-0.081829 |
R(47-54) |
1.336 |
A(26-25-30) |
109.8 |
|
F54 |
-0.07629 |
- |
- |
A(25-26-27) |
114.5 |
|
- |
- |
- |
- |
A(25-26-29) |
107.5 |
|
- |
- |
- |
- |
A(25-26-32) |
102.7 |
|
- |
- |
- |
- |
A(28-25-30) |
107.6 |
|
- |
- |
- |
- |
A(27-26-29) |
107.1 |
|
- |
- |
- |
- |
A(27-26-32) |
113.4 |
|
- |
- |
- |
- |
A(26-27-31) |
111.0 |
|
- |
- |
- |
- |
A(26-27-33) |
109.4 |
|
- |
- |
- |
- |
A(26-27-34) |
110.6 |
|
- |
- |
- |
- |
A(29-26-32) |
111.7 |
|
- |
- |
- |
- |
A(26-32-35) |
126.2 |
|
- |
- |
- |
- |
A(31-27-33) |
108.0 |
|
- |
- |
- |
- |
A(31-27-34) |
108.9 |
|
- |
- |
- |
- |
A(33-27-34) |
108.8 |
|
- |
- |
- |
- |
A(32-35-36) |
105.7 |
|
- |
- |
- |
- |
A(32-35-38) |
111.2 |
|
- |
- |
- |
- |
A(32-35-39) |
112.7 |
|
- |
- |
- |
- |
A(36-35-38) |
109.4 |
|
- |
- |
- |
- |
A(36-35-39) |
109.8 |
|
- |
- |
- |
- |
A(35-36-37) |
115.6 |
|
- |
- |
- |
- |
A(35-36-40) |
108.5 |
|
- |
- |
- |
- |
A(35-36-44) |
108.1 |
|
- |
- |
- |
- |
A(38-35-39) |
107.9 |
|
- |
- |
- |
- |
A(37-36-40) |
107.7 |
|
- |
- |
- |
- |
A(37-36-44) |
107.8 |
|
- |
- |
- |
- |
A(36-37-41) |
111.0 |
|
- |
- |
- |
- |
A(36-37-42) |
108.6 |
|
- |
- |
- |
- |
A(36-37-43) |
110.9 |
|
- |
- |
- |
- |
A(40-36-44) |
109.1 |
|
- |
- |
- |
- |
A(41-37-42) |
108.7 |
|
- |
- |
- |
- |
A(41-37-43) |
108.9 |
|
- |
- |
- |
- |
A(42-37-43) |
108.7 |
|
- |
- |
- |
- |
A(46-45-48) |
110.1 |
|
- |
- |
- |
- |
A(46-45-49) |
110.0 |
|
- |
- |
- |
- |
A(45-46-47) |
115.5 |
|
- |
- |
- |
- |
A(45-46-50) |
108.0 |
|
- |
- |
- |
- |
A(45-46-51) |
108.4 |
|
- |
- |
- |
- |
A(48-45-49) |
107.9 |
|
- |
- |
- |
- |
A(47-46-50) |
107.9 |
|
- |
- |
- |
- |
A(47-46-51) |
107.8 |
|
- |
- |
- |
- |
A(46-47-52) |
108.6 |
|
- |
- |
- |
- |
A(46-47-53) |
110.8 |
|
- |
- |
- |
- |
A(46-47-54) |
110.9 |
|
- |
- |
- |
- |
A(50-46-51) |
109.1 |
|
- |
- |
- |
- |
A(52-47-53) |
108.8 |
|
- |
- |
- |
- |
A(52-47-54) |
108.7 |
|
- |
- |
- |
- |
A(53-47-54) |
109.0 |
Also, it has been found that in the structure of PFPE-216-2, all oxygen atoms are expected to have a positive charge as in the case of PFPE-216-1, as well as part of fluorine atoms (F8, F10, F11, F20, F21, F29) are positively charged. The percentage of positively charged fluorine atoms from the total number of fluorine atoms in the structure is about 18%. When all positively charged atoms, such as carbons, oxygen and fluorine atoms, are calculated from the total number of atoms of the molecule, the content of positively charged centers in the molecule is about 39%.
