Received: February 2023
DOI 10.17677/fn20714807.2023.02.02
Fluorine Notes, 2023, 147, 3-4
CASCADE THREE-COMPONENT SYNTHESIS OF 2-AMINO-4-(FLUOROALKYL)-4,5-DIHYDROPYRANO[3,2-B]INDOLE-3-CARBONITRILES WITH THE INVOLVEMENT OF ALIPHATIC FLUOROCARBONYL COMPOUNDS
O. Yu. Fedorovskii, N. D. Chkanikov
Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences,
Vavilova 28, Moscow, 119991, Russia (offskii@rambler.ru)
Abstract: A three-component cascade synthesis (3CCS) of 5-acetyl-2-amino-4-(fluoroalkyl)-4,5-dihydropyrano[3,2-b]indole-3-carbonitriles 1a-e based on aliphatic fluorocarbonyl compounds 2a-e with N-acetylindoxyl and malonitrile was carried out. The yields of 3CC synthesis of 1a-e are 51‑76% when carried out at room temperature in the presence of a catalytic amount of organic base. Removal of N-acetyl protection group in 1a-e leads to the formation of indolepyrans 4a-e with high yields of 84-88%. The structure of the compounds was confirmed by 1H, 13C and 19F NMR spectroscopy, mass spectrometry, and elemental analysis data.
Keywords: 2-(2,2,2-trifluoroethylidene)malonitrile, fluoro-substituted dicyanoethylenes, three-component cascade synthesis, C2-alkylation, aliphatic fluorocarbonyl compounds, N-acetylindoxyl, malonitrile.
Introduction
Currently, almost 25% of the known biologically active compounds used in pharmaceuticals contain a fluorine atom [1-3]. Organofluorine compounds have been widely used as medicines, and herbicides, plant growth stimulants and antidotes to sulfonylureas in agrochemistry [4, 5].
For the construction of polycyclic heteroatomic structures with biological activity, multicomponent reactions involving such compounds are very attractive, in which, after passing the key stage of the formation of a C-C bond according to the type of Michael reaction, the following reaction could take place, for example, the formation of a pyran cycle. Such multicomponent reactions are commonly called cascade reactions [6].
To date, cascade multicomponent reactions have reached the level of highly effective synthetic methods, the search and development of which is stimulated by the principles of waste-free chemical production, that is, the principles of "green" chemistry [7, 8]. In this case, the application of the multicomponent synthesis procedure avoids the stage of preparation, purification and use of fluorinated cyanoethylenes, known for their high reactivity and toxicity [11].
Earlier we reported about a three-component reaction involving derivatives of indole, pyrrole, trifluoroacetaldehyde ethyl semiacetal 2a and malonitrile in the presence of an organic base. Nitriles of 3-(1H-indole-3-yl)-2-cyano-4,4,4-trifluorobutanoic acid are formed with good yields [9, 10]. Further studying of multicomponent reactions involving fluorinated carbonyl compounds, we introduced N-acetylindoxyl into them.
In this work, a cascade three-component synthesis of fluorinated derivatives of indolepyranes 1a-e was carried out with the participation of N-acetylindoxyl, malonitrile and aliphatic fluorocarbonyl compounds 2a-e.
Results and discussion
Three-component cascade synthesis of indolepyranes 1a-e was carried out according to Scheme 1. All reagents were introduced into the reaction sequentially in equimolar amounts: fluorocarbonyl compound 2a-e, malonitrile, N‑acetylindoxyl, molecular sieves and a catalytic amount of organic base – N(Et)3. 3CC synthesis was carried out in a DCM solution at room temperature for 16 hours. The completion of 3CC synthesis was controlled by TLC method.
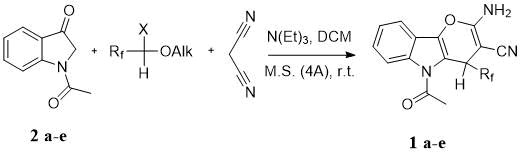
Scheme 1. Cascade three-component synthesis of indolepyranes 1a-e.
a) Rf= CF3, X= OH, Alk= Et; b) Rf= C2F5, X= OH, Alk= Me; c) C3F7, X= OH, Alk= H;
d) C4F9, X= OH, Alk= H; e) Rf= CHF2(CF2)5, X= O, OAlk is absent.
