Received: October 2023
DOI 10.17677/fn20714807.2023.06.02
Fluorine Notes, 2023, 151, 3-4
OPTICAL PROPERTIES OF COPOLYMERS OF PERFLUORO-2,2-DIMETHYL-1,3-DIOXOLE AND PERFLUOROHEX-1-ENE OBTAINED AT ULTRAHIGH PRESSURE
V.I. Sokolov1,2, I.O. Goryachuk1, S.I. Molchanova1, E.V. Polunin3
1Federal Scientific Research Center "Crystallography and Photonics" RAS, 119333, Leninsky ave. 59, Moscow, Russia
2Federal Scientific Center, Scientific Research Institute for System Research of RAS, 117218, Nakhimovsky ave. 36, bldg. 1, Moscow, Russia
3N. D. Zelinsky Institute of Organic Chemistry RAS, 119991, Leninsky ave. 47, Moscow, Russia
Annotation: The method of ultrahigh pressure involves synthesis of copolymers of perfluoro-2,2-dimethyl-1,3-dioxole and perfluorohex-1-ene with different molar content of x links of perfluorohex-1-ene in macromolecule without using initiators. The resulting copolymers are amorphous if x < 35 mol% and partially crystalline otherwise. Amorphous copolymers have high optical transparency in the visible and near infrared regions of the spectrum and low refraction index n = 1.29-1.3 (at wavelength λ= 632.8 nm), wherein n increases with growth of x. The copolymers are dissolved in perfluorinated solvents capable of film formation and can be used to create various waveguide elements of integrated optical devices.
Keywords: amorphous perfluorinated copolymers, perfluorohex-1-ene, ultrahigh pressure polymerization, refractive index, light-conducting films.
Introduction
The amorphous perfluorinated polymers exhibit a high optical transparency, a low refractive index n, a low material dispersion dn/dλ≤ in telecommunication regions of the wavelengths close to λ= 0.85, 1.3, 1.55 μm, and therefore are promising for the creation of different waveguide elements of integrated optical devices [1 - 4]. Furthermore, the substitution of hydrogen atoms with fluorine atoms results in a small coefficient of light absorption in the polymer, and the amorphism-to small light scattering.
Furthermore, the perfluorinated polymers are hydrophobic, have high chemical resistance and are more resistant to environmental effects (primarily temperature and humidity) than their hydrocarbon analogues. The range of commercially available amorphous perfluoropolymers is currently quite limited. Such polymers are known to be commercially available only from DuPont (perfluoropolymers of the type TeflonAF), Asachi Glass (perfluoropolymer Cytop), Solvay (Hyflon AD) and Chromis Technologies (CyclAFlor) [5, 6]. As a result, the synthesis of novel amorphous perfluorinated polymer materials having an improved complex of beneficial properties is an urgent problem.
Amorphous perfluoropolymers are prepared by radical homopolymerization or copolymerization of two or some perfluorinated monomers in solutions or emulsions. Furthermore, many perfluorinated monomers, for example perfluorostyrene [7], perfluoropropyl vinyl ether [8], and also perfluorinated olefins of the type CF2 = CF-Rf, where Rf is an aliphatic perfluorinated radical, and which are highly difficult to react in radical polymerization under normal conditions. As a result, said monomers are polymerised at high pressure (10-20 thousand atm.) and a temperature of 100-200°C [7-12].
The perfluorinated copolymers produced by radical polymerization can be amorphous or polycrystalline, wherein the degree of crystallinity depends not only on the chemical structure of the monomers used, but also on the molar ratio of the links of said monomers in the copolymer macromolecule. For example, copolymers of perfluoro-2,2-dimethyl-1,3-dioxole and perfluoropropylvinyl ethers are generally amorphous if the molar concentration of ether links in the macromolecule does not exceed 30% [13]. On the other hand, copolymers of perfluoro-2,2-dimethyl-1,3-dioxole and perfluorobutenyl vinyl ether are amorphous at any molar ratio of links from these monomers [14]. Synthesis was carried out by super-high pressure synthesis of amorphous copolymers of perfluoro-2,2- dimethyl-1,3-dioxole and perfluorohexa-1-ene with different molar content of x links of perfluorohexa-1-ene in macromolecule. These copolymers are shown to be amorphous at x < 35 mol% and partially crystalline at x > 35 mol%. The amorphous copolymers have a high optical transparency in the near-infrared region of the spectrum and a low refractive index n = 1.29-1.30. They are dissolved in perfluorinated solvents capable of film formation and can be used for creation of waveguide elements of integrated optical devices.
