Received: June 2023
DOI 10.17677/fn20714807.2023.04.02
Fluorine Notes, 2023, 149, 3-4
DECAY SEQUENCES - IONIC SERIES OF MASS SPECTRA FOR 2,4,6‑TRIS(PERFLUOROALKYL)-1,3,5-TRIAZINES
N. D. Kagramanov
A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 119991, GSP-1, Moscow, B-334, Vavilov St. 28
e-mail: ndkagram@gmail.com
Abstract: This report presents the ion series of mass spectra for eight homologues of 2,4,6‑tris(perfluoroalkyl)-1,3,5-triazines from NIST libraries, including the ion series of two isomers: triazine (C3F7)3C3N3 with symmetrical substituents C3F7 and triazine (CF3)2C3N3(C7F15) with unsymmetrical substituent C7F15. The influence of molecular weight of substituents and their symmetry on fragmentation of 1,3,5-triazines is discussed. Using [(CF3)3CO]3C3N3 homologue with oxaperfluoroalkyl substituents as an example, the effect of (CF3)3C groups and oxygen atoms on fragmentation is discussed.
Keywords: 2,4,6-tris(perfluoroalkyl)-1,3,5-triazines, ion series of mass spectra, effect of molecular weight of substituents and their symmetry on peak intensities of fragment ions.
Ionic series of mass spectra for 2,4,6-trifluoro-1,3,5-triazine and 1,3,5-triazine
The first triazine homologues - 1,3,5-triazine and 2,4,6-trifluoro-1,3,5-triazine - are trimers of HCN [1] and, respectively, FCN [2]. Its ionic series are presented separately from other homologues, since it reflects the decays of triazine rings, not complicated by fragmentation of perfluoroalkyl substituents, which have large masses. Figs. 1 and 2 show the ion series of mass spectra for 1,3,5‑triazine and 2,4,6-trifluoro-1,3,5-triazine.
In Fig. 1 the main ionic series of trifluorotriazine is marked with red lines, and two side series - with dotted lines.
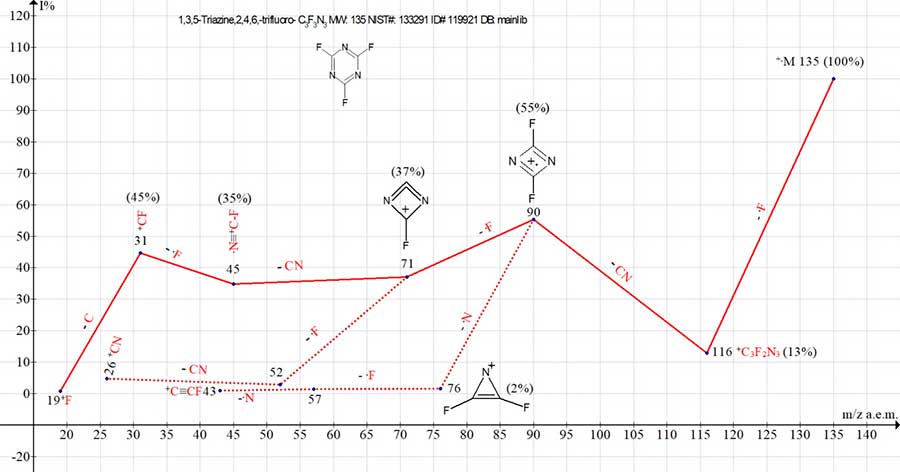
Figure 1. Mass spectrum of ion series for2,4,6-trifluoro-1,3,5-triazine, C3F3N3 MW: 135
NIST#: 133291 ID#: 119921 DB: mainlib.
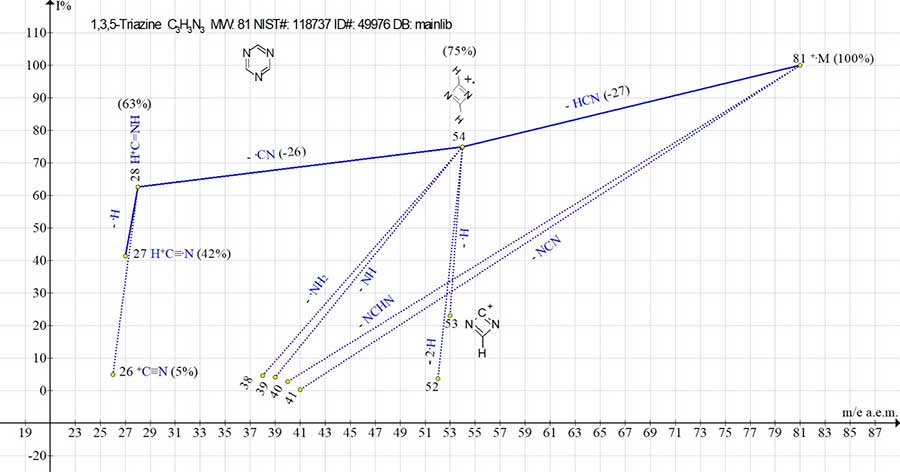
Figure 2. Mass spectrum of ion series for 1,3,5-triazine, C3H3N3 MW: 81 NIST#: 118737
ID#: 49976 DB: mainlib.
In Fig. 2 the main ionic series of 1,3,5-triazine is marked with blue line, and the side fragmentation series - with dotted lines.
