Received: February 2023
DOI 10.17677/fn20714807.2023.02.03
Fluorine Notes, 2023, 147, 5-6
PREPARATION OF METHYL PERFLUOROALKYLKETONES
A.V. Sinko, S.M. Igumnov, V.L. Don
A.N. Nesmeyanov Institute of Organoelement Compounds of the Russian Academy of Sciences, 28 Vavilova St., 119991 Moscow, Russia.
P@M-Invest Scientific Production Association, 119991, Leninsky ave. 47, Moscow, Russia.
e-mail: sinkoav@gmail.com
Abstract: A simple method has been found for the preparation of methyl perfluoroalkyl ketones Rf(CO)CH3 by the action of concentrated sulfuric acid on perfluoroacyl acetones Rf(CO)CH2(CO)CH3 while heating with simultaneous distillation of target product.
Keywords: perfluoroalkyl methyl ketone, perfluoroacyl acetone
Methyl perfluoroalkyl ketones are used both by themselves, for example, as the basis of fire extinguishing compositions that do not destroy the ozone layer and easily decompose [1], and, more importantly, as intermediates for the chemical and pharmaceutical industries, including the production of various ligands [ 2], in particular, β-diketones of the formula Rf(CO)CH2(CO)Rf. Their chelates with metals are highly volatile, which allows them to be used in microelectronics for the deposition of organometallic compounds from the vapor phase [3], as well as catalysts for polymerization [4] and oxidation [5].
General methods for the preparation of methyl perfluoroalkyl ketones are the reactions of alkyl Grignard reagents with perfluoroacids or their esters [6, 7], oxidation of the corresponding alcohols [8], and the cleavage of perfluoroacyl acetates under the action of sulfuric acid. Preparation of trifluoroacetone, methyl pentafluoroethyl ketone, and methyl heptafluoropropyl ketone by the last method has been described [9]. The corresponding perfluoroacylacetic acid esters are obtained by Claisen condensation of perfluoroacid esters and ethyl acetate. At the same time, the yields of ketoesters (except of trifluoroacetoacetic ester) are low (30–40%) [9, 10].
Preparation of methyl perfluoroalkyl ketones by cleavage of β-perfluoroacylvinyl ethers with sulfuric acid was proposed by Kurykin [11].
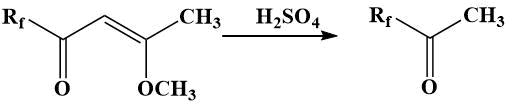
Starting β-perfluoroacylvinyl ethers were obtained by acylation of acetone dimethyl acetal with anhydrides of perfluoroacids or perfluoroacyl chlorides in the presence of pyridine according to method [12]; the yields of ketones were 60–80%.
The cleavage of C(O)–C bond in ketones and diketones, including trifluoroacetylacetone, was described by the action of potassium tert-butoxide in methanol [13], resulting in a mixture of products, among which about a third was trifluoroacetone. The authors were interested in cleavage of C(O)-C bond in ketones and diketones up to ester bond, however, the corresponding ketone was the second component in the decomposition of diketone, and in the case of trifluoroacetoacetone, the preferred cleavage route was the bond away from CF3 group (with a ratio of the products approximately 2:1).
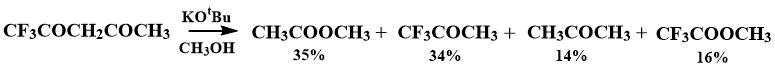
It was reported that when crystals of hexafluoroacetylacetone dihydrate are heated, CO–C bond is cleaved, and trifluoroacetone and trifluoroacetic acid are formed [14].

Diketones of Rf(CO)CH2(CO)CH3 formula containing a perfluoroalkyl and methyl substituent are obtained by Claisen condensation of acetone with perfluoroacyl esters with 70–85% yields [10,15,16].
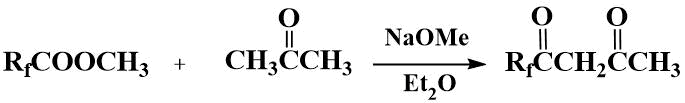
In an attempt to distill off diketones Rf(CO)CH2(CO)CH3 from concentrated sulfuric acid, the corresponding ketones were obtained in 85–95% yields.
It turned out that under the action of sulfuric acid, not only β-perfluoroacylvinyl ethers, but also the corresponding diketones are subjected to cleavage, as a result ketones are obtained even in higher yields.