Synthesis of perfluorinated copolymers at ultrahigh pressure
In order to obtain copolymers, monomers are used: perfluoro-2,2-dimethyl-1,3-dioxole D1 and perfluorohex-1-ene H1 of the Russian Company P&M Invest, see Figure 1. These monomers are transparent colorless liquids and have a degree of chemical purity greater than 88%. Synthesis of copolymers was carried out without the use of initiators in Teflon ampoules in a volume of 1-2 ml in cylinder-piston moulds at a pressure of 14-15 thousand atm. and a temperature of 150°C. The reaction time ranged from 168 to 336 hours. Note that monomer D1 is more active during radical polymerisation than monomer H1, and can play the role of a timely initiator when producing a copolymer starting from its concentration of about 35 mol%.
Prior to synthesis, the monomers were distilled in an argon atmosphere to purify the dioxole from the stabilizer, as well as to remove moisture and dissolved oxygen, which is known to be a radical polymerization inhibitor. The product obtained after completion of the reaction and opening of the ampoule is a solid rubber-like substance or a strongly viscous transparent gel containing, in addition to the linear copolymer, volatile components (for example, unreacted monomers) and various by-products of the reaction (dimers, oligomers, etc.). To remove these substances, the copolymers were vacuumized to a constant weight at 120°C. The typical yield of the useful product is 40-50%.
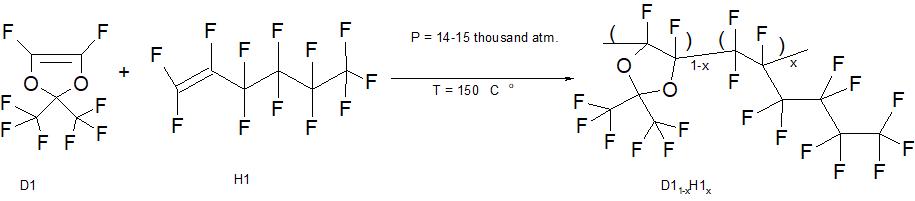
Figure 1. The synthesis scheme of copolymers of perfluoro-2,2-dimethyl-1,3-dioxole D1 and perfluorohex-1-ene H1 by radical polymerisation at ultrahigh pressure without the use of initiators. X is the molar concentration of H1 units in the macromolecule of the copolymer D11-xH1x.
Copolymers D11-xH1x with different molar content of perfluorohex-1-ene units in macromolecule from x = 5 mol% to 75 mol% have been synthesized by described method. This content was determined from 19F NMR spectra of the obtained copolymers. Figure 2a and Figure 2b shows NMR spectra of monomers D1 and H1, respectively, and in Figure 2b is the spectrum of the copolymer D11-xH1x with x≈ 0.4. All spectra were obtained on a Bruker AM-300 device (282.40 MHz), wherein the spectra of D11-xH1x copolymers were measured in hexafluorobenzene.