Unlike two consecutive emissions of ·F and ·CN occurring during decomposition of fluorine-substituted triazine cycle (see Fig. 1), the fragmentation of molecular +·M radical cation of hydrogen-substituted cycle (see Fig. 2) occurs in three parallel ways, namely with detachments of HCN, NCN and NCHN.
The intensities of peaks for trimeric, dimeric and monomeric ions in the spectra of 1,3,5‑triazine and 2,4,6-trifluoro-1,3,5-triazine have similar values (see Table 1).
Table 1
|
[C3H3N3]+· |
MW: 81 |
100% |
[C3F3N3]+· |
MW: 135 |
100% |
|
[C2H2N2]+· |
MW: 54 |
75% |
[C2F2N2]+· |
MW: 90 |
55% |
|
[HCN]+· |
MW: 27 |
42% |
[FCN]+· |
MW: 45 |
35% |
In NIST library#: 284289 ID#: 17428 DB: mainlib also presents the mass spectrum of (E)‑2,3‑difluoroprop-2-enenitrile, which is a formal dimer of FCN.
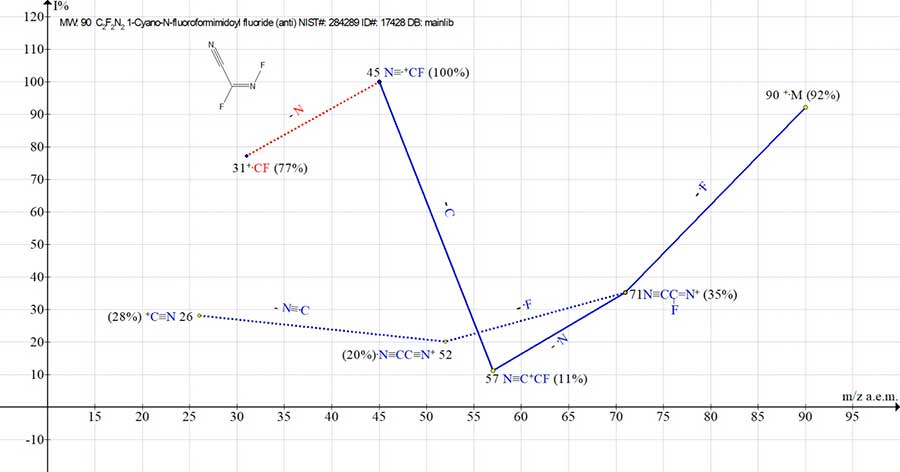
Figure 3. Mass spectrum of ion series for (E)-2,3-difluoroprop-2-ennitrile, MW:90 C2F2N2
NIST#: 284289 ID#: 17428 DB: mainlib.
The peak intensity of molecular ion for such a dimer (m/z 90) is (92%), and the peak intensity of monomeric ion [FCN]+· (m/z 45) is (100%).
Ionic series of mass spectra of homologues of 2,4,6-tris(perfluoroalkyl)-1,3,5-triazines
Fig. 4 shows the ion series of the mass spectrum of 2,4,6-tris(trifluoromethyl)-1,3,5-triazine.
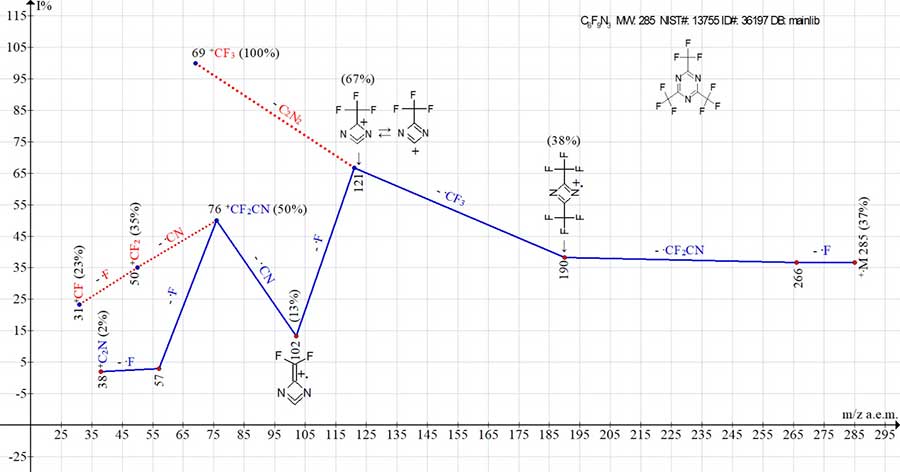
Figure 4. Ion series of the mass spectrum for 2,4,6-tris(trifluoromethyl)-1,3,5-triazine,
C6F9N3 MW: 285 NIST#: 13755 ID#: 36197 DB: mainlib.
As a result of successive detachments of fluorine atom and ·CF2CN, [CF3(CN)2CF3]+· dimeric ion with m/z 190 (38%) appears. Surprisingly, the intensities of +.M, +.M-.F and ‑.CF2CN peaks do not change and are equal to 37-38%. At the next detachment, the ion-monomer [CF3CN]+. with m/z 95 is not formed. The ejection of ·CF3 leads to the ion with m/z 121, which loses a fluorine atom, turning into +.C3F2N2 with m/z 102. As a result, instead of monomeric ion, the +CF2CN ion with m/z 76 (50%) appears. Peaks of ions with m/z 69 +CF3 (100%), 50 +CF2 (35%) and 31 +CF (23%) result from decay of ions with m/z 121 and m/z 76 (marked with red dotted lines).