Upon consideration of the PFPE-216-2 molecule, it has been found that, as is the case with PFPE-216-1, positively charged fluorine atoms are linked to the central atom of the carbon skeleton of the molecule. An ether oxygen bridge and a CF3 group are connected to the same carbon atom, the electron density being shifted towards said group.
Presence of said positive and negative centers in structure of PFPE-216-1 and PFPE-216-2 makes it possible to assume possibility of electrostatic interactions with small molecules and stability of retention in time of PFPE-216 gases [20] and small organic molecules, allowing it to be used as a gas transfer medium resistant to impurities and fluctuations in external conditions, as well as liquid dielectrics.
Table 4. The value of bond lengths, the angles of atoms arrangement relative to each other and each atom charge value of the PE-1 molecule of PFPE-216-1 hydrocarbon analogue.
|
PE-1 hydrocarbon analogue of PFPE-216-1 |
|||||
|---|---|---|---|---|---|
|
Atom type and number |
Atom charge, Mulleken |
Designation of bond atoms |
Bond length value, Å |
Designation of angle atoms |
Bond angles value, degrees |
|
C1 |
-0.153408 |
R(1-2) |
1.539 |
A(2-1-4) |
106.3 |
|
C2 |
-0.190649 |
R(1-4) |
1.429 |
A(2-1-6) |
107.5 |
|
C3 |
-0.441739 |
R(1-6) |
1.101 |
A(2-1-50) |
112.3 |
|
O4 |
0.013733 |
R(1-50) |
1.528 |
A(1-2-3) |
112.9 |
|
O5 |
0.038501 |
R(2-3) |
1.527 |
A(1-2-5) |
106.0 |
|
H6 |
0.179052 |
R(2-5) |
1.432 |
A(1-2-7) |
107.9 |
|
H7 |
0.161348 |
R(2-7) |
1.100 |
A(4-1-6) |
108.9 |
|
H8 |
0.166522 |
R(3-8) |
1.090 |
A(4-1-50) |
112.1 |
|
H9 |
0.158036 |
R(3-9) |
1.094 |
A(1-4-12) |
115.3 |
|
H10 |
0.115296 |
R(3-10) |
1.093 |
A(6-1-50) |
109.6 |
|
C11 |
-0.202572 |
R(4-12) |
1.419 |
A(1-50-51) |
111.5 |
|
C12 |
-0.252886 |
R(5-20) |
1.419 |
A(1-50-52) |
110.2 |
|
C13 |
-0.364687 |
R(11-12) |
1.528 |
A(1-50-53) |
110.0 |
|
O14 |
-0.03675 |
R(11-13) |
1.527 |
A(3-2-5) |
111.1 |
|
H15 |
0.159003 |
R(11-14) |
1.428 |
A(3-2-7) |
109.7 |
|
H16 |
0.140242 |
R(11-16) |
1.100 |
A(2-3-8) |
110.5 |
|
H17 |
0.132807 |
R(12-15) |
1.100 |
A(2-3-9) |
110.2 |
|
H18 |
0.138064 |
R(12-54) |
1.096 |
A(2-3-10) |
110.8 |
|
H19 |
0.164044 |
R(13-17) |
1.092 |
A(5-2-7) |
109.1 |
|
C20 |
-0.38789 |
R(13-18) |
1.091 |
A(2-5-20) |
114.9 |
|
C21 |
-0.127816 |
R(13-19) |
1.094 |
A(8-3-9) |
108.7 |
|
C22 |
-0.372806 |
R(14-40) |
1.421 |
A(8-3-10) |
108.2 |
|
H23 |
0.176773 |
R(20-21) |
1.528 |
A(9-3-10) |
108.3 |
|
H24 |
0.135478 |
R(20-23) |
1.096 |
A(4-12-11) |
108.6 |
|
H25 |
0.172347 |