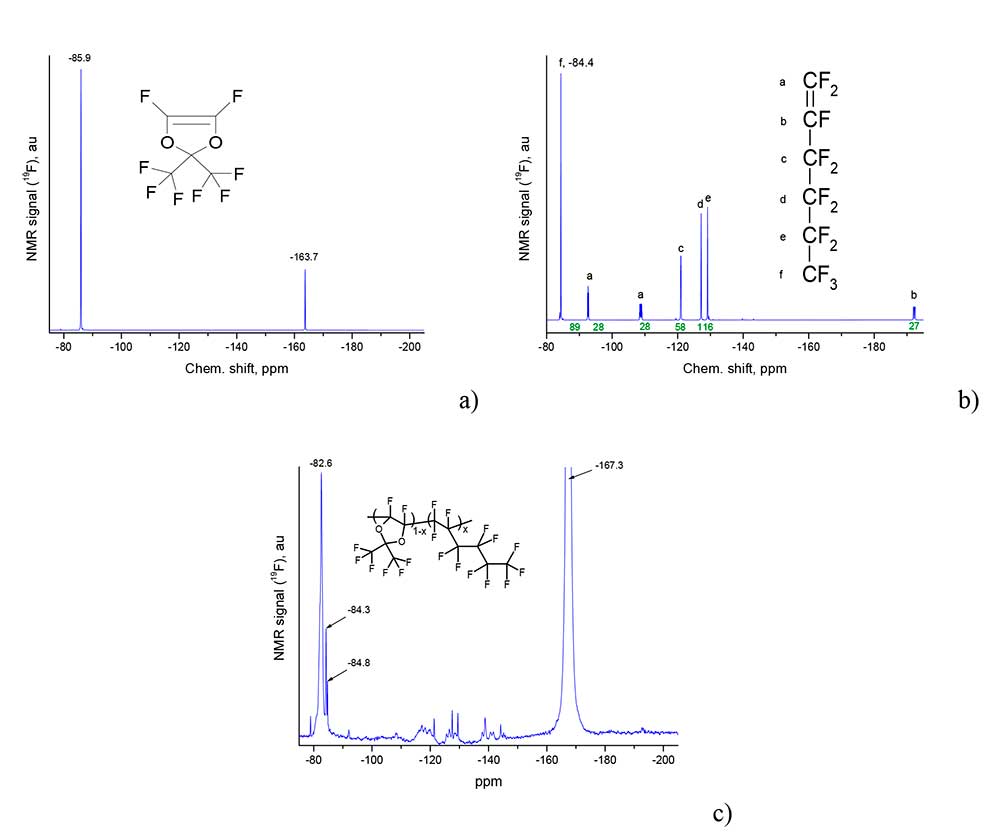
Figure 2. 19F NMR spectra of monomers D1 (a) H1 (b) and copolymer D11-xH1x with x≈0.4 (c), obtained on a Bruker AM-300 device (282.40 MHz).
Signal -85.9 ppm in Figure 2a corresponds to six fluorine atoms in two trifluoromethyl groups, and signal -163.7 ppm corresponds to two separate atoms F with double C = C bond in monomer D1. Reference of signals a, b, c, d, e, f in monomer H1 and their position is represented by Figure 2b.
Let us analyze of positions 19F of NMR signals and their association with molecular groups for copolymer D11-xH1x with x≈ 0.4, see Figure 2c. First of all, it is noted that the signal -167.3 ppm is related to the solvent (hexafluorobenzene) in which the copolymer was dissolved. The broadened singlet at -82.6 ppm corresponds to six fluorine atoms in the methyl groups of perfluorodioxolane D1, and the signal at -84.3 ppm corresponds to the terminal trifluoromethyl group of perfluorohex-1-ene H1 in the copolymer macromolecule. The multiplets at -125 (2F), -131 (4F) and -135 ppm correspond to CF2 groups in the side chains of the polymer in different versions of the coupling of hexenoid and dioxole fragments. Broadened signals of fluorine atoms in the main chain of polymer are located in the same field from -110 to -150 ppm. From the analysis of Figure 2b it is possible to evaluate the ratio of the units of dioxole and hex-1-ene in the copolymer as D1:H1≈3:2 (x≈0.4).
In order to estimate the molecular weight of the copolymers D11-xH1x, the average hydrodynamic diameter of the macromolecular globules in the perfluorooctane is measured. The measurements were made by dynamic light scattering using 90Plus_Zeta particles/proteins analyzer (Brown Instruments Corp, USA) under illumination with a laser beam with a wavelength of 640 nm. A typical histogram of globule size distribution is shown in Figure 3. It is seen that the average diameter of the globules is equal to 37 nm. Consequently, copolymers D11-xH1x synthesized by radical copolymerization at ultrahigh pressure without the use of initiators can be attributed to the class of high molecular weight substances.