The key step of synthesis 1 is the C2-alkylation of N-acetylindoxyl by reactive
alkenes – 2‑(fluoroalkylidene)malonitriles 3a-e, i.e. the formation
of a C-C bond by the Michael reaction. The corresponding alkenes 3 are formed in situ by the interaction of fluorocarbonyl compounds 2a-e and malononitrile
in the presence of a catalytic amount of base (Scheme 2).

Scheme 2. Formation of 2-(fluoroalkylidene)malonitriles 3a-e in the reaction mass in situ.
a) Rf= CF3, X= OH, Alk= Et; b) Rf= C2F5, X= OH, Alk= Me; c) C3F7, X= OH, Alk= H;
d) C4F9, X= OH, Alk= H; e) Rf= CHF2(CF2)5, X= O, OAlk is absent.
The resulting C2-alkylation adducts under the conditions of a three-component reaction undergoes
a "cascade" transformation with the formation of a 1,4-dihydropyrane cycle in the final compounds
1 (Scheme 3).
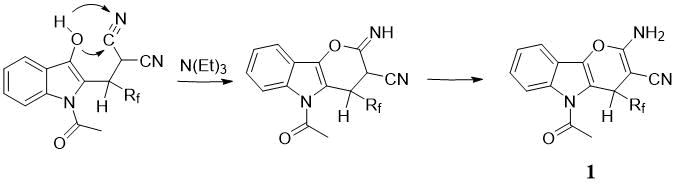
Scheme 3. Probable mechanism of cascade cyclization with the formation of 1,4-dihydropyrane cycle in 1.
In the 1H NMR spectra of 1a-e, there are signals of protons located at the sp3-hybridized carbon atom at position 4 of the pyrane ring. These signals have a characteristic detection interval at 5.2-5.7 ppm (DMSO-d6, CDCl3) with a multiplicity of doublets of doublets and have SSCC equal to 5-6 and 15-18 Hz.
Experimental data show that with an increase in the chain length of the fluorine-containing fragment CF3→C6F12, the yields of compounds 1a-e increase. It is possible that with the growth of the chain of the fluorine-containing fragment, higher synthons 3 become less reactive due to the steric factor and react more selectively [14].
It should be noted that a competing reaction C2-hydroxyalkylation of Nacetylindoxyl by fluorine
carbonyl compounds 2a-e with the formation of secondary alcohols
is not observed in this 3CC synthesis [10].
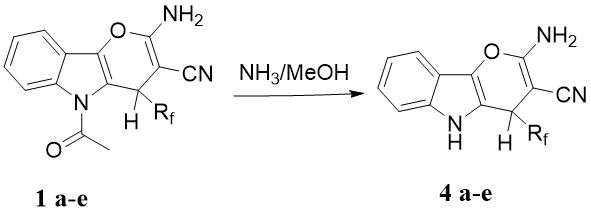
Scheme 4. Removal of the N-acetyl group.
a) Rf= CF3; b) Rf= C2F5; c) C3F7; d) C4F9; e) Rf= CHF2(CF2)5.
The removal of N-acetyl protection group proceeds smoothly in a solution of MeOH saturated with ammonia. Indolepyranes 4a-e are formed with high yields of 84-87% (Scheme 4).
In the 1H NMR spectra of compounds 4a-e, the absence of the N-acetyl group causes a shift of their signals into a strong field by ~0.5 ppm, compared with compounds 1. The signal of such a proton is detected in the region of 5 ppm (DMSO-d6, CDCl3) with a multiplicity of triplet and has SSCC equal to 5-6 Hz.
Our indolepyranes 1 and 4 have a carbon skeleton similar to the compounds described in [12, 13] as having anti-tuberculosis activity. The described compounds were obtained on the basis of aromatic and heterocyclic aldehydes derivatives according to the traditional step-by-step method [12].
