Received: October 2023
DOI 10.17677/fn20714807.2023.06.01
Fluorine Notes, 2023, 151, 1-2
RATIOS OF PRIMARY SEPARATIONS IN IONIC SERIES OF MASS-SPECTRA OF PERFLUOROALKANES, PERFLUOROCYCLOHEXANE, EICOSANE, CYCLOTRIACONTANE, CONTAINING REGULAR FRAGMENT GROUPS (C2H4 or CF2)
N. D. Kagramanov
A.N. Nesmeyanov Institute of Organoelement Compounds RAS, Russian Federation, 119334, Moscow, Vavilov str. 28.
e-mail: ndkagram@gmail.com
Abstract: In ion series of mass spectra of alkanes, cycloalkanes, perfluoroalkanes and perfluorocycloalkanes, containing regular fragment groups (C2H4 or CF2), primary separation of one or more radicals occurs by a similar mechanism. The subsequent emissions of the regular fragment groups in all ionic series of the mass spectrum of the compound, as a rule, have the same or similar total masses. That is, the relative excitation energy costs +·M of several ion series of the spectrum differ by the energies of the primary tearing and the number of radicals to be separated. Primary separation of radicals is substantially equalizing the energies of all ion series. As a result, practically identical breaks of regular fragment groups take place in all series. Ionic series of n-alkanes and cycloalkanes, as well as perfluoroalkanes have been presented earlier. In order to compare the primary tearing occurring in them, it is necessary to return to them again. The difference in excitation energies, molecular cation-radicals of ion series is a result of dispersion of ion source electrons energy. Ratio of maximum and minimum excitation energy of molecular cation-radicals of n-perfluoroalkanes, perfluorocyclohexane and eicosane equal to 3: 1 corresponds to maximum - 3 and minimum -1 number of primary, synchronously detachable radicals. It can also be estimated by the ratio of the energies of the rupturable connections. Analysis of mass spectra of compounds containing regular fragmental groups makes it possible to conclude that triple difference of energies of primary breaks in ion series of mass spectrum does not conflict with postulate of quasi-equilibrium theory, describing uniform distribution of energy before beginning of decomposition. Synchronous primary separation of three fluorine atoms (C2, C1 and C20F3) in one of series of perfluoroeicosane, and also two hydrogen atoms and a radical .CH3 (C2, C1 and C20H3) in one of the eicosane series confirm the alignment of the excitation energy +.M corresponding series, even before the onset of decay.
Keywords: primary synchronous detachment of radicals, decay sequences, ionic series of eicosane, perfluoroeicosane, cyclotriacontane, perfluorocyclohexane.
Introduction
Compounds containing regular fragmental groups CF2 or CH2 are simple and therefore the best object for studying primary detachments occurring in their ionic series. Primary detachment of radicals and subsequent emissions of regular fragment groups with formation of corresponding ionic series in mass spectra of eicosane, 1-bromoeicosane and 1,20-dibromoeicosane was reported earlier [1].
Ionic series of perfluoroeicosane, perfluorocyclohexane, cyclohexane and cyclotriacontane were presented in article [2]. The aim of the present invention is to compare and estimate the relative energies of primary detachments in ionic batches of linear and cyclic alkanes, as well as perfluoroalkanes and perfluorocyclohexane.
The method of establishing primary detachments of ionic series
In order to determine the primary or several primary detachments occurring in one of the ionic series of the compound containing the regular fragment groups C2H4 or CF2, it is necessary to install the first fragment ion, the resulting at detachment of one, two or three primary radicals. In the spectra of higher homologs of n-alkanes and n-perfluoroalkanes, the peaks of molecular ions and also of the first fragment ions are either absent or have an extremely low intensity. To establish the first fragment ion of the ion series, a "reverse" sequence of fragmentation of any intense peak of the "final" ion of one of the ionic series can be used. By adding to the mass of the selected fragment ion, regular fragment groups (28) C2H4 in alkanes or (50) CF2 in perfluoroalkanes can be produced in several variants of mass, smaller than the mass of the molecular ion by the amount of detachment of one or more primary radicals.
In the spectra of perfluoroalkanes, depending on the ionic series, the number of primary fluorine atoms to be detached varies from one to three. Primary detachments of .CH3 and .C2H5 radicals occur in n-alkane spectra, as well as combined detachments of one or two hydrogen atoms and .CH3 and .C2H5 radicals. The final stage in the decrypt of the original detachments is a comparison of total masses of primary detached radicals of all ion series and establishment of their separation sequence. To decrypt primary detachments, containing several radicals, there is desirable to analyze the fragmentation of the nearest homologues. However, the fragmentation and ionic series of the C1‑C5 homologues may differ from the C9-C20 homologues fragmentation. Thus, only one series of consecutive separations of four fluorine atoms occurs in the mass spectrum of tetrafluoromethane.
Primary detachments and ionic series of eicosofluoronane, perfluoro-2,4-dimethyl-3- ethylpentane, perfluoroeicosane and perfluorocyclohexane
Published mass spectra of higher perfluoroalkanes are few. In the NIST libraries, only the perfluoroeicosane C20F42 spectrum is presented from higher perfluoroalkanes. The mass spectrum of eicosofluorinane published in [3,4] is presented in Table 1.