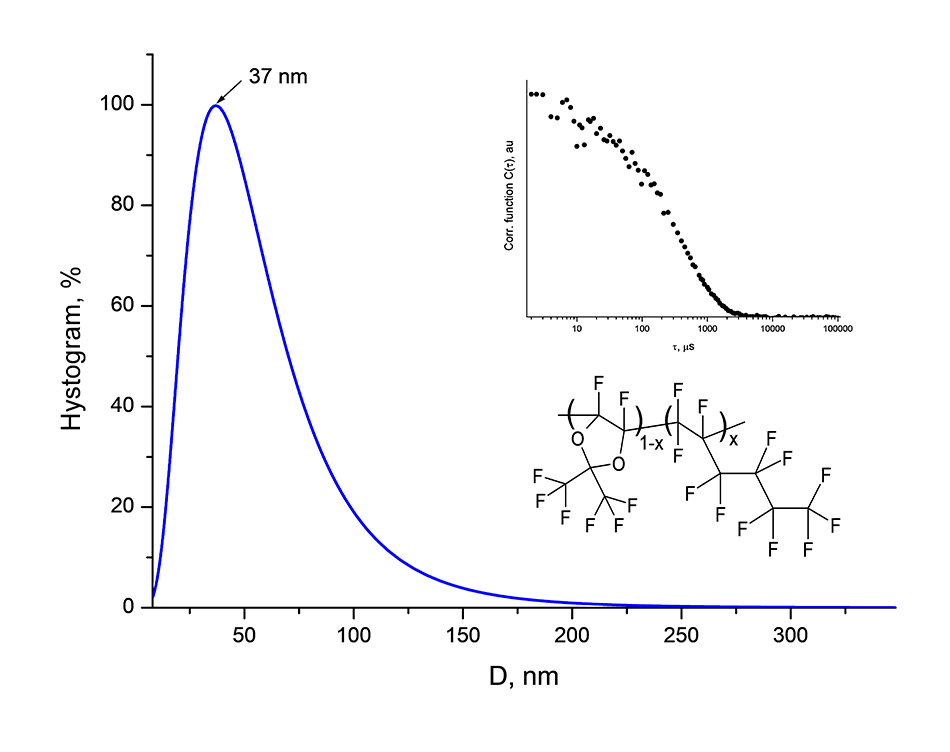
Figure 3. Histogram of distribution of macromolecular globules of copolymer D11 -xH1x with x = 0.5 in size, measured by dynamic light scattering in perfluorooctane. D is the diameter of the globule. The inserts are in the form of an auto-correlation function C (τ), where τ is the time in msec, and also a fragment of the copolymer’s structure.
Analysis of structure of perfluorinated copolymers by method of wide-angle X-ray scattering
Synthesis of copolymers at ultrahigh pressure in the absence of initiator is carried out by radical mechanism [8-12]. Formation of an ordered structure capable of causing polycrystallites in the copolymer and, accordingly, increased light scattering, is unlikely for the following reasons. First, the coupling of the perfluorohex-1-ene links H1 in the polymer macromolecule can be either head-to-head and head-to-tail. Secondly, links D1 and H1 in the polymer chain can occur in different sequences. Furthermore, all asymmetric carbon atoms can have either R- or S-configuration. These factors increase the degree of disorder of the links in the macromolecule and lead to the formation of amorphous materials.