Fig. 5 shows the ion series of mass spectrum for 2,4,6-tris(pentafluoroethyl)-1,3,5-triazine.
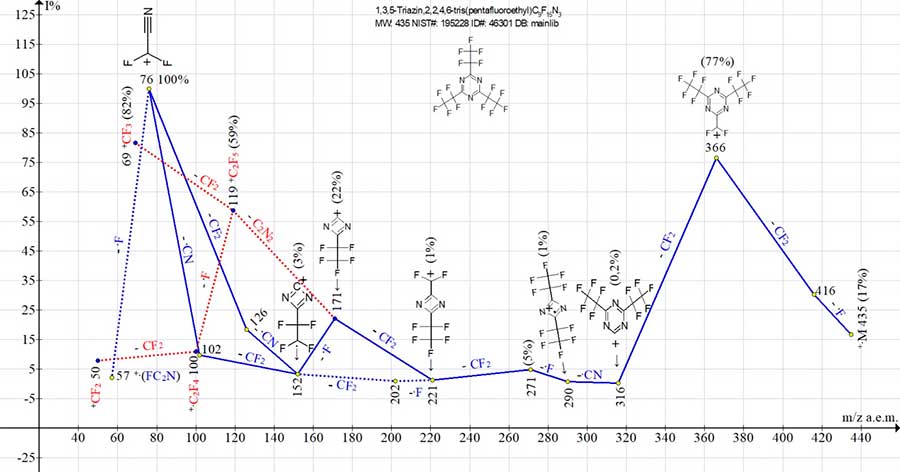
Figure 5. Mass spectrum of ion series for 2,4,6-tris(pentafluoroethyl)-1,3,5-triazine,
C9F15N3 MW: 435 NIST#: 195228 ID#: 46301 DB: mainlib.
As a result of detachments of .F and CF2, the first intense peak of spectrum appears with m/z 366 (C2F5)2[C3N3]+CF2 (77%). Subsequent ejections of CF2 and .CN are completed by dimeric ion +.[C2F5(CN2C)C2F5] with m/z 290 (1%).
The ion with m/z 290 ejects .F and CF2 with formation of C2F5(C2N2)+CF2 ion weak peak with m/z 221 (1%). The ion with m/z 221 fragments both by successive detachments of CF2 and .F, and .F and CF2, forming a radical cation with m/z 152 (3%). However, the detachment from the ion with m/z 221 with only CF2 (without .F radical) leads to a peak (with medium intensity) of C2F5-CN2+C ion with m/z 171 (22%).
The ion with m/z 171 fragments in two ways. As a result of detachment of .F,.CN and CF2 atoms from it, the base peak of mass spectrum with m/z 76 appears. As a result of ejection of ethanedinitrile NC-CN molecule, the +C2F5 ion, its fragment ion +CF3 and so the +.C2F4 and +CF2 ions (marked with red dotted line) are formed (marked with a red dotted line).
Figure 6 shows the ion series of mass spectrum of symmetrical (relative threefold symmetry axis) 2,4,6-tris(heptafluoropropyl)-1,3,5-triazine.
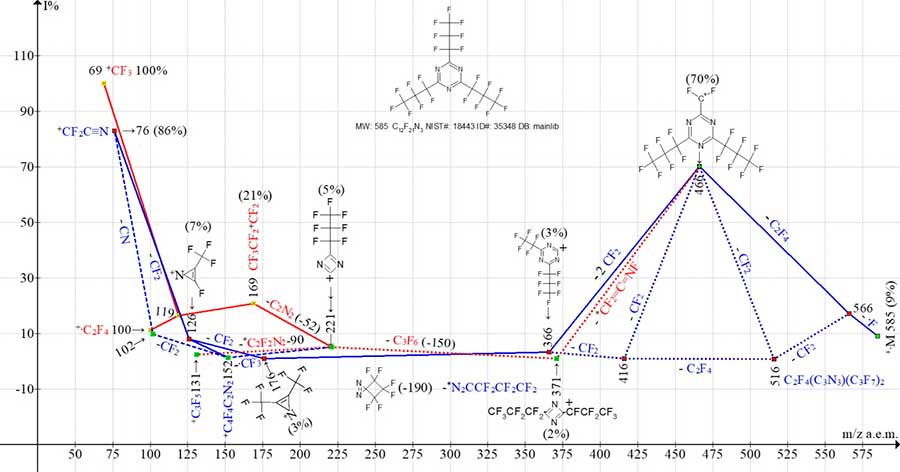
Figure 6. Mass ion series of 2,4,6-tris(heptafluoropropyl)-1,3,5-triazine, C12F21N3 MW:
585 NIST#: 18443 ID#: 35348 DB: mainlib.
The ionic series C12F21N3 (see Fig. 6) are similar to C9F15N3 series (see Fig. 5).
As a result of detachment of .F and C2F4, the first intense peak appears with m/z 466 (C3F7)2[C3N3]+CF2 (70%). It fragments in two ways. Detachment from the ion with m/z 466 (-2 CF2) leads to ion with m/z 366 (3%), forming a series of ions: with m/z 176 (3%), 126 (7%) and 76 (83%) (marked with blue lines).
As a result of detachment of rearrangement molecule (-*CF2=C=NF) from the ion with m/z 466, the ion with m/z 371 (2%) appears. It fragments with ejection of - C3F6 , forming the C3F7-CN2+C ion with m/z 221 (5%). The ion with m/z 221 fragments to form both a perfluoroallyl ion with m/z 131 (2%) and a series of perfluoroalkyl ions ending in +CF3 base ion (marked with red lines).