Table 1. The mass spectrum of eicosofluorinane C20F42 [3,4].
|
Ion |
Formula |
m/z |
|||
|
+.M |
C9F20 |
488 0 % |
488 0 % |
||
|
+.M -.F |
C9F19 |
469 0 % |
|||
|
+.M -3.F |
C9F17 |
431 0,2% |
|||
|
+.M -3.F -CF2 |
C8F15 |
381 0,3% |
|||
|
+.M -.F -2CF2 |
C7F15 |
369 0,1% |
|||
|
+.M -3.F-2CF2 |
C7F13 |
331 0,4% |
|||
|
+.M -.F -3CF2 |
C6F13 |
319 0,1% |
|||
|
+.M -3.F-3CF2 |
C6F11 |
281 1,6% |
|||
|
+.M -.F -4CF2 |
C5F11 |
269 2,6% |
|||
|
+.M -3.F-4CF2 |
C5F9 |
231 2,4% |
|||
|
+.M -.F -5CF2 |
C4F9 |
219 7,1% |
|||
|
+.M -3.F-5CF2 |
C4F7 |
181 5,6% |
|||
|
+.M -.F -6CF2 |
C3F7 |
169 17,2% |
|||
|
+.M -.F-6CF2 -.F |
C3F6 |
150 1,8% |
|||
|
+.M -3.F-6CF2 |
C3F5 |
131 17,6% |
|||
|
+.M -3.F-6CF2 -2.F |
C3F3 |
93 2,5% |
|||
|
+.M -.F-7CF2 |
C2F5 |
119 13,8% |
|||
|
+.M -.F-6CF2 -.F -CF2 |
C2F4 |
100 7,1% |
|||
|
+.M -3.F-7CF2 |
C2F3 |
81 0% |
|||
|
+.M -.F-8CF2 |
CF3 |
69 100% |
|||
|
+.M -.F-6CF2-.F-2CF2 |
CF2 |
50 0,9% |
|||
|
+.M -3.F-6CF2-2.F-C2F2 |
CF |
31 3,6% |
|||
|
+.M -3.F-8CF2 |
CF |
31 3,6% |
In linear perfluoroalkanes because of the dense "packing" of the chain with fluorine atoms, the primary detachments of the perfluoroalkyl radicals .CF3 and .C2F5 are not possible. For this reason, only the detachments of fluorine atoms [5] occurs in the perfluoroalkanes. The two primary separations from which the fragmentation of eicosofluorinonane begins are the detachments of one, as well as three fluorine atoms.
As a result of these detachments, two main ion series of spectrum occur: Perfluoroalkyl (peaks are marked with red color, the last significant digit of mass 9) +.M-.F -8CF2 → +69 and perfluoroalkenyl (peaks are marked with blue color, the last significant digit of mass 1) +.M -F(C2) -F(C1),-F(C9) -6CF2→+131 -2CF2→+31. Both ionic series are branched. When a fluorine atom is detached from the alkyl ion with m/z 119, an olefin ion is formed +.C2F4 m/z 100 (marked with a violet color and the last significant digit of mass 0), and ion +C3F3 with m/z 93 is generated in case of detachment of two fluorine atoms from alkenyl ion with m/z 131. Since the total number of CF2 detachments in the perfluoroalkyl and perfluoroalkenyl series is equal to eight, it can be concluded that the alkenyl series (detachment of three fluorine atoms) is three times more energy-consuming than the alkyl series which begins with the detachment of one fluorine atom. The ionic series of the non - linear structural isomer of eicosofluorinonane, perfluoro -2,4 - dimethyl -3 - ethylpentane is given in Figure 1.
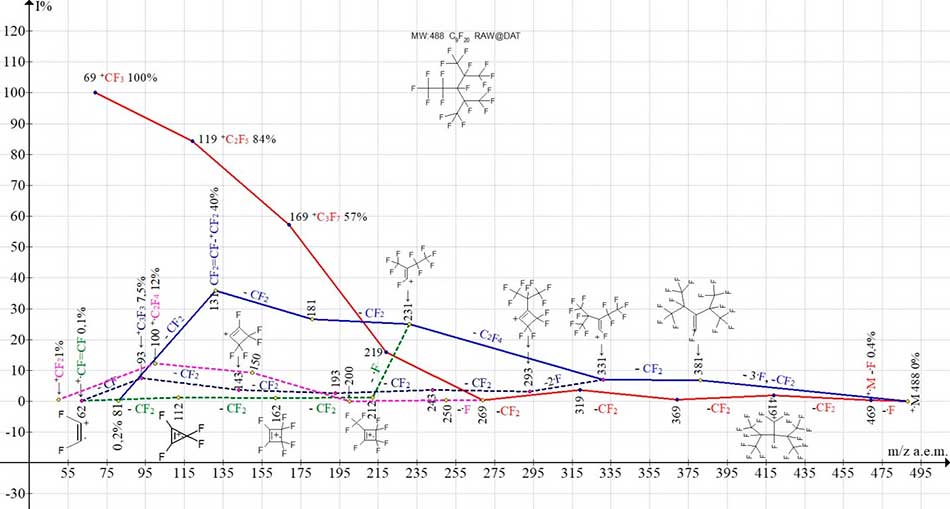
Figure 1. Ionic series of mass spectrum of perfluoro-2,4-dimethyl-3-ethylpentane C9F20 MW:488 (ineos.ac.ru ).