Structural diagnostics of perfluorinated copolymers was carried out on a wide-angle X-ray diffractometer Rigaku Miniflex600 (Cu, λ= 1.54184 A) in the range of angles from 3 to 100 degrees. Diffractogram of the film from D11-xH1x с x = 0.5 is presented in Figure 4a. As follows from this figure, only wide halo-age of 2 ˜ 9.0, 14.6, 24.0 and 37.4 degrees are observed in the diffractogram, which indicates the amorphism of this material. Similar form had diffractograms of films with x < 0,35. Note that the films with x < 0.35 appear to be transparent. Figure 4b shows the diffractograms of the film from the copolymer D11-xH1x с x = 0.67. It can be seen that besides halo in the diffractogram there is a sharp peak at 2 = 4.2 degrees, as well as other peaks with a smaller amplitude. A film made from this copolymer is not transparent (matt), which leads to a conclusion about its polycrystallinity. Films made from copolymers D11-xH1x с x > 0.5 were also matt. From the analysis of the obtained data, it can be concluded that the copolymers D11-xH1x are amorphous if x < 0.35 and partially crystalline otherwise.
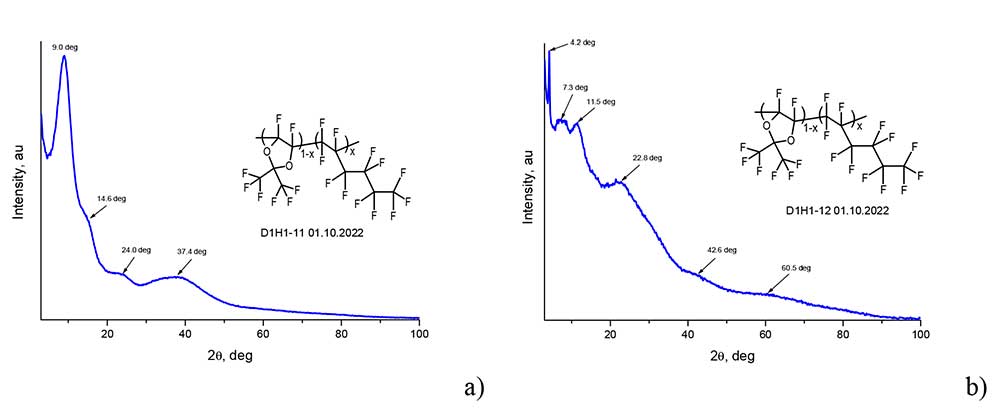
Figure 4. Diffractograms of films made from copolymers D11-xH1x with x = 0.50 (а) and x = 0.67 (b), obtained on an X-ray diffractometer Rigaku Miniflex600.
Synthesized perfluorinated copolymers at all values x are dissolved at room temperature in perfluorinated solvents such as perfluorohexane, perfluorooctane, hexafluorobenzene, perfluoro-(1,3-dimethyl)-cyclohexane (carbogal), perfluorodecaline and are capable of film formation.
Study of IR spectra of absorption of perfluorinated copolymers
IR absorption spectra of copolymers D11-xH1x с x < 0.5 were measured on a Shimadzu8400 Fourier spectrometer. For this purpose, polymer films with a thickness of 1-10 µm on KBr substrates were formed by centrifugation. The transmission spectra of a pure substrate and a substrate with a polymer film applied thereto were measured. The absorption spectrum of the copolymers is determined from their ratio. Figure 5 shows an absorption spectrum of the film of copolymer D11-xH1x с x = 0.5.
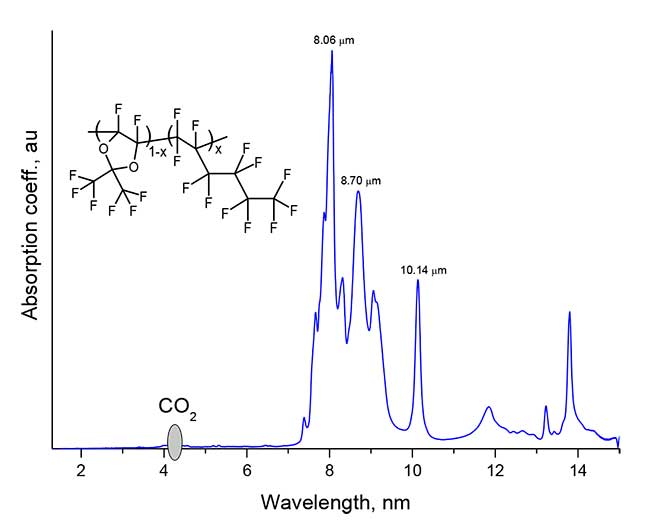
Figure 5. The absorption spectrum of the film from the perfluorinated copolymer D11-xH1x with x = 0.5, applied to the KBr substrate by centrifugation from the solution.