Conclusions
Thus, not only trifluoroacetaldehyde takes part in the three-component cascade synthesis of indolepyranes 1a-e, but also higher aliphatic fluorocarbonyl compounds, both in the form of free aldehydes and in the form of their less reactive derivatives - semi-acetals and hydrates with good yields of 51-76%. Aliphatic fluorocarbonyl compounds 2 allow for 3CC synthesis of indolepyranes 1 at room temperature in the presence of a catalytic amount of organic base in an inert solvent. Increasing the chain length of the fluorinated fragment increases the yields of compounds 1a-e. The removal of N‑acetyl protection group by the action of ammonia in MeOH effectively proceeds at ‑30°C with the formation of indolepyranes 4a-e with high yields of 84-88%. Heterocyclic compounds 4a-e, apparently, may be of interest as potential anti-tuberculosis agents.
Experimental part
All starting compounds were purchased from Merck, Sigma-Aldrich and used without purification. All solvents: petroleum ether Tbp= 40-70°C (PE), EtOAc (EA), DCM and triethylamine were purified by distillation before use. Merck Kieselgel 60 F254 TLC plates were used to monitor the reaction and detect substances. The products were purified by column chromatography using Merck Kieselgel 60 silica gel (0.06-0.20 mm). The 1H, 13C and 19F NMR spectra were recorded on a Bruker Avance 400 spectrometer with an operating frequency of 400, 100 and 376 MHz, respectively. Chemical shifts of 1H and 13C nuclei were determined relative to the residual CDCl3 and DMSO-d6 signals (7.26, 2.50 and 77.16, 39.52 ppm, respectively) and recalculated to the SiMe4 signal. The signals of 13C atoms containing an odd and even number of protons have the opposite polarity in the JMODECHO mode. The 19F nuclei spectra were recorded both with and without suppression of the H-F spin-spin coupling. Chemical shifts of 19F atoms are determined relative to CFCl3 as an external standard. The mass spectra were recorded on a FINNIGAN MAT INCOS 50 quadrupole mass spectrometer, direct sample input, ionization energy - 70 eV. The melting points of the obtained compounds have values above 235°C.
General 3CC method of synthesis of 5-acetyl-2-amino-4-(fluoroalkyl)-4,5-dihydropyrano[3,2-b]indole-3-carbonitriles 1a-e.
Into a round-bottomed single-necked flask equipped with a magnetic stirrer, fluorine carbonyl compound 2a-e (1 mmol), malonitrile (0.066 g, 1 mmol), N-acetylindoxyl (0.175 g, 1 mmol), freshly distilled DCM (5 ml), 5-7 granules of dried molecular sieves with a pore size of 4A are loaded. The flask is closed with a septa, vacuumed, filled with argon, cooled to +5°C, triethylamine (0.05 g) is injected with a syringe. The reaction mass is kept at room temperature for 16 hours. The completion of the 3CC reaction is controlled by the TLC method. 10 ml of a mixture of DCM and EA (8 and 2 ml) is added to the contents of the flask and transferred to a chromatographic column containing 20 ml of silica gel. The reaction mass is eluted with the same mixture of solvents and, if necessary, the gradient of the eluent is increased by adding EA. Solutions of purified products 1a-e are evaporated at reduced pressure and re-evaporated with 20 ml of DCM.
General method of synthesis of 4a-e, removal of N-acetyl protection in 1a-e.
Into a round-bottomed single-necked flask equipped with a magnetic stirrer 1a-e (1 equivalent), MeOH (1 ml) are loaded, a septa is closed, vacuumed, filled with argon, cooled to -30 °C and an ammonia-saturated MeOH (~15% by weight, 2 ml) is injected into the flask with a syringe cooled to -30 °C. The reaction mass is kept at a temperature of -30 °C for 16 hours. The completion of the reaction is controlled by TLC. The contents of the flask are diluted with DCM (10 ml) and transferred to a glass filter containing 10 ml of silica gel. Indolepyrans 4a-e are eluted with a mixture of solvents DCM and EA (4/1). Solutions 4a-e are evaporated at reduced pressure and re-evaporated with 20 ml of DCM.
(1a) 5-acetyl-2-amino-4-(trifluoromethyl)-4,5-dihydropyrano[3,2-b]indole-3-carbonitrile,
C15H10F3N3O2, MМ: 321.