Two main ionic series of asymmetric isomer C9F20 (Figure 1): perfluoroalkyl (marked with a red color, the last significant number of masses 9) and perfluoroalkenyl (marked with a blue color, the last significant digit of masses 1), as well as a series of linear isomer, begin with primary detachments of one and, respectively, three fluorine atoms. At detachment of one fluorine atom there is the first ion of perfluoroalkyl series with m/z 469 (0,4 %). When detachment from the perfluoroalkyl ion with m/z 269, the second in the alkyl series of the fluorine atom is branched to form an olefin series of ions with m/z 250, 200, 150, 100, 50 (marked with a violet color, the last significant digit of masses is 0). The perfluoroalkenyl series is branched twice. When two fluorine atoms detachment from the ion with m/z 331, there is a series of ions with m/z 293, 243, 193, 143, 93 and 62. When one fluorine atom is detached from ion 231, a series of ions with m/z 212, 162, 112, 62 occurs (marked with a dotted blue line). Branching of ionic series with additional fluorine atoms detachment is probably the result of energetically advantageous cyclization of ions with m/z 250, 293 and 212. Fragmentation of two ion series with m/z 293 and 212 is completed with ion +CF.CF with m/z 62. Total number of ionic series of mass spectrum is equal to five.
In the mass spectrum of perfluoroeicosane C20F42 MW 1038 NIST #: 239239 ID #: 36518 DB: mainlib (Figure 2), two main ion series (perfluoroalkyl and perfluoroalkenyl) differ in the topology of electron removal (C1 and C2), the number of primary detachments of fluorine atoms 1 and 3 and the number of emissions of CF2 19 and 17. When the electron of the terminal group of CF3 is detached, one fluorine atom is emitted. In contrast to the mass spectrum of the asymmetric isomer C9F20 (Figure 1) in which there is a peak +.M-.F, in the C20F42 (Figure 2) spectrum, it is not manifested. The first peak of the perfluoroalkyl series C20F42 is a peak with m/z 769 (0.1%) corresponding to the detachment of one fluorine atom and the five CF2 groups.
Another variant of the primary detachment leading to the release of two more fluorine atoms is the detachment of one fluorine atom from the CF2 -group of the terminal perfluoroethyl group. When the electron is detached from the C2F5 group, one fluorine atom is emitted to form a CF3(CF2)n+CF-CF3* cation. This emission "provokes" the additional energetically advantageous detachment of the fluorine atom of the group (1).
[M]+. -1.F → CF3(CF2)n+CF-CF3* -2.F → CF3(CF2)n+CF-.CF2 (1)
CF3(CF2)n+CF-.CF2 -3.F → +CF2 (CF2)nCF=CF2 (2)
The detachment of the third fluorine atom from the opposite terminal group CF3 results in the transfer of a positive charge and the occurrence of a double bond (2) which protects one of the flanks of the chain from fragmentation. The possibility of transferring a positive charge or a "hole" is reported in [6]. Then, the regular fragment groups of CF2 are detached.
The first detectable ion of an alkenyl series with m/z 931 0.5% (M-107) (series is marked with blue, the last significant digit of masses 1) corresponds to the primary detachment of three fluorine atoms M-57 (M-3F) and the emission of CF2.
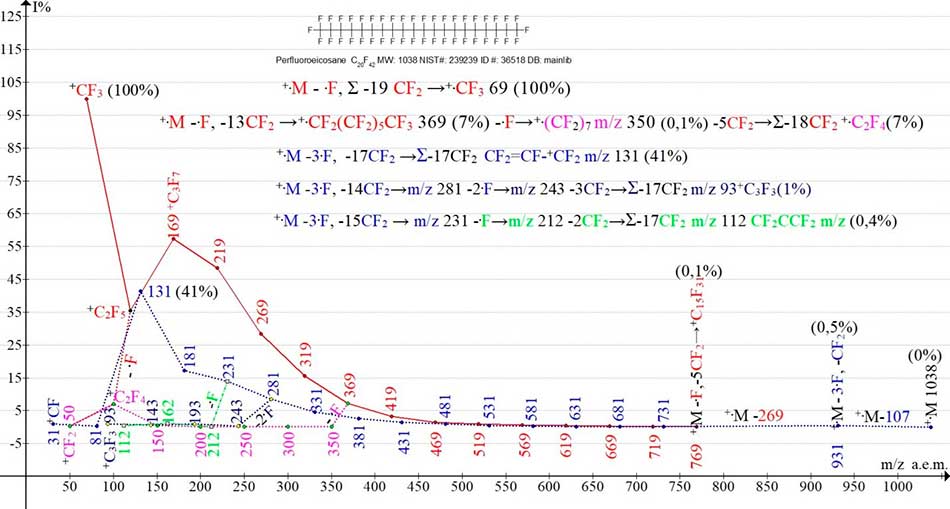
Figure 2. Five ionic series of mass spectrum of perfluoroeicosane C20F42 MW:1038 NIST# 239239 ID: 36518 DB:mainlib.