Fig. 7 shows the ion series of mass spectrum for asymmetric (relative first-order symmetry axis) 2-perfluoroheptyl-4,6-bis(trifluoromethyl)-1,3,5-triazine.
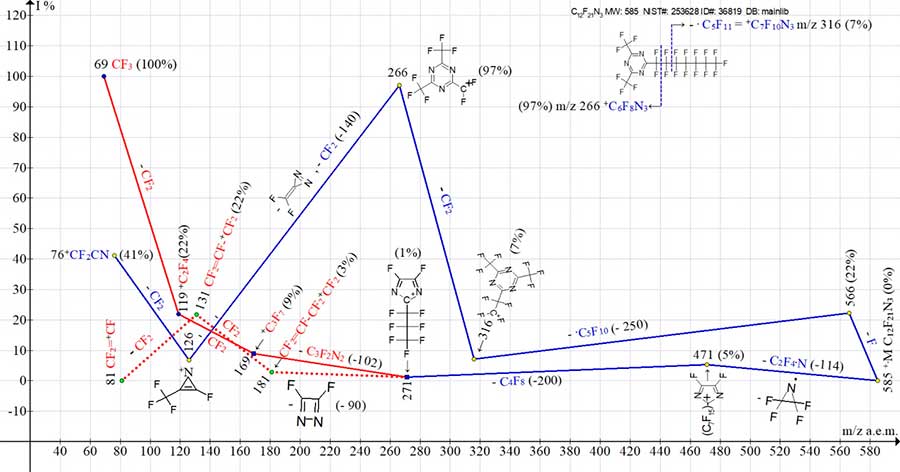
Figure 7. Mass spectrum ion series of 2-perfluoroheptyl-4,6-bis(trifluoromethyl)-1,3,5-triazine,
C12F21N3 MW: 585 NIST#: 253628 ID#: 36819 DB: mainlib.
The ionic series of unsymmetrical homologue C12F21N3 (see Fig. 7) differ from those of symmetrical triazine C12F21N3 (see Fig. 6). The molecular radical cation (see Fig. 7) fragments in two ways. Upon detachment of rearrangement radical (CF2)2N.. the rearrangement ion appears with m/z 471 (5%), which retains the C7F15 substituent. The subsequent ejection of C4F8 leads to formation of the ion +С6F9N2 with m/z 271 (1%), which fragments both with formation of a series of perfluoroalkyl ions +C3F7, +C2F5 with the base peak +CF3, and the series of perfluoroalkenyl ions with perfluoroallyl ion +C3F5 (m/z 131) (22%) (marked with red lines). Another way of M+ fragmentation includes the detachment of fluorine atom and subsequent ejections of +.C5F10 and CF2 with formation of intense ion peak with m/z 266 (97%). Detachment of -CF2CN from the ion with m/z 266 (as it happens in spectrum of (CF3)3C3N3, where the intensity of peak with m/z 266 is only 38%, see Fig. 4), does not occur in spectrum of unsymmetrical isomer (see Fig. 7). As a result, the dimeric ion [CF3(CN)2CF3]+. with m/z 190 is not formed.
The ion with m/z 266 (100%) (see Fig. 7) rejects -CF2= СN2 and -CF2 (-140), turning into C3F4N+ ion with m/z 126 (7%) and fragment ion +CF2СN with m/z 76 (41%).
Fig. 8 shows the ion series of mass spectrum for 2,4,6-tris(perfluoro-tert-butoxy)-1,3,5-triazine C15F27O3, the compound with three substituents -OC(CF3)3 (m/z 235).
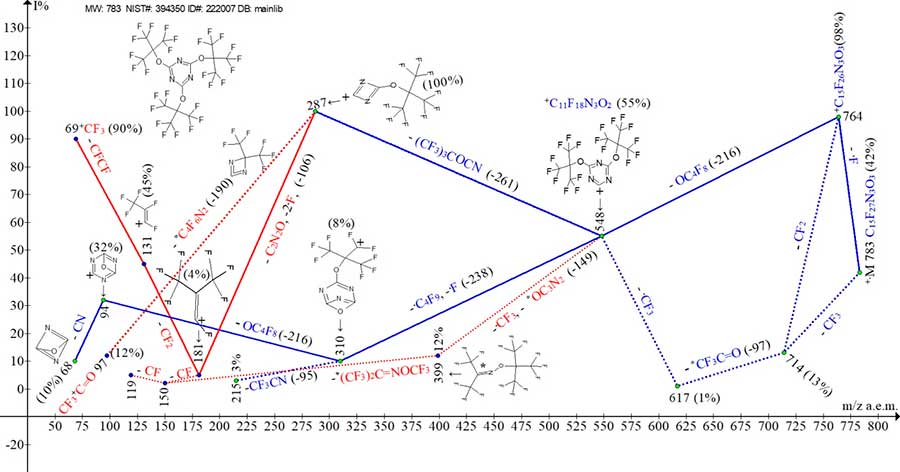
Figure 8. Mass spectrum of ion series for 2,4,6-tris(perfluoro-tert-butoxy)-1,3,5-triazine
C15F27N3O3 MW: 783 NIST#: 394350 ID#: 222007 DB: mainlib.