R(20-25) |
1.100 |
A(4-12-15) |
110.5 |
|
H26 |
0.160108 |
R(21-22) |
1.527 |
A(4-12-54) |
111.6 |
|
O27 |
-0.035204 |
R(21-24) |
1.100 |
A(5-20-21) |
108.8 |
|
H28 |
0.128973 |
R(21-27) |
1.429 |
A(5-20-23) |
111.6 |
|
H29 |
0.162358 |
R(22-26) |
1.092 |
A(5-20-25) |
110.5 |
|
C30 |
-0.312009 |
R(22-28) |
1.092 |
A(12-11-13) |
112.7 |
|
C31 |
-0.111039 |
R(22-29) |
1.094 |
A(12-11-14) |
104.9 |
|
C32 |
-0.57889 |
R(27-30) |
1.421 |
A(12-11-16) |
107.8 |
|
H33 |
0.112629 |
R(30-31) |
1.522 |
A(11-12-15) |
109.6 |
|
H34 |
0.129289 |
R(30-33) |
1.099 |
A(11-12-54) |
108.4 |
|
H35 |
0.14641 |
R(30-34) |
1.102 |
A(13-11-14) |
112.4 |
|
H36 |
0.135474 |
R(31-32) |
1.531 |
A(13-11-16) |
109.6 |
|
H37 |
0.150932 |
R(31-35) |
1.095 |
A(11-13-17) |
111.7 |
|
H38 |
0.132753 |
R(31-39) |
1.095 |
A(11-13-18) |
109.9 |
|
H39 |
0.138488 |
R(32-36) |
1.095 |
A(11-13-19) |
110.1 |
|
C40 |
-0.321191 |
R(32-37) |
1.093 |
A(14-11-16) |
109.2 |
|
C41 |
-0.10461 |
R(32-38) |
1.095 |
A(11-14-40) |
115.4 |
|
C42 |
-0.581808 |
R(40-41) |
1.522 |
A(15-12-54) |
108.1 |
|
H43 |
0.128801 |
R(40-43) |
1.102 |
A(17-13-18) |
108.0 |
|
H44 |
0.113384 |
R(40-44) |
1.099 |
A(17-13-19) |
108.4 |
|
H45 |
0.139842 |
R(41-42) |
1.531 |
A(18-13-19) |
108.7 |
|
H46 |
0.145346 |
R(41-45) |
1.095 |
A(14-40-41) |
108.8 |
|
H47 |
0.151184 |
R(41-46) |
1.095 |
A(14-40-43) |
109.7 |
|
H48 |
0.133088 |
R(42-47) |
1.093 |
A(14-40-44) |
110.8 |
|
H49 |
0.135519 |
R(42-48) |
1.095 |
A(21-20-23) |
108.4 |
|
C50 |
-0.447427 |
R(42-49) |
1.095 |
A(21-20-25) |
109.5 |
|
H51 |
0.141363 |
R(50-51) |
1.092 |
A(20-21-22) |
112.8 |
|
H52 |
0.163876 |
R(50-52) |
1.094 |
A(20-21-24) |
108.0 |
|
H53 |
0.142867 |
R(50-53) |
1.091 |
A(20-21-27) |
104.8 |
|
H54 |
0.179452 |
- |
- |
A(23-20-25) |
108.0 |
|
- |
- |
- |
- |
A(22-21-24) |
109.5 |
|
- |
- |
- |
- |
A(22-21-27) |
112.4 |
|
- |
- |
- |
- |
A(21-22-26) |
109.9 |
|
- |
- |
- |
- |
A(21-22-28) |
111.5 |
|
- |
- |
- |
- |
A(21-22-29) |
110.2 |
|
- |
- |
- |
- |
A(24-21-27) |
109.2 |
|
- |
- |
- |
- |
A(21-27-30) |
115.4 |
|
- |
- |
- |
- |
A(26-22-28) |
108.0 |
|
- |
- |
- |
- |
A(26-22-29) |
108.8 |
|
- |
- |
- |
- |
A(28-22-29) |
108.4 |
|
- |
- |
- |
- |
A(27-30-31) |
108.7 |
|
- |
- |
- |
- |
A(27-30-33) |
110.8 |
|
- |
- |
- |
- |
A(27-30-34) |
109.7 |
|
- |
- |
- |
- |
A(31-30-33) |
109.9 |
|
- |
- |
- |
- |
A(31-30-34) |
110.4 |
|
- |
- |
- |
- |
A(30-31-32) |
112.4 |
|
- |
- |
- |
- |
A(30-31-35) |
108.5 |
|
- |
- |
- |
- |
A(30-31-39) |
108.6 |
|
- |
- |
- |
- |
A(33-30-34) |
107.4 |
|
- |
- |
- |