In fragmentation and a decrease in the molecular weight of the perfluoroalkyl series ions, the probability of structural isomerization thereof increases, with a positive charge being transferred to the centre of symmetry. The structural isomerization of linear ions is also possible in a perfluoroalkenyl series (ion with m/z 131). Branching of ionic series in spectra C9F20 and C20F40 occur at different masses of fragmentizing ions, except for detachment of one fluorine atom in perfluoroalkenyl series from ion with m/z 231. When a fluorine atom is detached from the perfluoroalkyl series ion with m/z 369 (having a mass per 100 Da that is greater than the C9F20 spectrum), said fluorine atom is branched to form an olefin series of ions with m/z 350, 300, 250, 200, 150, 100, 50 (marked with a violet color, the last significant digit of ionic masses is 0). When two fluorine atoms are detached from ion with m/z 281 (with a mass by 50 Da less than in spectrum C9F20), as well as one fluorine atom from ion with m/z 231 of perfluoroalkenyl series two additional low-intensity series of ions occur: with m/z 243, 193, 143, 93, as well as with m/z 212, 162, 112, 62. Branches of a perfluoroalkenyl series with 3+2 and 3+1 fluorine atoms are likely to result from two energetically advantageous over-grouping of perfluoroalkenyl ions.
The present examples of five ionic series of C9F20 isomers, as well as perfluoroeicosane C20F42, confirm the same primary fluorine atom detachment, ionic series and the release sequences of regular fragment groups CF2. Since the binding energy of C-F to n-perfluoroalkanes is 116 kcal/mol (5.03 eV) [5], the energy required for the primary detachment of three fluorine atoms is about 348 kcal/mol (15.09 eV). Ratio of primary detachment of fluorine atoms of two main ionic series is 1:3, probably, is a result of two versions of electron removal, acquisition and consumption of excitation energy. However, considering the energy gain of the double bond formation, this ratio may be somewhat less. The detachment of the fluorine atom in the branching of the perfluoroalkyl series, and the three additional detachments of the fluorine atoms as a result of the two branches of the alkenyl series do not change the energies ratios of the fluorine atom detachments in the two main ionic series (alkyl and alkenyl). The ratio of the sums of the detached fluorine atoms in the two main ionic series, considering the branches thereof, is 2:6, which is also equal to 1:3. The number of CF2 cuts occurring in three ionic series: in perfluoroalkyl - 19, in perfluoroalkenyl – 17, and in perfluorolevine - 18. It should be noted that synchronous detachment of three fluorine atoms from three terminal groups CF3, followed by emission of ten groups (CF2) 1-10 takes place also in one of three main fragmentation series of perfluorotert-butylamine (PFTBA) [7]. This series is terminated by formation of ion CF2+N CF2 with m/z 114 (0.4%).
The mass spectrum of perfluorocyclohexane C6F12 MW 300 (NIST#: 34431 ID#: 115484 DB: mainlib), as well as the mass spectrum of perfluoroeicosane, includes five ionic series (Table 2) [2].
Table 2. Five ionic series of perfluorocyclohexane C6F12 MW 300.
|
Series |
C6F12 |
M 300 0,7% |
-CF2 |
-CF2 |
-CF2 |
-CF2 |
-CF2 |
∑I %= 308,8 |
I series current, % |
|
1 |
M-.F |
281 3.7% |
231 20.9% |
181 21.4% |
131 100.0% |
81 2.1% |
31 21.8% |
170.6 |
55.2 |
|
2 |
M-2.F |
262 Tr |
212 0.1% |
162 3.9% |
112 2.5% |
62 1.2% |
7.7 |
2.5 |
|
|
3 |
M-3.F |
243 Tr |
193 2.7% |
143 2.0% |
93 15.0% |
43 0.3% |
20.0 |
6.5 |
|
|
4 |
M/2 M- C3F6 |
150 0.4% |
100 29.2% |
50 5.1% |
34.7 |
11.3 |
|||
|
5 |
*M-.C3F5 |
169 Tr |
119 5.5% |
69 70.3% |
75.8 |
24.5 |
Tr – the peak intensity in % at the trace level.
In the mass spectrum of perfluorocyclohexane, as in the spectra of n-perfluoroalkanes, primary synchronous detachments of one, two and three fluorine atoms occur, with the formation of three ionic series N1-N3 (Table 2). Formation of three ion series at detachments of one, two and three fluorine atoms of perfluorocyclohexane is difficult to explain various topologies of electron removal. In linear perfluoroalkanes, the primary separation of perfluoroalkyl radicals does not typically occur [5] because of the "packing" of the chain with fluorine atoms. In fragmentation, the non-linear perfluorocyclohexane molecule C-C of the cycle link becomes available for separation. Two ionic series (olefinic N4 and alkyl N5) (Table 2) occur as a result of symmetric decomposition of M/2 with rupture of two C-C bonds as well as asymmetric, regrouping decomposition with detachment of perfluoroallyl radical +.M- .C3F5 *+CF2CF2CF3 m/z 169 Tr.
As a result of symmetric decomposition, olefin series ions +C3F6 m/z 150 0.4%, +C2F4 m/z 100 29% and +CF2 m/z 50 5% occur.
As a result of asymmetric rearrangement decomposition +.C6F12 with separation of .C3F5 there is the first ion of perfluoroalkyl series *+C3F7 m/z 169, fragmentation with formation of ions +C2F5 m/z119 5.5% and +CF3 m/z 69 70%. Returning to the N1-N3 ionic series, it can be assumed that the first act preceding the detachments of one, two and three fluorine atoms is the break of the cycle to form three linear cation radicals with different excitation energies.