As can be seen from Figure 5, the copolymer does not have intense absorption bands below 2 µm, which indicates high optical transparency thereof in the telecommunications C-wavelength range of 1530 -1565 nm. The most intense absorption bands of the copolymer are in the range of 7.5-10 microns, due to the valence fluctuations of the CF, CF2 and CF3 groups in the macromolecule.
Measurement of refractive index of perfluorinated copolymers by refractometric method
Refractive index n of light guide films made from copolymers D11-xH1x on KBr substrates, as well as on glass substrates, was carried out by a refractometric method at a wavelength of λ= 632.8 nm at TE and TM polarization of the probing laser beam using a Metricon 2010/M prism communication device. The films were produced by spin-coating method from solutions of copolymers in perfluorooctane and heated at 120°C for 24 hours to completely remove the solvent. Measurements were carried out either by determining the critical angle of total internal reflection, or (for films having a thickness of 4 to 7 μm) using the m-line method. The measurement results are shown in Figure 6. As follows from Figure 6, values of refractive indices nTE and nTM in directions along and across film are close, which testifies to its isotropy. Index of refraction depends on molar concentration of x links of perfluorohexa-1-ene in macromolecule and increases with growth of x. Figure 6 also follows that the refractive index of the copolymer with low values of x < 0.35 lies in the range of n = 1.29-1.30.
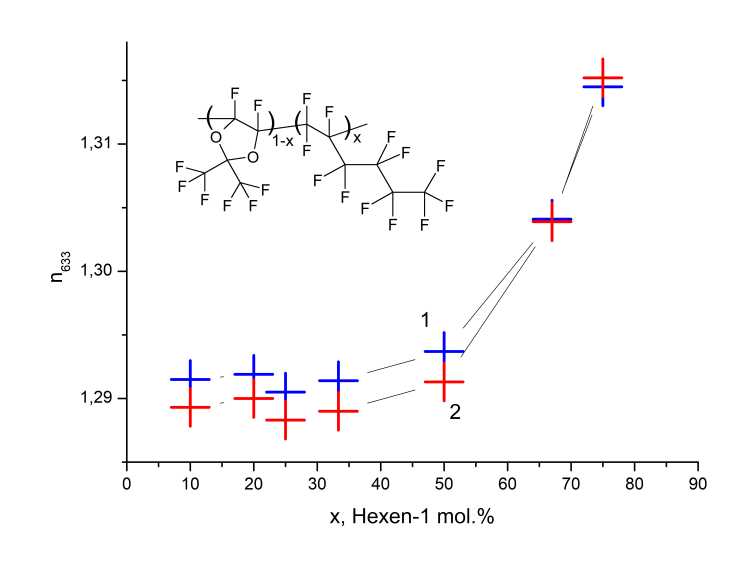
Figure 6. The refractive indices nTE (1) and nTM (2) of the copolymers D11-xH1x at a wavelength of 632.8 nm, depending on the molar concentration of x perfluorohex-1-ene in the initial mixture of monomers.
Thus, the perfluorinated copolymers D11-xH1x are capable of film forming and can be used to form waveguides and other elements of photonic devices. In particular, they can be used as a waveguide shell in the manufacture of optical data transmission buses on printed circuit boards. Low refractive index values of these copolymers make it possible to use them as envelope of high-aperture quartz fibers.