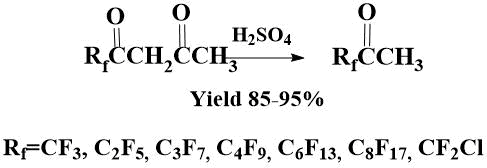
Thus, a simple preparative method for the preparation of methyl perfluoroalkyl ketones was found, since β-diketones of the formula Rf(CO)CH2(CO)CH3 themselves, containing perfluoroalkyl and methyl substituents, are obtained by condensation of perfluoroacyl ethers with acetone in good yields [10,15,16], and under the action of concentrated sulfuric acid when heated, ketones are obtained with yields of 85-95% (the resulting ketones do not require additional purification). This method is especially relevant for ketones with a perfluoroalkyl chain longer than C3.
Experimental part
1. Methyl perfluoropropyl ketone
Into a 150 ml three-necked flask equipped with thermometer, dropping funnel and 25 cm long deflegmator, connected through descending condenser to receiving flask equipped with dry-ice condenser, to sulfuric acid (70 g), preheated to 100°C, 5,5,6,6,7,7,7-heptafluoroheptan-2,4-dione (70 g (0.28 mol)) is added dropwise with stirring, simultaneously distilling off methyl perfluoropropyl ketone boiling at 63-64°C. This process is carried out until the end of product distillation, heating the cube to 130°C. Methyl perfluoropropyl ketone (51 g) is obtained as a colorless liquid with purity of 97% (according to GC and NMR). Bp 63-64°C Yield 86%.
1H NMR, δ, ppm: 2.9 (s, 3H, CH3).
19F NMR, δ, ppm: -128.23 (m, 2F, CF2), -122.51 (m, 2F, CF2), -82.51 (s, 3F, CF3).
Hereinafter, 1H and 19F NMR spectra were recorded via Bruker AVANCE-400 spectrometer (at 400 and 376.50 MHz, respectively; external standard - CDCl3). Chemical shifts for 1H NMR spectra are given in ppm relative to TMS, and for 19F NMR spectra - relative to CFCl3.
2. Methyl perfluorobutyl ketone
Into a 150 ml three-necked flask equipped with a thermometer, dropping funnel and 25 cm reflux condenser with a descending condenser connected to a receiving flask, equipped with a dry ice condenser, to sulfuric acid (80 g), preheated to 100°C, 5,5,6,6,7,7,8,8,8-nonafluorooctane-2,4-dione (79 g (0.26 mol)) is added dropwise with stirring, while distilling off methyl perfluorobutyl ketone. This process is carried out until the end of the distillation of the product, heating the cube to 140°C. Methyl perfluorobutyl ketone (60 g) is obtained as a colorless liquid boiling at 85-87°C with a purity of 97% (according to GC and NMR). Yield 88%.
1H NMR, δ, ppm: 2.8 (s, 3H, CH3).
19F NMR, δ, ppm: -127.10 (m, 2F, CF2), -124.57 (m, 2F, CF2), -121.82 (m, 2F, CF2), 82.82 ( c, 3F, CF3).
3. Methyl perfluorohexyl ketone
Into a 100 ml three-necked flask equipped with thermometer, dropping funnel and 25 cm long deflegmator connected through descending condenser to receiving flask, equipped with dry-ice condenser, to 66 g sulfuric acid preheated to 100°C, perfluoroheptanoyl acetone (66 g (0.16 mol)) is added dropwise, simultaneously distilling off in vacuo the product boiling at 51-52°C/39 Torr After addition of diketone this process is carried out until the end of product distillation, heating the cube to 100-110°C. Methyl perfluorohexyl ketone (56 g) is obtained as a colorless liquid with a purity of 97%. Yield 95%.
1H NMR, δ, ppm: 2.73 (s, 3H, CH3).
19F NMR, δ, ppm: 127.41(m, 2F, CH2)-122.57-123.84(m, 6F, (CH2)3), -121.54(m, 2F, CH2), 82.62 (s, 3F, CH3).
4. Methyl perfluorooctyl ketone
Into a 100 ml three-necked flask equipped with thermometer, dropping funnel and 25 cm long deflegmator connected through descending condenser to receiving flask, equipped with dry-ice condenser, to sulfuric acid conc. (60 g), preheated to 100°C, perfluoronanoyl acetone (60 g (0.12 mol)) is added dropwise with stirring, simultaneously distilling off the methyl perfluorooctylketone boiling at 52-53°C/13 Torr. After addition of the diketone this process is carried out until the end of product distillation, heating the cube to 100-110°C. Methyl perfluorooctylketone (51 g) is obtained as a colorless liquid with a purity of 98%. Yield 93%.