Yield 51% (0.165 g), light beige powder, Rf= 0.2 (PE+EA, 8+2).
1Н NMR (DMSO-d6, δ, ppm., J/Hz): 2.81 (3H, s, CH3); 5.25 (1H, q, 6.3 Hz, CHCF3); 7.40 (1H, t, 7.4 Hz, Ar); 7.50 (1H, t, 7.7 Hz, Ar); 7.58 (1H, d, 7.7 Hz, Ar); 7.73 (2H, br. s, NH2); 7.99 (1H, d, 8.5 Hz, Ar).
13С NMR (DMSO-d6, δ, ppm., J/Hz): 26.92 (СH3); 38.62 (q, 30.3 Hz, СНCF3); 47.55 (q, 2.4 Hz); 110.68 (q, 1.7 Hz, CN); 115.63; 116.94; 118.64; 119.45; 124.01; 125.42 (q, 283.7 Hz, CF3); 126.83; 133.67; 137.64; 163.39; 170.21.
19F NMR (DMSO-d6, δ, ppm., J/Hz): -72.08 (s, CF3).
MS (EI, 70 eV), m/z (Irel (%)): 322 [М+Н]+ (5.79); 252 [M-СF3]+ (17.57); 210 [M-СF3-Ac]+ (37.74).
Calculated: C, 56.08; H, 3.14; N, 13.08. Found: С, 56.04; H, 3.19, N, 13.05.
(4a) 2-amino-4-(trifluoromethyl)-4,5-dihydropyrano[3,2-b]indole-3-carbonitrile,
C13H8F3N3O, MМ: 279.
Yield: 84 % (0.11 g), light beige powder, Rf= 0.3 (PE+EА, 8+2).
1Н NMR (DMSO-d6, δ, ppm., J/Hz): 4.83 (1H, q, 6.6 Hz, CHCF3); 6.78 (2H, br. s, NH2); 7.07 (1H, t, 7.4 Hz, Ar); 7.18 (1H, t, 7.6 Hz, Ar); 7.40 (1H, d, 8.3 Hz, Ar); 7.49 (1H, d, 7.8 Hz, Ar); 11.12 (1H, br. s, NH).
13С NMR (DMSO-d6, δ, ppm., J/Hz): 38.17 (q, 30.1 Hz, СНCF3); 47.21; 110.34 (CN); 115.65; 116.73; 118.31; 119.96; 124.11; 125.11 (q, 282.1 Hz, CF3); 126.;53 133.15; 137.94; 163.77.
19F NMR (DMSO-d6, δ, ppm., J/Hz): -71.55 (d, 6.4 Hz, CF3).
MS (EI, 70 eV), m/z (Irel (%)): 279 [М]+ (12.77); 210 [M-СF3]+ (75.63).
Calculated: C, 55.92; H, 2.89; N, 15.05. Found: С, 55.87; H, 2.94; N, 15.01.
(1b) 5-acetyl-2-amino-4-(perfluoroethyl)-4,5-dihydropyrano[3,2-b]indole-3-carbonitrile,
C16H10F5N3O2, MМ: 371.
Yield: 63 % (0.235 g), light beige powder, Rf= 0.2 (PE+EА, 8+2).
1Н NMR (DMSO-d6, δ, ppm., J/Hz): 2.82 (3H, s, CH3); 5.40 (1H, dd, 6.6 Hz, 15.3 Hz, CHCF2); 7.40 (1H, t, 7.5 Hz, Ar); 7.50 (1H, t, 7.8 Hz, Ar); 7.58 (1H, d, 7.7 Hz, Ar); 7.83 (2H, br. s, NH2); 7.97 (1H, d, 8.5 Hz, Ar).
13С NMR (DMSO-d6, δ, ppm., J/Hz): 26.91 (СH3); 37.43 (t, 23.2 Hz, СНCF2); 47.13 (t, 4.6 Hz); 110.89 (t, 1.0 Hz, CN); 114.57 (tq, 257.5 Hz, 35.3 Hz, CF2); 115.55; 116.93; 118.68; 118.75 (qt, 287.4 Hz, 36.6 Hz, CF3); 119.54; 123.71; 126.60; 133.64; 138.29; 164.33; 170.41.