The difference between the alkenyl series of perfluorocyclohexane and the alkenyl series of perfluoroalkanes is that it does not require separation of three fluorine atoms for its formation, and the detachment of one fluorine atom is sufficient. The first ion of the alkenyl series with m/z 281 CF2=CF-(CF2)3-+CF2 (3.7%) appears as a result of the break of the cycle, separation of the fluorine atom, from the second CF2 group of the broken cycle, rearrangement to form a terminal vinyl group and transfer of the positive charge to the opposite "flank". It fragments to form the series N1 (Table 2): 231, 181, 131, 81, 31.
As a result of the cycle decay and two symmetrical detachments of 2-5 fluorine atoms, occurs a linear cation-radical with m/z 262 decafluorohexa-1,5-dien CF2 =CF-(CF2)2-.CF=+CF2. It fragments to form the series N2 (Table 2): 212, 162, 112, 62.
At maximum excitation energy of molecular cation-radical there is a break of cycle and detachment of three fluorine atoms from terminal group C3F6 with formation of ion with m/z 243 Tr % CF2=C=CF-(CF2)2-+CF2, or the cycle -(C3F3)(CF2)2+CF2. It fragments to form the series N3 (Table 2): 193, 143, 93, 43. Double bonds strengthening one or both flanges of the perfluoroalkyl chain occur in the ion series N1-N3 after detachment of fluorine atoms (1F,2F,3F).
When total ionic currents of series 1-5 (Тable 3) are compared, maximum current intensity is 55.2% in series 1 (separation of one fluorine atom). It is followed by the series 5-24.5% и 4-11.3% (detachments .C3F5 и C3F6). The most energy-consuming ionic series N3 and N2, with separation of three and two atoms, fluorine have minimum intensity of ion current 6.5 and 2.5%, respectively.
Sequences of formation and graphs of five ionic series of perfluorocyclohexane with intensities of formed ions are presented in Figure 3.
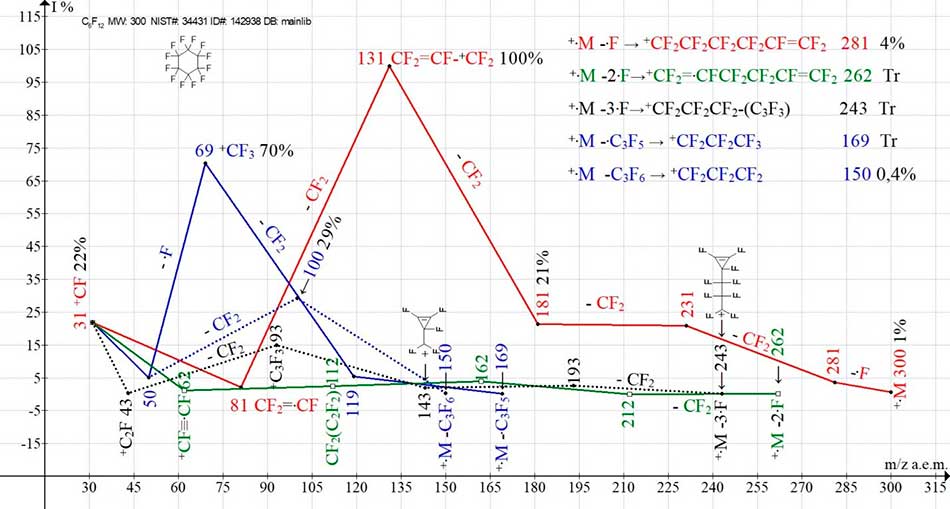
Figure 3. Five ionic series of mass spectrum of perfluorocyclohexane C6F12 MW:300 NIST#: 34431 ID#: 142938 DB: mainlib
In the ionization of perfluorocyclohexane, five variants +.M1-5 with different excitation energies are produced, while only two +.M1-2 are used for ionizing the perfluorocyclohexane. It is possible that this result is that the ratio of their molecular weights is 300:1038 = 1:3.4. An increase in the molecular weight of the compound helps to reduce the specific energy of the chain links when equalizing the excitation energy. If there are no branches in five series of perfluorocyclohexane, the third branches of two main series (1+2) occur in the series of perfluoroeicosane. So, if the excitation energy after decay of cycle in the spectrum of perfluorocyclohexane is sufficient for occurrence of the five "independent" ionic series, then in the spectrum of perfluoroeicosane due to the possible energy deficit the additional separation of fluorine atoms takes place as a result of energetically favorable isomerization and cyclization of emerging ions.
Primary detachments and ionic series of eicosane and cyclotriacontane
In the mass spectra of eicosane, its halogen-derivatives [1], as well as cyclotriaftane [2], decay sequences-their ionic series were established. Including six ionic series of eicosane C20H42, eight series of 1-bromoeicosane C20H41Br and 1,20-dibromoeicosane C20H40Br2 [1] and six series of cyclotriacontanе [2].
The eicosane spectrum, С20H42 MW 282 NIST#: 341441 ID#: 25337 DB: mainlib, includes six series of ions. The sequences of separations in six ionic series are presented in Figure 4.