- |
A(32-31-35) |
110.2 |
|
- |
- |
- |
- |
A(32-31-39) |
110.3 |
|
- |
- |
- |
- |
A(31-32-36) |
111.5 |
|
- |
- |
- |
- |
A(31-32-37) |
111.0 |
|
- |
- |
- |
- |
A(31-32-38) |
111.5 |
|
- |
- |
- |
- |
A(35-31-39) |
106.7 |
|
- |
- |
- |
- |
A(36-32-37) |
107.5 |
|
- |
- |
- |
- |
A(36-32-38) |
107.7 |
|
- |
- |
- |
- |
A(37-32-38) |
107.5 |
|
- |
- |
- |
- |
A(41-40-43) |
110.3 |
|
- |
- |
- |
- |
A(41-40-44) |
109.9 |
|
- |
- |
- |
- |
A(40-41-42) |
112.4 |
|
- |
- |
- |
- |
A(40-41-45) |
108.6 |
|
- |
- |
- |
- |
A(40-41-46) |
108.5 |
|
- |
- |
- |
- |
A(43-40-44) |
107.4 |
|
- |
- |
- |
- |
A(42-41-45) |
110.3 |
|
- |
- |
- |
- |
A(42-41-46) |
110.2 |
|
- |
- |
- |
- |
A(41-42-47) |
111.0 |
|
- |
- |
- |
- |
A(41-42-48) |
111.5 |
|
- |
- |
- |
- |
A(41-42-49) |
111.4 |
|
- |
- |
- |
- |
A(45-41-46) |
106.7 |
|
- |
- |
- |
- |
A(47-42-48) |
107.5 |
|
- |
- |
- |
- |
A(47-42-49) |
107.6 |
|
- |
- |
- |
- |
A(48-42-49) |
107.7 |
|
- |
- |
- |
- |
A(51-50-52) |
108.3 |
|
- |
- |
- |
- |
A(51-50-53) |
108.0 |
|
- |
- |
- |
- |
A(52-50-53) |
108.8 |
Table 5. The value of bond lengths, the angles of atoms arrangement relative to each other and each atom charge value of the PE-2 molecule of PFPE-216-2 hydrocarbon analogue.
|
PE-2 hydrocarbon analogue PFPE-216-2 |
|||||
|---|---|---|---|---|---|
|
Atom type and number |
Atom charge, Mulleken |
Designation of bond atoms |
Bond length value, Å |
Designation of angle atoms |
Bond angles value, degrees |
|
C1 |
-0.493093 |
R(1-2) |
1.524 |
A(2-1-5) |
108.8 |
|
C2 |
-0.061703 |
R(1-5) |
1.424 |
A(2-1-7) |
109.5 |
|
C3 |
-0.127388 |
R(1-7) |
1.100 |
A(2-1-10) |
110.9 |
|
C4 |
-0.6522 |
R(1-10) |
1.099 |
A(1-2-3) |
115.1 |
|
O5 |
0.098723 |
R(2-3) |
1.537 |
A(1-2-8) |
107.9 |
|
O6 |
0.024396 |
R(2-8) |
1.094 |
A(1-2-9) |
108.6 |
|
H7 |
0.137678 |
R(2-9) |
1.096 |
A(5-1-7) |
109.6 |
|
H8 |
0.16629 |
R(3-4) |
1.524 |
A(5-1-10) |
110.8 |
|
H9 |
0.157403 |
R(3-6) |
1.435 |
A(1-5-16) |
115.4 |
|
H10 |
0.124733 |
R(3-11) |
1.097 |
A(7-1-10) |
107.2 |
|
H11 |
0.151271 |
R(4-12) |
1.093 |
A(3-2-8) |
109.1 |
|
H12 |
0.138254 |
R(4-13) |
1.094 |
A(3-2-9) |
108.6 |
|
H13 |
0.160143 |
R(4-14) |
1.092 |
A(2-3-4) |
113.3 |
|
H14 |
0.14899 |
R(5-16) |
1.431 |
A(2-3-6) |
110.2 |
|
C15 |
-0.107713 |
R(6-25) |
1.419 |
A(2-3-11) |
108.8 |
|
C16 |
-0.364537 |
R(15-16) |
1.532 |
A(8-2-9) |
107.1 |
|
C17 |
-0.430553 |
R(15-18) |
1.417 |
A(4-3-6) |
106.3 |
|
O18 |
-0.066372 |
R(15-19) |
1.100 |
A(4-3-11) |
109.4 |
|
H19 |
0.122218 |
R(15-21) |
1.101 |
A(3-4-12) |
111.3 |
|
H20 |