After primary detachment of fluorine atom, the +C15F26O3 ion with m/z 764 (98%) ejects ОС4F8, losing one of the three OC(CF3)3 substituents and turning into [(CF3)3CO]2C2N3+C ion with m/z 548 (55 %).
The loss of ·OC(CF3)3 substituent also occurs in three successive detachments: -·CF3, -CF3CO and ·CF3 (marked with dotted blue lines).
The ion with m/z 548 fragments in three ways:
- with detachment of second substituent together with CN group of triazine ring of (CF3)3COCN molecule, turning into the ion with m/z 287(100%);
- with detachment of .C4F9 from second substituent, and .F atom from third substituent, turning into the ion with m/z 310 (8%);
- with detachment of .CF3 and O-C3N2 group of triazine ring or F3COC3N2 rearrangement molecule (marked with dotted red lines), forming the ion with m/z 399 (12%).
The ion with m/z 287 (100%) fragments as if with ejection of C4F6N2 (-190) rearrangement molecule, turning into CF3+C=O ion with m/z 97(12%), which is characteristic of spectra for polyoxaperfluoroalkanes [3] (marked with dotted red lines), and with detachment of CN2C=O molecule and two fluorine atoms, turning into rearrangement ion CF3-CF=+CF with m/z 181 (4%).
Fragmentation of ion with m/z 181 yields an analogue of CF3-CF=+CF perfluoroallyl ion with m/z 131 (45%) and its fragment ion +CF3 (90%).
The ion with m/z 310 fragments with detachment of OC4F8, forming the ion with m/z 94 (32%) with C3N3O triazine ring containing an additional oxygen atom. The ion with m/z 94 fragments with detachment of CN and formation of C2N2O ion with m/z 68 (10%).
The ion with m/z 399 fragments with ejection of (CF3)2C=N-OCF3 molecule and formation of perfluoroolefin ions, i. e. + С3F6 and +C2F4 (marked with red dotted lines).
Fig. 9 shows the ion series of mass spectrum for 2,4,6 tris(pentadecafluoroheptyl)-1,3,5-triazine.
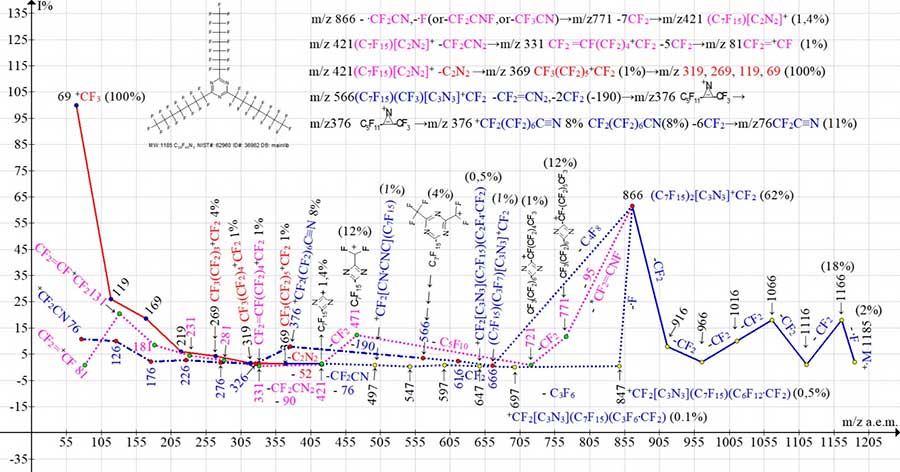
Figure 9. Mass spectrum of ion series for 2,4,6-tris(pentadecafluoroheptyl)-1,3,5-triazine
C24F45N3 MW: 1185 NIST#: 62960 ID#: 36982 DB: mainlib.
As a result of primary detachment of fluorine atom and subsequent ejections of six CF2 groups, the first intense peak of triazine ion with m/z 866 (62%) with two C7F15 substituents and one +CF2group is formed. The ion with m/z 866 fragments in three ways (marked in Fig. 9 with dotted lines), with formation of three ionic series.
The first way of fragmentation of the ion with m/z 866 begins with ejection of C4F8 molecule from second substituent C7F15, which has not yet fragmented, to form the ion (C7F15)(C3F7)[C3N3]+CF2 with m/z 666 (1%).
The second way of fragmentation of the ion with m/z 866 is detachment of ·CF2CN (with ring break and detachment of CN) from the first already fragmented substituent and additional ejection of the ·F atom from second substituent, or ejection of CF2C=NF or CF3CN molecule with formation of rearrangement ion with m/z 771 (12%). The additional detachment of ·F atom probably comes from CF2 group of C7F15 substituent, which is directly linked to fragmenting triazine ring. The subsequent ejection of -CF2 ends with C7F15[C3N3](C3F6.CF2)+CF2 ion with m/z 721(1%).
The third way of fragmentation of ion with m/z 866 is detachment of fluorine atom from second, not yet fragmented substituent, with formation of radical C7F15[C3N3](C6F12.CF2)(+CF2) with m/z 847 (0.5%) . The next detachment of C3F6 molecule probably also occurs from the second one, which began to fragment the C6F12.CF2 substituent. Detachment of C3F6 also leads to radical cation C7F15[C3N3](C3F6.CF2)+CF2 with m/z 697 (0.1%).