Conclusion
Copolymers of perfluoro-2,2-dimethyl-1,3-dioxole D1 and perfluorohex-1-ene H1 are synthesized by the method of ultrahigh pressure. Copolymers of D11-xH1x are shown to be amorphous at x < 0.35 and partially crystalline otherwise. The amorphous copolymers D11-xH1x have high optical transparency in telecommunication wavelength ranges close to 0.85, 1.3 and 1.5 microns, have a low refractive index n = 1.29-1.30, are capable of film formation and are promising for the manufacture of various waveguide elements of integrated optical devices. This expands the class of amorphous perfluorinated polymers for various uses in photonics. The synthesized copolymers can be used as a shell in the production of waveguide amplifiers for the telecommunication S-wavelength band of 1530 - 1565 nm based on polymers with embedded fluoride nanocrystals doped with erbium, as well as for manufacturing the shell of waveguide optical light modulators.
Acknowledgements
This work was financially supported by the Ministry of Science and Higher Education of the Russian Federation within the framework of the state task of the Federal Research Center «Crystallography and Photonics» of the Russian Academy of Sciences. The equipment of the Center for Collective Use of the Federal Research Center «Crystallography and Photonics» of the Russian Academy of Sciences was used in the work.
References
- W. Groh, Overtone absorption in macromolecules for polymer optical fibers, Makromol. Chem., 1988, 189, 2861-2874.
- W. Groh, A. Zimmermann, What is the lowest refractive index of organic polymers, Macromolecules, 1991, 24, 6660-6663.
- M. Zhou, Low-loss polymeric materials for passive waveguide components in fiber optical telecommunication, Opt. Eng., 2002, 41(7), 1631-1643.
- A. Vitale, R. Bongiovanni, B. Ameduri, Fluorinated Oligomers and Polymers in Photopolymerization, Chemical Reviews, 2015, 115(16), 8835-8866.
- B. Ameduri, The Promising Future of Fluoropolymers, Macromolecular Chemistry and Physics, Wiley-VCH Verlag, 2020, 221(8), 1900573.
- M.A. El-Okazy, L. Liu, C.P. Junk, E. Kathmann, W. White, S.E. Kentish, Gas separation performance of copolymers of perfluoro(butenyl vinyl ether) and perfluoro(2,2-dimethyl-1,3-dioxole), Journal of Membrane Science, 2021, 634, 119401.
- L.A. Wall, D.W. Brown, High pressure polymerization of perfluorostyrene, Journal of Fluorine Chemistry, 1972/73, 2, 73-85.
- E.V. Polunin, S.I. Molchanova, J. E. Pogodina, V.I. Sokolov, I. V. Zavarzin, Homo- and co-polymerisation of perfluoroisopropylvinyl ether under high pressure, Fluorine Notes, 2017, 114, 5‑6.
- A.A. Zharov, I.A. Guzyaeva, Kinetics and mechanism of thermal polymerization of hexafluoropropylene under high pressures, Russian Chemical Bulletin, 2010. 59(6), 1225-1231.
- H.S. Eleuterio, Polymers of perfluoropropylene, US 2958685, 1960.
- V.I. Sokolov, V.E. Boyko, I.O. Goryachuk, S.M. Igumnov, S.I. Molchanova, Yu.E. Pogodina, E.V. Polunin, Synthesis and optical properties of copolymers of perfluoro-2,2-dimethyl-1,3-dioxole and perfluoropropyl vinyl ether, Russian Chemical Bulletin, International Edition, 2017, Vol. 66, 66(7), 1284-1289
- V. I. Sokolov, I. O. Goriachuk, I. V. Zavarzin, S. I. Molchanova, Yu. E. Pogodina, E. V. Polunin, and A. A. Yarosch. New copolymers of perfluoro-2-ethyl-2-methyl-1,3-dioxole and perfl uorovinyl ether with low non-monotonic refractive index. Russian Chemical Bulletin, International Edition, 2019, 68(3), 569-564.
- E.N. Squire, Amorphous copolymers of perfluoro-2,2-dimethyl-1,3-dioxole, US 4754009, 1988.
- P.R. Resnick, Amorphous copolymers of two fluorinated ring monomers, US 5276121, 1994.
ARTICLE INFO
Received 16 October 2023
Accepted 11 December 2023
Available online December 2023
Recommended for publication by PhD V.L. Don
eLIBRARY Document Number (EDN) PWLITX

Fluorine Notes, 2023, 151, 3-4