1H NMR, δ, ppm: 2.51 (s, 3H, CH3).
19F NMR, δ, ppm: -127.71 (m., 2F, CH2), -123.13-124.02 (m., 8F, (CH2)4, -122.64 (m., 2F, CH2), 82.95 (s., 3F, CH3).
5. Methyl chlorodifluoromethyl ketone
Into a 100 ml three-necked flask equipped with thermometer, dropping funnel and 25 cm long deflegmator connected through descending condenser to receiving flask, equipped with dry-ice condenser, to sulfuric acid (100 g), preheated to 100°C, 1-chloro-1,1-difluoropentane-2,4-dione (100 g (0.59 mol)) is added dropwise with stirring, simultaneously distilling off methyl chlorodifluoromethyl ketone boiling at 56-60°C .
After addition of the diketone process is carried out until the end of product distillation. Methyl chlorodifluoromethyl ketone (72 g) is obtained with a purity of 98% (according to GC and NMR) as a colorless liquid. Yield 95%.
1H NMR, δ, ppm: 2.93 (s, 3H, CH3).
19F NMR, δ, ppm: -69.22 (m, 2F, CH2).
6. 1,1,1-Trifluoroacetone
Into a 100 ml three-necked flask equipped with thermometer, dropping funnel and 25cm long deflegmator connected through descending condenser to receiving flask, equipped with dry-ice condenser, to sulfuric acid (30 g), preheated to 100°C, 1,1,1-trifluoroacetylacetone (33 g (0.21 mol)) is added dropwise with stirring, simultaneously distilling off 1,1,1-trifluoroacetone. After addition of diketone this process is carried out until the end of product distillation. 1,1,1-Trifluoroacetone (21 g) is obtained as a colorless liquid Bp 23°C. Yield 86%.
1H NMR, δ, ppm: 2.97 (s, 3H, CH3).
19F NMR, δ, ppm: -82.16 (m, 3F, CH3).
Acknowledgments
This work was supported by the Ministry of Science and Higher Education of the Russian Federation (Contract/agreement No. 075-00697-22-00 and was performed employing the equipment of «Center for molecular composition studies» of INEOS RAS.
References
- Patent JP2893038(B1), 1999.
- Kolaříková V., Šimůnek O., Rybáčková M., Cvačka J., Březinová A., Kvíčala J., Dalton Transactions, 2015, 44(45), 19663-19673.
- Croxtall B., Fawcett J., Hope E. G., Stuart A. M., Journal of Fluorine Chemistry, 2003, 119(1), 65‑73.
- Benvenuti F., Carlini C., Marchetti F., Marchionna M., Galletti A. M. R., Sbrana G., J. Organomet. Chem., 2001, 622, 286.
- Klement I., Lütjens H., Knochel P., Angew. Chem. Int. Ed. Engl., 1997, 36, 14543.
- Dishart K. T., Levine R., J. Am. Chem. Soc., 1956, 78, 10, 2268-2270.
- Haszeldine R. N., Journal of the Chemical Society, 1953, 1748-1757.
- McBee E. T., Higgins J. F., Pierce O. R., J. Am. Chem. Soc., 1952, 74, 6, 1387-1390.
- Burdon J., McLoughlin V. C. R., Tetrahedron, 1964, 20, 2163-2166.
- Syntheses of organofluorine compounds, Part 2, Moscow, 2011, Publishing house Trovant, ISBN 978-5-89513-238-8, 351 p. (in Russian)
- Patent RU 2143420, 1998.
- Hojo M., Masuda R., Okada E., Synthesis, 1986, 12, 1013.
- Subaramanian M., Ramar P.M., Rana J., Gupta V.K., Balaraman E., Chem. Commun., 2020, 56, 8143.
- Johnson D.A., Waugh A.B., Hambley T.W., Taylor J.C., Journal of Fluorine Chemistry, 1985, 27, 371-378.
- Park J. D., Brown H. A., Lacher J. R., J. Am. Chem. Soc., 1953, 75(19), 4753-4756.
- Büttner S., Lubbe M., Reinke H., Fischer C., Langer P., Tetrahedron, 2008, 64, 7968-7976.
ARTICLE INFO
Received 17 February 2023
Accepted 22 March 2023
Available online April 2023
Recommended for publication by PhD M.A. Manaenkova
eLIBRARY Document Number (EDN) NMTFPY

Fluorine Notes, 2023, 147, 5-6