The ion with m/z 666 (C7F15)(C3F7)[C3N3]+CF2 (first way) after detachment of two CF2 groups, is formed by (C7F15)(CF3)[C3N3]+CF2 ion with m/z 566 (4%). The ion with m/z 566 fragments with ejection of CF2CN2 (-90) molecule and detachment of C2F4, turning into C5F11C+NCCF3 ion, which rearranges into +CF2C6 F12CN ion with m/z 376 (8%). The series of six further CF2 detachments: (326, 276, 226, 176) is completed by +CF2CN ion with m/z 76 (11%).
The ion with m/z 721(1%) (second way) ejects the C5F10 (-250) molecule turning into +CF2(CN2C)(CF2)6CF3 ion with m/z 471 (12%). The next detachment of CF2 gives rise to the ion with m/z 421 (1%), which fragments in two ways: both with ejection of cyanogen NC-CN and formation of +C3F7 ion with m/z 369 (1%), a series of perfluoroalkyl ions, with detachment of .CF2CN2, leading to +C7F13 ion with m/z 331(1 %) and series of perfluoroalkenyl ions, the most intense peak of which is the peak of perfluoroallyl ion CF2=CF-+CF2 with m/z 131 (21%).
The cation-radical with m/z 697 (0.1%) (third way) C7F15[C3N3](C3F6.CF2)+CF2 ejects four CF2 groups, transforming it into cation-radical C7F15[CN.CNCN]+CF2 497 (1%). The detachment of .CF2CN (-76) with breakdown of triazine ring terminates C7F15[CN2+С] ion with m/z 421 (1%), which fragments in two ways, which we have considered above.
Fig. 10 shows the ion series of mass spectrum for 2,4,6-tri-(perfluorononyl)-1,3,5-triazine C30F57N3. Like C24F45N3 homologue (see Fig. 9).
The spectrum of C30F57N3 consists of three ionic series: perfluoroalkyl ion series +CF3 with m/z 369-69, CF2=CF-+CF2 perfluoroalkenyl ion series with m/z 381-131 and +CF2 (CF2)nCN ion series with m/z 476-76. Its difference is that the peak intensities of perfluoroalkyl ions and +CF2CN ion series in C30F57N3 spectrum (see Fig. 10) are one and a half times weaker than in C24F45N3 spectrum (see Fig. 9), while the peak intensities of its perfluoroalkenyl series are the same.
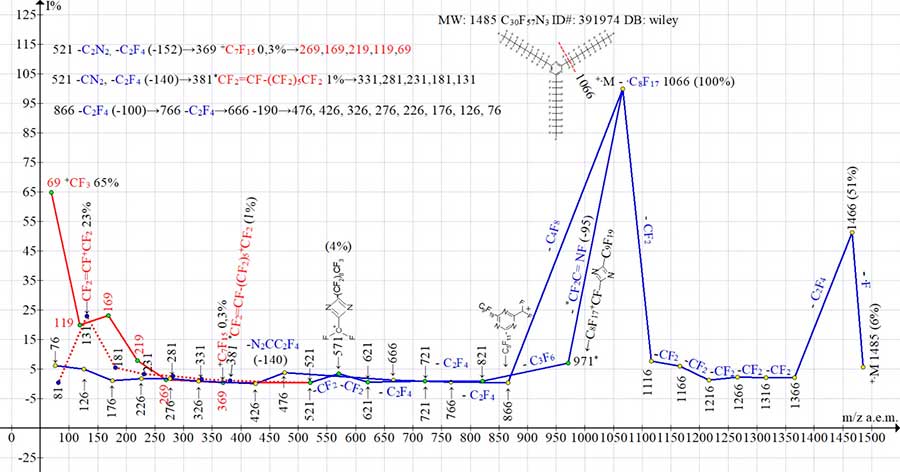
Figure 10. Mass spectrum of ion series for 2,4,6-tris(perfluoronyl)-1,3,5-triazine
C30F57N3 MW: 1485 ID#: 391974 DB: wiley_nist98.
Detachment of fluorine atom from first intense ion with m/z 866, which occurs in spectrum of C24F45N3 (see Fig. 9), does not occur (or does not appear) in spectrum of C30F57N3 (see Fig. 10) with m/z 1066 -F.
Conclusions
A review of ion series of mass spectra for 2,4,6-tris(perfluoroalkyl)-1,3,5-triazines allows us to compare the primary processes of its fragmentation, as well as the intensities of resulting peaks, depending on substituents of triazine ring and its molecular weights.
Fig. 11 shows: the intensities graph of molecular peaks +.M of triazine homologues (marked with red dotted lines), the graph of +.M-.F peaks (marked with blue dotted lines), as well as the graph of first intense fragment peaks after the ejection of fluorine atom (marked with green dotted lines).
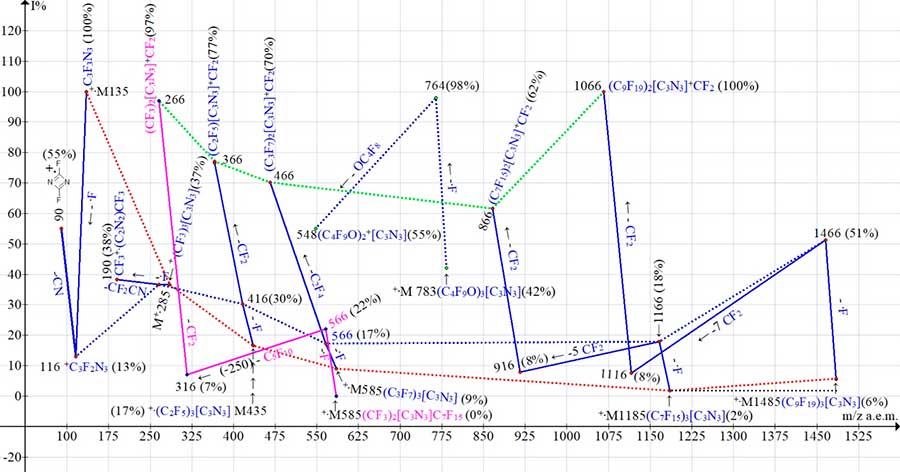
Figure 11. Intensity graph of triazine homologue molecular ion peaks,
+.M-.F peaks and first intense fragment peaks.