0.151721 |
R(16-17) |
1.521 |
A(3-4-13) |
110.4 |
|
H21 |
0.150215 |
R(16-20) |
1.099 |
A(3-4-14) |
110.1 |
|
H22 |
0.153202 |
R(17-22) |
1.092 |
A(6-3-11) |
108.7 |
|
H23 |
0.141749 |
R(17-23) |
1.092 |
A(3-6-25) |
115.3 |
|
H24 |
0.152055 |
R(17-24) |
1.094 |
A(12-4-13) |
108.5 |
|
C25 |
-0.328332 |
R(18-45) |
1.420 |
A(12-4-14) |
108.2 |
|
C26 |
-0.019417 |
R(25-26) |
1.528 |
A(13-4-14) |
108.2 |
|
C27 |
-0.437254 |
R(25-28) |
1.098 |
A(5-16-15) |
109.6 |
|
H28 |
0.167414 |
R(25-30) |
1.099 |
A(5-16-17) |
107.8 |
|
H29 |
0.145543 |
R(26-27) |
1.527 |
A(5-16-20) |
109.4 |
|
H30 |
0.173251 |
R(26-29) |
1.100 |
A(6-25-26) |
108.5 |
|
H31 |
0.158869 |
R(26-32) |
1.429 |
A(6-25-28) |
110.6 |
|
O32 |
-0.034559 |
R(27-31) |
1.092 |
A(6-25-30) |
111.8 |
|
H33 |
0.126925 |
R(27-33) |
1.093 |
A(16-15-18) |
108.7 |
|
H34 |
0.164999 |
R(27-34) |
1.094 |
A(16-15-19) |
110.1 |
|
C35 |
-0.281761 |
R(32-35) |
1.420 |
A(16-15-21) |
109.4 |
|
C36 |
-0.12604 |
R(35-36) |
1.522 |
A(15-16-17) |
112.4 |
|
C37 |
-0.579369 |
R(35-38) |
1.100 |
A(15-16-20) |
108.1 |
|
H38 |
0.117508 |
R(35-39) |
1.102 |
A(18-15-19) |
110.1 |
|
H39 |
0.125129 |
R(36-37) |
1.531 |
A(18-15-21) |
110.8 |
|
H40 |
0.143585 |
R(36-40) |
1.095 |
A(15-18-45) |
113.6 |
|
H41 |
0.135303 |
R(36-44) |
1.095 |
A(19-15-21) |
107.8 |
|
H42 |
0.15027 |
R(37-41) |
1.095 |
A(17-16-20) |
109.6 |
|
H43 |
0.132739 |
R(37-42) |
1.093 |
A(16-17-22) |
110.1 |
|
H44 |
0.140069 |
R(37-43) |
1.095 |
A(16-17-23) |
110.5 |
|
C45 |
-0.18965 |
R(45-46) |
1.521 |
A(16-17-24) |
110.1 |
|
C46 |
-0.147664 |
R(45-48) |
1.101 |
A(22-17-23) |
109.1 |
|
C47 |
-0.567507 |
R(45-49) |
1.102 |
A(22-17-24) |
108.7 |
|
H48 |
0.1313 |
R(46-47) |
1.531 |
A(23-17-24) |
108.3 |
|
H49 |
0.118716 |
R(46-50) |
1.095 |
A(18-45-46) |
109.1 |
|
H50 |
0.142905 |
R(46-51) |
1.095 |
A(18-45-48) |
109.8 |
|
H51 |
0.142953 |
R(47-52) |
1.093 |
A(18-45-49) |
109.8 |
|
H52 |
0.151806 |
R(47-53) |
1.094 |
A(26-25-28) |
109.0 |
|
H53 |
0.133133 |
R(47-54) |
1.094 |
A(26-25-30) |
109.0 |
|
H54 |
0.133655 |
- |
- |
A(25-26-27) |
112.5 |
|
- |
- |
- |
- |
A(25-26-29) |
108.0 |
|
- |
- |
- |
- |
A(25-26-32) |
104.9 |
|
- |
- |
- |
- |
A(28-25-30) |
107.9 |
|
- |
- |
- |
- |
A(27-26-29) |
109.5 |
|
- |
- |
- |
- |
A(27-26-32) |
112.5 |
|
- |
- |
- |
- |
A(26-27-31) |
109.8 |
|
- |
- |
- |
- |
A(26-27-33) |
111.6 |
|
- |
- |
- |
- |
A(26-27-34) |
110.1 |
|
- |
- |
- |
- |
A(29-26-32) |
109.2 |
|
- |
- |
- |
- |
A(26-32-35) |
115.4 |
|
- |
- |
- |
- |
A(31-27-33) |
108.2 |
|
- |
- |
- |
- |
A(31-27-34) |