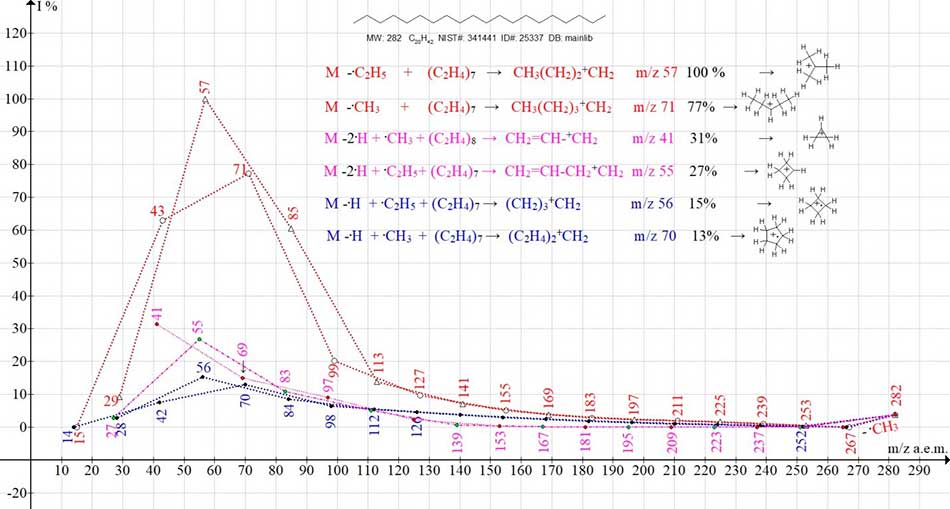
Figure 4. Six ion series of mass spectrum of eicosane C20H42 NIST#: 341441 ID#: 25337 DB: mainlib
Two alkyl series of ions are marked with red lines, two alkenyl series with violet lines, two olefinic series with blue lines. According to the calculated reference data on the bond energies of n‑alkanes [8] energy of separation of alkyl radicals and hydrogen atom increase in the row:
C-C2H5 80,8 ; С-СН3 83 ; C-H 96,2 kcal/mol (3)
Using data [5], the C-H bond energy in n-alkanes is 99.5 kcal/mol (4.3 eV), which is 16.5 kcal/mol less than the C-F bond energy in n-perfluoroalkanes (116 kcal/mol (5.03 eV)).
In comparison with ease of detachment of CF2 in mass-spectra of perfluoroalkanes, separation of CH2 in spectra of n-alkanes does not occur [9], and release of molecules C2H4 and C3H6 takes place.
In contrast to primary detachments of perfluoroalkanes, in which only fluorine atoms participate, in the spectrum of alkanes there are primary detachments -.C2H5 and .CH3, as well as synchronous combined separation of two and three radicals. In particular, the separation of .Н + .CH3 and .Н + .C2H5 atoms, as well as 2.Н + .CH3 and 2.Н + .C2H5.
Six ionic series of eicosane C20H42 involve:
- two series of alkyl ions terminating in intense, probably regrouping peaks with m/z 57 +C(CH3)3 (100%) and m/z 71 +CH(C2H5)2 (77%);
- two series of alkenyl ions terminating in intense peaks with m/z 41 +C3H5 (31%) and m/z 55 +C4 H7 (27%), probably with cyclopropylium and cyclobutyllium structures;
- two series of olefin ions terminating in peaks with m/z 56 cyclobutane +.(CH2)4 (15%) and m/z 70 cyclopentane +.(CH2)5 (13%);
The energies of six variants of radicals primary separation (in kcal/mol and eV) +.M1-6 eicosane vary (from 81 to 275 kcal/mol) or (from 3.5 eV to 11.9 eV). So, the ratio of the minimum and maximum energies of the primary separations in the n-alkane spectra, as in the n-perfluoroalkane spectra, is equal to 1: 3.
M* -.C2H5 (-81 kcal/mol; 3,5 eV) -196 (C2H4)7 → +CH2(CH2)2CH3 m/z 57 (100%);
M* -.CH3 (- 83 kcal/mol; 3,6 eV -196 (C2H4)7 → +CH2(CH2)3CH3 m/z 71 (77%);
M* -2.H,-.CH3(-275 kcal/mol; 11,9 eV) -224(C2H4)8 → +CH2-CH=CH2 m/z 41 (31%);
M* -2.H,-.C2H5 (-273 kcal/mol;11,8 eV)-196(C2H4)7 → +CH2-CH2CH=CH2 m/z 55(27%);
M* -.H,-.C2H5 (-177 kcal/mol;7,7 eV) -196 (C2H4)7 → +CH2(CH2)2.CH2 m/z 56 (15%);
M* -.H -.CH3 ( -179 kcal/mol;7,8 eV) -196 (C2H4)7 → +CH2(CH2)3.CH2 m/z 70 (13%);
As a result of the primary detachments -2.H, ions with a terminal vinyl group occur. The subsequent separation of the methyl or ethyl radical is completed by forming two series of alkenyl ions.
Two olefin series of ions are formed as a result of primary -(.Н + .CH3) and -(.Н + .C2H5).
After primary separation of radicals, in five series, the same total weight of ethylene (C2H4)7=196 Da is released. In the sixth ion series, after separation of two hydrogen atoms and CH3 radical, eight ethylene molecules (C2H4)8=224 Da are released. The detachment of eight ethylene molecules, in one of the six series of eicosane, compared to the detachment of seven ethylene molecules in five other series, makes it possible to conclude that the total energy of the regular fragment groups detachment in all six series is practically the same.