The graphs of primary detachments in spectra of two C12F21N3 isomers with m/z 585 differ in color: the graph of symmetric isomer (C3F7)3C3N3, like all graphs of symmetric isomers, is marked with blue lines). The plot of unsymmetrical (CF3)2C3N3(C7F15) isomer is marked with purple lines.
With rising in molecular weight of homologue (i. e. in total mass of substituents in triazine ring), the intensity of molecular ion peak decreases from 100% for C3F3N3 to 2% for C7F15)3C3N3. However, for (C9 F19)3C3N3 homologue, the intensity of molecular ion peak increases to 6% (marked with red dotted lines in Fig. 11).
During ionization, the peak of +.C3F3N3 molecular ion (which has a minimum mass of 135 a.m.u.) acquires the maximum energy and maximum intensity. However, detachment of one fluorine atom from +.C3F3N3 leads to a sharp decrease in peak intensity of +.M-.F with m/z 116 (13%). The reason for destabilization of triazine cycle is the loss of one of three symmetrical fluorine atoms. The next ejection of.CN radical leads to formation of a stable symmetrical dimer +.[FCNNCF] with m/z 90 (55%).
The peak intensity of molecular ion +.(CF3)3[C3N3] with a mass of 285 a.m.u., 2.1 times greater than that of +.C3F3N3, decreases in 2.7 times to 37%. However, when a fluorine atom is detached from +.(CF3)3[C3N3], the intensity of resulting +.M-.F peak remains at level of 37% and only slightly increases to 38% upon detachment of CF2CN and in appearance of dimeric ion CF3+.(C2N2)CF3. For homologs of (C2F5)3C3N3 and (C3F7)3C3N3 (C2F5) the intensity of +.M-.F peak decreases from 30% to 17%. It remains constant at 17%-18% for homologue (C7F15)3C3N3.
After detachment of fluorine atom from +.(C9F19)3C3N3 with molecular weight of 1485 a.m.u. (see Fig. 11) there is unprecedented increase in peak intensity +.M-.F with m/z 1466 (51%) - three times greater than that of (C7F15)3C3N3 homologue. It is possible that this is the result of threefold increase in intensity of +.(C9F19)3C3N3 molecular ion peak (6%) compared to (2%) for (C7F15)3C3N3 molecular peak with m/z 1166. After appearance of M–F peaks the possibility of subsequent stabilizing detachments of CF2 is realized, up to formation of most intense peaks of fragment ions with two initial substituents and one +CF2 group. With successive detachments of CF2, the insignificant successive increase in peaks of emerging ions occurs. However, only the final ejection of CF2 with formation of ion with two initial triazine ring substituents and one +CF2 group leads to the first intense fragmentary peaks.
Comparison of +.M peaks intensities of two isomers (symmetric (C3F7)3[C3N3] (9%) and unsymmetrical (CF3)2[C3N3](C7F15) (0%)) allows us to conclude that, in contrast to +.M symmetrical isomer, the +.M of unsymmetrical isomer with substituent C7F15 is unstable (0%).
The minimum difference between the masses of its C3F7 and CF3 substituents is 100 a.m.u., and the maximum difference between the masses of C3F7 and C7F15 is 200 a.m.u. for C4F8.
In mass spectra of (C2F5)3[С3N3] with m/z 435, (C3F7)3[С3N3] with m/z 585, as well as in mass spectra of unsymmetrical isomer (CF3)2[C3N3](C7F15) with m/z 585 after detachment of fluorine atom and ejection CF2, C2F4, and, accordingly, C5F10 +CF2 molecules, the first intense peaks of stable fragment ions appear: with m/z 366 (77%), m/z 466 (70%) and m/z 266 (97% ) with two symmetrical substituents and one +CF2 group. The maximum intensity of 97% (stability) is acquired by peak of the (CF3)2[C3N3]+CF2 ion with a minimum mass of m/z 266, which occurs during fragmentation of asymmetric isomer (CF3)2[C3N3](C7F15) with m/z 585. That is, with the same molecular weight of two isomers with different substituents of triazine ring, it is precisely the minimum mass of fragment ion with m/z 266 a.m.u. corresponds to maximum intensity of emerging peak (97%). The lower a molecular weight of fragment ion with two initial substituents and one +CF2group, the greater a peak intensity of its fragment ion. Decreasing the intensities of first fragment peaks with m/z 366 (77%), 466 (70%) and 866 (62%) correspond to detachments of increasing masses: CF2 (50), C2F4 (100) and C5F10 +CF2 (300) (see Fig. 11). However, the dependence graph is disturbed by the peak of fragment ion with m/z 1066 (100%), since expected decrease in its intensity (by analogy with fragment ions of other homologues) does not occur. The peak intensity of fragment ion +.M-.F with m/z 1466 (51%), which is three times higher than peak intensity of +.M-.F with m/z 1166 (18%), seems strange at first glance.