108.7 |
|
- |
- |
- |
- |
A(33-27-34) |
108.4 |
|
- |
- |
- |
- |
A(32-35-36) |
108.7 |
|
- |
- |
- |
- |
A(32-35-38) |
110.8 |
|
- |
- |
- |
- |
A(32-35-39) |
109.7 |
|
- |
- |
- |
- |
A(36-35-38) |
109.9 |
|
- |
- |
- |
- |
A(36-35-39) |
110.3 |
|
- |
- |
- |
- |
A(35-36-37) |
112.5 |
|
- |
- |
- |
- |
A(35-36-40) |
108.5 |
|
- |
- |
- |
- |
A(35-36-44) |
108.6 |
|
- |
- |
- |
- |
A(38-35-39) |
107.4 |
|
- |
- |
- |
- |
A(37-36-40) |
110.2 |
|
- |
- |
- |
- |
A(37-36-44) |
110.3 |
|
- |
- |
- |
- |
A(36-37-41) |
111.5 |
|
- |
- |
- |
- |
A(36-37-42) |
111.0 |
|
- |
- |
- |
- |
A(36-37-43) |
111.5 |
|
- |
- |
- |
- |
A(40-36-44) |
106.7 |
|
- |
- |
- |
- |
A(41-37-42) |
107.5 |
|
- |
- |
- |
- |
A(41-37-43) |
107.6 |
|
- |
- |
- |
- |
A(42-37-43) |
107.5 |
|
- |
- |
- |
- |
A(46-45-48) |
110.4 |
|
- |
- |
- |
- |
A(46-45-49) |
110.4 |
|
- |
- |
- |
- |
A(45-46-47) |
112.4 |
|
- |
- |
- |
- |
A(45-46-50) |
108.6 |
|
- |
- |
- |
- |
A(45-46-51) |
108.5 |
|
- |
- |
- |
- |
A(48-45-49) |
107.3 |
|
- |
- |
- |
- |
A(47-46-50) |
110.2 |
|
- |
- |
- |
- |
A(47-46-51) |
110.2 |
|
- |
- |
- |
- |
A(46-47-52) |
110.9 |
|
- |
- |
- |
- |
A(46-47-53) |
111.5 |
|
- |
- |
- |
- |
A(46-47-54) |
111.5 |
|
- |
- |
- |
- |
A(50-46-51) |
106.7 |
|
- |
- |
- |
- |
A(52-47-53) |
107.5 |
|
- |
- |
- |
- |
A(52-47-54) |
107.5 |
|
- |
- |
- |
- |
A(53-47-54) |
107.7 |
The simplest and repeating group of atoms in PFPE-216-1 is CF3 with a bond length C-F equal to (1.336 ± 0.003) Å, which is consistent with the literature data [18] and can be considered to be the same within the accuracy of calculations.
Energetically bond between carbon and fluorine is approximately 443 kJ/mol [21], and between hydrogen and carbon 414 kJ/mol [21], –rather close values (approximately 7% of difference), especially in comparison with difference in distance between atoms C-F and C-H. Similar CH3 group in PE-1 analogue with a bond length of C-H equal to (1.093 ± 0.003) Å, has a bond length of almost 20% less. This observation is logical because the fluorine atom has atomic membranes completely filled with electrons, but a longer bond is assumed to be less strong. Experimental data on the reactivity of perfluorinated ethers of PFPE-216 contradicts this conclusion: the reaction of substitution of individual fluorine atoms in a molecule is not noted, and destruction of the molecule occurs, as a rule, on oxygen ether bonds.
Let us consider other groups of atoms in PFPE-216-1 and PE-1, which numerical characteristics are given in Table 2 and Table 4.