The cyclotriacontane spectrum С30H60 MW#:420 also includes six ionic series [2] as the eicosane spectrum. The sequences of excerpts occurring in the ionic series С30H60 are shown in Figure 5.
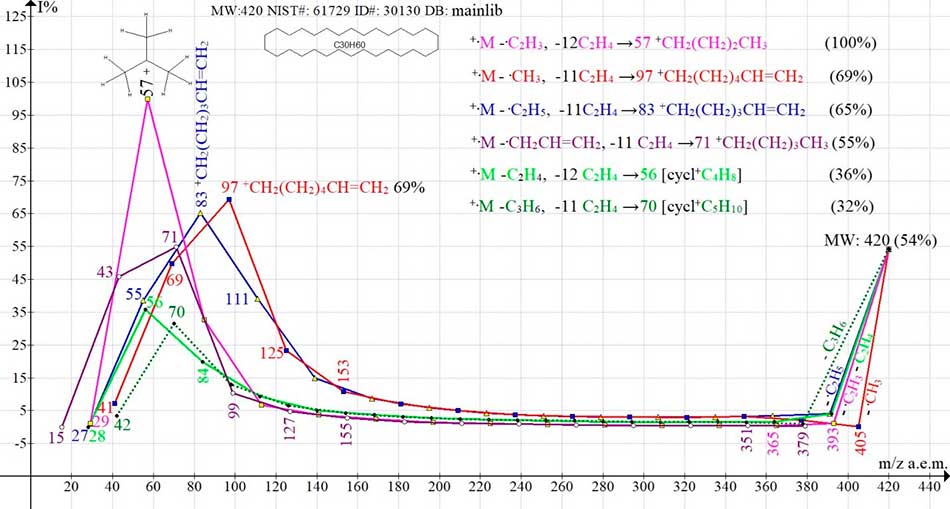
Figure 5. Six ion series of mass spectrum of cyclotriacontane C30H60 MW#: 420 NIST#: 61729 ID#: 26169 DB: mainlib
Four ionic series of cyclotriacontane occur at break of cycle and primary detachments of four regrouping radicals: vinyl, methyl, ethyl and allyl. Two more olefin series occur in the event of a break in the cycle and in the primary detachments of two olefin fragments of the chain: ethylene and propylene. Unlike perfluorocyclohexane, decomposition +.M/2→m/z 210 of cyclotriacontane to form olefin series terminating in peaks 98, 70, 42 does not occur. In four ionic batches of six, terminating with peaks with m/z 97, 83, 71 and 70, the detachment of eleven ethylene molecules occurs. Separation of twelve ethylene molecules takes place in two other ionic series terminating in formation of base peak with m/z 57 and intensive peak with m/z 56. When the cycle is broken and the alkenyl radicals C2H3 or C3H5 are detached, one of the CH2 groups of the broken chain acquires "unused by the broken radical" hydrogen atom, turning into the terminal group CH3.
M* -27 [M -.C2H3]+ -336 (12C2H4) → +CH2(CH2)2CH3 m/z 57 (100%);
M* -15 [M -.CH3]+ -308 (11C2H4) → +CH2(CH2)4CH=CH2 m/z 97 (69%);
M* -29 [M -.C2H5]+ -308 (11C2H4) → +(CH2)4CH=CH2 m/z 83 (65%);
M* -41 [M -.C3H5]+ -308 (11C2H4) → +(CH2)4CH3 m/z 71 (55%);
M -28 [M - C2H4]+ -336 (12C2H4) → +CH2(CH2)2CH2 m/z 56 (36%);
M -42 [M - C3H6]+ -308 (11C2H4) → +CH2(CH2)3CH2 m/z 70 (32%);
When the cycle is broken and the alkyl radicals .CH3 or .C2H5 are separated, one of the CH2CH2 groups of the broken chain loses an additional hydrogen atom, "necessary" for detached CH2 или C2H4 groups, turning into the terminal group CH=CH2.
Detachment of radicals: -.CH3 and -.C2H5 can be explained also as a rearrangement of the excited molecular cation-radical with formation of linear rearrangement ions, in which one of terminal CH2CH2 groups of the broken cycle at the moment of rupture loses a hydrogen atom, turning into a vinyl group, and the other acquires a hydrogen atom, turning into the detachable terminal group CH3 or C2H5.
[M]+.cycle → *CH3(CH2)26CH2+CH .CH2 -.CH3→ .CH2(CH2)25CH2+CH .CH2
.CH2(CH2)25CH2+CH .CH2 →+CH2(CH2)25CH2CH=CH2 (4)
When separating the methyl or ethyl radical from the linear rearrangement cation-radical, a fragmenting with C2H4 detachments alkenyl cation is formed.
The radicals: -.C2H3 and -.C3H5 confirm the rearrangement of the molecular cation of the cyclotriacontane radical to form linear rearrangement ions, in which, when the cycle is broken, one of the terminal groups of CH2CH2 or CH2CH2CH2 of the broken cycle loses a hydrogen atom, turning into a vinyl or propenyl radical, and the other acquires a hydrogen atom, turning into a methyl or ethyl group.