However, this increase in intensity is probably the result of threefold increase in intensity of peak for molecular ion (C9F19)3+.[C3N3] (6%) compared to peak intensity of molecular ion (C7F15)3+.[C3N3] (2%). The reason for intensity increasing of molecular peak with m/z 1485 a.m.u. there may be an increase in its effective ionization cross section compared to homologue with m/z 1185.
In spectra of homologues C24F45N3 and C30F57N3 after detachment of fluorine atom, only the ejection of six CF2 groups and, accordingly, the ejection of C2F4 and six CF2 groups leads to intense peaks of fragment ions with two symmetrical substituents and one CF2 group: ((C7F15)2[C3N3]+CF2 with m /z 866 (62%) and (C9F19)2[C3N3]+CF2 with m/z 1066 (100%). The increase in peak intensity with m/z 1066 (100%) compared to intensity of peak at m/z 866 (62%) is the result of difference in intensities of its molecular and fragment peaks +.M-.F. Ions with intense peaks are fragmented in several ways, which leads to branching of ionic series.
The branching of ionic series of fragment ions is apparently the result of variants of its isomerization that are close in energy.
In most of presented spectra, the branching of ionic series begins only after detachment of one of triazine ring substituents. The exceptions are: the mass spectrum of triazine with unsymmetrical substituent (CF3)2C3N3(C7F15), as well as the mass spectrum of triazine (C4 F9O)3C3N3 with three .OC(CF3)3 groups. In its spectra, the branching of ion series begins immediately upon fragmentation of molecular ions. This may also be the result of isomerization of its +.M. Reliable examples of isomerization of molecular ion are: five ion series of benzene spectrum, resulting from rearrangement of its carbon backbone, in accordance with five variants of π-conjugations, eight ion series of 1,3,5,7‑cyclooctatetraene and 15 ion series of 18-annulene [4].
It should be noted that peak intensities graph of mass spectrum (C4F9O)3C3N3 (see Fig. 11), as it were, falls out of the graphs of triazines that do not contain oxaperfluoroalkyl groups.
The intensity of peak of (C4 F9O)3C3N3 molecular ion with m/z 783 (42%) is higher than the intensity of peak of (CF3)3C3N3 molecular ion with m/z 285 (37%), but less than that for С3F3N3 with m/z 135 (100 %). In contrast to linear perfluoroalkyl substituents, three OC(CF3)3 groups probably promote more efficient ionization processes and redistribution of excitation energy. The efficiency of ionization and transfer of excitation energy depends on effective ionization cross section of molecule, but its determination requires special equipment [5].
In (C4 F9O)3C3N3 spectrum, the detachment of fluorine atom leads to a sharp increase in intensity of +.M-.F peak (98%). There is no such intense +.M-.F peak in spectra of any considered homologues.
It is possible that such stabilization, which occurs when a fluorine atom is detached, is associated either with increase in effective ionization cross section of molecule, or with appearance of interaction between the +CF2 terminal group and oxygen atom of substituent. This is indicated by ejection of OC4F8 molecule, although successive but less intense detachments also occur along with CF2, CF3C=O and CF3. As a result, the ion (C4F9O)2+C3N3 with m/z 548 (55%) appears with preserved triazine cycle (see Fig. 8). Its analogs are the low-intensity peak (C2F5)2+C3N3 with m/z 316 (0.2%), mass-spectrum of (C2F5)3+C3N3 and (C3F7)2+С3N3 peak with m/z 416 (1%) of symmetrical (C3F7)3+С3N3 mass spectrum. The +CF2CN ion with m/z 76, characteristic for spectra of triazines with perfluoroalkyl substituents that do not contain oxygen atoms, is not formed in spectrum of C15F27N3O3.
Acknowledgements
This work was supported by the Ministry of Science and Higher Education of the Russian Federation and was performed employing the equipment of Center for Molecular Composition Studies of INEOS RAS.
References
- Nekrasov B.V., Fundamentals of General Chemistry, Moscow, Chemistry, 1967, v. 2, 33. (in Russian)
- Nekrasov B.V. Fundamentals of General Chemistry, Moscow, Chemistry, 1973, v.1, 523. (in Russian)
- Kagramanov N.D., Sterlin S.R., Tyutyunov A.A., Decomposition sequences - Ion series of mass spectra of polyoxaperfluoroalkanes and polyoxaperfluoroalkyls with terminal halide atoms, Fluorine notes, 2023, 2(147), 1-2.
- Kagramanov N.D., Decay sequences - Ion series of mass spectra of benzene, 1,3,5,7‑cyclooctatetraene, [18]-annulene, hexafluorobenzene and its isomers, Fluorine notes, 2022, 3(142), 5-6.
- Shafranyosh I.I., Svida Yu.Yu., Sukhoviya M.I., Shafranosh M.I., Minaev B.F., Baryshnikov G.V., Minaev V.A. Journ. of Techn. Phys. 2015, 85(10), 16-22. (in Russian)
ARTICLE INFO
Received 29 June 2023
Accepted 03 August 2023
Available online August 2023
Recommended for publication by PhD M.A. Manaenkova
eLIBRARY Document Number (EDN) QKPHPF

Fluorine Notes, 2023, 149, 3-4