Based on the results of quantum-chemical calculations, the difference in the values of the bond lengths between oxygen and carbon, for example, the coupling length C1-O4 for PFPE-216 is 1.397 Å, and for PE is 1.429 Å, it is greater than about 2%. This testifies to the fact that the hydrocarbon polyethers are more friable in these groups of atoms, while longer bonds between the carbon atoms in the molecule lead to practically the same external dimensions of the molecule. This aspect is important for the processes of formation of donor-acceptor bonds during oxygen using complex formation.
At the same time, if the bond angles between the corresponding C2-C1-O4 atoms are compared 104.4° for PFPE-216 and 106.3° for PE, the difference in the angles is 1.8%, - close values, but reduction of the bond angles near the heteroatoms will make it difficult to complex oxygen complexation in PFPE-216 both sterically and due to the displacement of the electron density to the fluorine atoms of the molecule.
In the case of hydrocarbon polyethers PE-1 and PE-2, mainly negative charges are concentrated on binding oxygen atoms, additionally passing the electron density of the nearest hydrogen and carbon atoms. Charge of positive and additional electron density of PFPE-216 on oxygen atoms is impossible to obtain from fluorine atoms, that is, therefore, molecules with oxygen bridges are more unstable and PFPE-216 is more prone to destruction than perfluoroparaffins without inclusion into the functional groups structure. Quantum-chemical calculations confirm that the ratio of the bond length C-O PE and PFPE-216, redistribution of electronic density in favor of trifluoromethyl groups in PFPE-216 leads to a decrease in the effective bond strength with oxa-atoms in the structure of perforated compounds and reduces the chemical and thermal stability of molecules with such groups, increasing the possibilities of their degradation under natural conditions. So, the result of the quantum-chemical calculation by a program that has proven to be used for hydrocarbons, for fluoropolyethers, also demonstrates good convergence of experimental and literary data.
Conclusions
- By NMR spectroscopy, the predominant structure is a PFPE-216 -1 molecule.
- The glass transition temperature PFPE-216 = -108°C is set. The temperature of the beginning of evaporation by DSC and CTA was 45°C. The decomposition temperature in the sealed crucible was 254°C.
- The calculated values of the energies of PFPE-216 -1 and PFPE-216 -2 are almost identical to each other, as well as the calculated values of PE practically coincide, whereas the differences in the energy of the molecules between the PFPE-216 and PE are several times as evidenced by the significantly greater chemical stability of the PFPE molecules in the experiment.
- The calculated dipole moments of the PFPE and PE differ stronger than would be expected.
- The discussion of charges on specific atoms can serve as the basis for predicting the interaction with large organic molecules and its functional groups, and therefore on the dissolving capacity of PFPE-216 and the possibility of coordinating its molecules.
- Comparison of the data of quantum chemical calculations of perfluorinated and hydrocarbon esters proved the correctness of this type of calculation of the properties of fluorinated molecules: comparison of bond lengths and angles in the structures of molecules corresponds to the literature data for similar compounds.
Proposed method for quantum chemical calculations has shown high convergence with literature data for lengths and angles of bonds, as well as on the distribution of charges of atoms of the molecule, which indicates the expediency of using and the reliability of this method of quantum calculations for fluoroorganic molecules.
Acknowledgements
The research was supported by the Perm Research and Education Centre "Rational Subsoil Use", 2024.