In case of cycle breaks, a primary separation of the rearrangement radicals of the chain takes place in four of the six series of cyclotriacontane, and only two olefin ionic series begin with the separation of ethylene and propylene.
Conclusion
Upon ionization with electrons, molecular cation-radicals acquire excess internal energy of from 0 to 20 eV. The branching of the ionic series +.M can be the cause of the energy-close isomerization variants +·M. An example of a molecular ion isomerization is six ion series of benzene spectrum, five of which occur as a result of restructuring its carbon backbone, according to five variants of π-conjugations [10]. In the spectra of considered compounds, containing regular fragmental groups C2H4 or CF2, isomerization +.M does not occur. Analysis of primary separations in ionic series of mass-spectrum makes it possible, according to the number of radicals to be detached, fluorine atoms or hydrogen atoms and alkyl radicals, to evaluate the energy consumption of its ionic series. The detachments of one, two or three radicals depend on the value of the acquired energy of the molecular ion. In some cases, radical separation may be associated with a specific electron removal topology and a radical detachments sequence. The number of occurring ionic series corresponds to variants of acquiring different excitation energies. The spread of the excitation energies +.M1-3 n-perfluoroalkanes, perfluorocyclohexane and eicosane, which is estimated at 1:3, the result of the energies dispersion of ion source ionizing electrons, as well as possibly unequal costs of electron removal energies.
The analysis of the spectra containing regular fragmental groups makes it possible to conclude that the triple difference in the excitation energies of ionic series molecular ions and that of primary separations does not conflict with the quasi-equilibrium theory postulate (QET) [11,12]. According to this theory, 10-12 sec excess energy is evenly distributed among all bonds of the molecular ion. So, the quasi-equilibrium is achieved before the decomposition begins.
Synchronous primary separation of three atoms ·F (C2,C1 and C20F3) in one of series of perfluoroeicosane, as well as two atoms .H and radical ·CH3 (C2,C1 and C20H3) in one of eicosane series, complete alignment of excitation energy С2-С20 +.M is confirmed even before decomposition.
The difference in the energies of the primary detachments of one, two and three radicals in the compounds containing the regular fragment groups, and an almost equal number of emissions from the regular fragment groups makes it possible to conclude that the excitation energy +.M1-6 of the ionic series differs mainly by the energies of the primary separations, after which the energy of the ionic series is averaged.
Acknowledgments
This work was supported by the Ministry of Science and Higher Education of the Russian Federation and was performed employing the equipment of Center for Molecular Composition Studies of INEOS RAS.
References
- Kagramanov N.D., Fragmentation sequences - ion mass spectra series n-alkylhalgenides, α,ω-dihaloalkanes, α,ω-dihaloperfluoroalkanes. Effect of terminal halide mass and chain molecular weight, Fluorine notes, 2022, 1(140), 5-6.
- Kagramanov N.D., A Series of Fragment Ions of Cycloalkanes, Perfluorocyclohexane, Perfluoropolycycloalkanes, Fluorine notes, 2021, 3(136), 3-4.
- “Advances in Fluorine Chemistry”, Vol.2, London, Butterworth, 1961, “Mass spectrometry of fluorine compounds” p.55-103, Majer J.R., p.58, Table 1.
- Mohler F.L., Dibeler, V.H. and Reese, R.M., J.Res.Nat. Bur.Stand., 1952, 49, 343.
- Fluorine compounds. Synthesis and application, Trans. from Japan/Edited by N. Ishikawa, Moscow, Mir, 1990, 407p., p.10 (table 1.1.1.), p.43. (In Russian)
- Polyakova A.A., Khmel'nickij R.A., Mass spectrometry in organic chemistry, Leningrad, Khimiya,1972, р.13 (Dissociation and localization of charge). (In Russian)
- Kagramanov N.D., Three series ions of perfluorotributylamine mass-spectrum (PFTBA), Fluorine notes, 2020, 3(130), 1-2.
- The energy of breaking chemical bonds. Ionization potentials and electron affinity, Gurvich L. V., Karachentsev V.K., Kondrat'ev Yu.A., Lebedev V.A., Medvedev V.A., Potapov Yu.S., Moscow, Nauka,1974, с. 351. (In Russian)
- Takhistov V.V., Organic mass spectrometry. Thermochemical description of isomerization and fragmentation of ions and radicals in the gas phase, Leningrad, Nauka, 1990, 24-25. (In Russian)
- Kagramanov N.D., Decay sequences - ion series of mass spectra of benzene, 1,3,5,7-cyclooctatetraene, [18]-annulene, hexafluorobenzene and its isomers, Fluorine notes, 2022, 3(142), 5-6.
- Н. М. Rosenshtock, Н. В. Wallenstein, А. Warhaftig, Н. Eyring, Proc. Natl. Acad. Sci USA, 1952, 38, 667.
- Lebedev А.Т., Mass spectrometry in organic chemistry, Moscow, BINOM Laboratoriya znaniya, 2003, 34. (In Russian)
ARTICLE INFO
Received 11 October 2023
Accepted 27 October 2023
Available online December 2023
Recommended for publication by PhD M.A. Manaenkova
eLIBRARY Document Number (EDN) OTAIBZ

Fluorine Notes, 2023, 151, 1-2