References
- Barabanov V.G., Maksimov B.N. Works of the Russian Research Center "Applied Chemistry" in the field of chemistry and technology of industrial fluorine-containing compounds // Russian Chemical Industry. 2019. Vol. 96, Iss. 6, p. 275–283
- Peganova N.V., Matalin V.A., Lyudikainen A.A., Puzanova N.V., Mikhailova T.V., Lesnevskaya N.B., Kaurova G.I., Bykov V.I., Petrova I.V., Lebedeva V.I., Volkov V.V., Tereshchenko G.F. Electrochemical synthesis of perfluoropolyethers by the Kolbe method // Russian Journal of Applied Chemistry. 2009, Vol. 82, Iss. 12, p. 1976–1984
- Litvinenko E.V., Matalin V.A., Peganova N.V., Lyudikainen A.A. Preparation of perfluoropolyether using electrochemical initiation of polymerization. In book: Innovative directions in the development of science on polymer fibrous and composite materials // Abstracts of reports of the International Scientific Conference, St. Petersburg, 2020, p. 84–85
- Lesnevskaya N.B., Litvinenko E.V., Lyudikainen A.A., Matalin V.A., Mikhailova T.V., Peganova N.V. Comparison of chemical initiation of polymerization and electrodimerization in the synthesis of perfluoro-5,8,11,12,15,18-hexamethyl-4,7,10,13,16,19-hexaoxadecosane // Russian Chemical industry, 2019, Vol. 96, Iss. 6, p. 306–312
- Pospelova N.B., Mokrushin I.G. Features of NMR analysis of perfluorinated compounds. Bulletin of Perm University. Series: Chemistry. 2016, № 3(23), p. 85–91
- http://engineering.technolog.edu.ru/pages/equipment_desc.php?itercount=%201 Date accessed 05.10.2023)
- Brief reference book of physical and chemical quantities // Ed. Ravdel A.A. and Ponomareva A. M. Ed. 8th. Leningrad: Chemistry, 1983, 496 p.
- Eskandari K., Lesani M. Does fluorine participate in halogen bonding? // Chemistry – A European Journal. 2015, Vol. 21, Iss. 12, p. 4739-4747
- Dovesi, R., et al., CRYSTAL14: A Program for the Ab Initio Investigation of Crystalline Solids // International Journal of Quantum Chemistry, 2014. Vol. 114(19), p. 1287–1318
- https://www.chetah.org/ Date accessed 27.12.2023
- Sashina E.S., Kashirsky D.A., Martynova E.V. Features of the molecular structure of pyridinium salts and their dissolving ability in relation to cellulose // Russian Journal of General Chemistry. 2012, Vol. 82, Iss. 4, p. 643–649
- Filimonov D.A., Poroikov V.V. PASS: Forecasting the spectrum of biological activity of organic compounds. RCJ, 2006, Vol. L, Iss. 2
- Alex A. Granovsky, Firefly version 8.0.0, Access mode http://classic.chem.msu.su/gran/firefly/index.html, Date accessed 20.12.2023
- Schmidt M.W., Baldridge K.K., Boatz J.A., Elbert S.T., Gordon M.S., Jensen J.H., Koseki S., Matsunaga N., Nguyen K.A., Su S., Windus T.L., Dupuis M., Montgomery J.A. // J. Comput. Chem. 1993, Vol. 14, p. 1347-1363
- Kashirsky D.A. The influence of the structure of ionic liquids based on 1-alkyl-3-methylpyridinium on their dissolving ability in relation to cellulose: specialty 02.00.04 “Physical chemistry”: PhD thesis / St. Petersburg, 2016, 155 p.
- Stepanov V.N., Nesterov I.A. Application of ab initio methods of quantum chemistry to determine intramolecular effects of interaction of substituents // News of the Samara Scientific Center of the Russian Academy of Sciences. 2006, Vol. 8, Iss. 3 – p. 645–651
- Batsanov S.S. Structural chemistry. Facts and dependencies. M. Dialog-MSU 2000, 292 p.: ill. Hardcover, Regular format. (ISBN: 5-89209-597-5 / 5892095975)
- Vishnevsky Yu.V., Shishkov I.F., Khristenko L.V., Rykov A.N., Vilkov L.V., Oberhammer H. Molecular structure of o- and m-fluoro(tri-fluoromethoxy)benzenes according to gas data electronography and quantum chemistry. Comparison of the structures of trifluoromethoxybenzene and its fluorine derivatives. Journal of Physical Chemistry. 2005. T. 79. No. 10. – P. 1735–1745
- Sina Ebnesajjad, Fluoroplastics// William Andrew, 2015, ISBN 978-1-4557-3199-2, DOI: https://doi.org/10.1016/C2012-0-05997-2
- Georgieff M., Drug containing lipophilic inert gas. Patent WO1998040083, 1998
- Fluorine Chemistry, ed. J. H. Simons// NY, Academic Press, 1950, 615 p.
ARTICLE INFO
Received 26 January 2024
Accepted 13 February 2024
Available online February 2024
Recommended for publication by Prof. S.M. Igumnov
eLIBRARY Document Number (EDN) VTGBJA

Fluorine Notes, 2024, 152, 1-2