19F NMR (DMSO-d6, δ, ppm., J/Hz): -77.22 (3F, s, CF3); -112.70 (1F, d, 266.4 Hz, CF2); -116.60 (1F, d, 266.0 Hz, CF2).
MS (EI, 70 eV), m/z (Irel (%)): 371 [М]+ (1.1); 252 [M- С2F5]+ (30.68); 210 [M- С2F5-Ac]+ (44.63).
Calculated: C, 51.76; H, 2.71; N, 11.32. Found: C, 51.70; H, 2.77; N, 11.28.
(4b) 2-amino-4-(perfluoroethyl)-4,5-dihydropyrano[3,2-b]indole-3-carbonitrile,
C14H8F5N3O, MМ: 329.
Yield: 85 % (0.12 g), light beige powder, Rf= 0.3 (PE+EА, 8+2).
1Н NMR (DMSO-d6, δ, ppm., J/Hz): 5.01 (1H, t, 9.9 Hz, CHCF2); 7.08 (1H, t, 7.5 Hz, Ar); 7.19 (1H, t, 7.6 Hz, Ar); 7.42 (1H, d, 8.3 Hz, Ar); 7.47 (1H, d, 8.4 Hz, Ar); 7.49 (2H, br. s, NH2); 11.26 (1H, s, NH).
13С NMR (DMSO-d6, δ, ppm., J/Hz): 36.97 (t, 24.9 Hz, СНCF2); 46.09 (t, 5.0, Hz); 109.61 (t, 4.4 Hz, CN); 112.35; 113.31 (tq, 257.4 Hz, 33.9 Hz, CF2); 115.19; 115.93; 119.32 (qt, 288.1 Hz, 36.7 Hz, CF3); 119.70; 120.27; 122.91; 130.98; 133.94; 164.49.
19F NMR (DMSO-d6, δ, ppm., J/Hz): -79.98 (3F, s, CF3); -114.76 (2F, d, 24.4 Hz, CF2).
MS (EI, 70 eV), m/z (Irel (%)): 329 [М]+ (100); 210 [M-C2F5]+ (77.54).
Calculated: C, 51.07; H, 2.45; N, 12.76. Found: C, 51.02; H, 2.51; N, 12.73.
(1c) 5-acetyl-2-amino-4-(perfluoropropyl)-4,5-dihydropyrano[3,2-b]indole-3-carbonitrile,
C17H10F7N3O2, MМ: 421.
Yield: 64 % (0.27 g), light beige powder, Rf= 0.2 (PE+EА, 8+2).
1Н NMR (DMSO-d6, δ, ppm., J/Hz): 2.82 (3H, s, CH3); 5.52 (1H, dd, 4.1 Hz, 18.6 Hz, CHCF2); 7.41 (1H, t, 7.5 Hz, Ar); 7.51 (1H, t, 7.9 Hz, Ar); 7.59 (1H, d, 7.8 Hz, Ar); 7.83 (2H, br. s, NH2); 7.97 (1H, d, 8.6 Hz, Ar).
13С NMR (DMSO-d6, δ, ppm., J/Hz): 26.95 (СH3); 37.83 (t, 22.9 Hz, СНCF2); 47.32 (dd, 3.5 Hz, 5.7 Hz); 108-118 (multiplets family of C3F7); 111.00 (CN); 115.59; 116.93; 118.75; 119.58; 123.72; 126.60; 133.66; 138.50; 164.68; 170.43.
19F NMR (DMSO-d6, δ, ppm., J/Hz): -80.23 (3F, t, 10.3 Hz, CF3); -113.77 (1F, m, CF2); -118.24 (1F, dd, 6.3 Hz, 271.6 Hz, CF2); -125.15 (1F, dd, 4.8 Hz, 8.4 Hz, CF2); -125.34 (1F, d, 9.5 Hz, CF2).
MS (EI, 70 eV), m/z (Irel (%)): 421 [М]+ (1.31); 252 [M-С3F7]+ (25.58); 210 [M-С3F7-Ac]+ (35.16).
Calculated: C, 48.47; H, 2.39; N, 9.97. Found: C, 48.53; H, 2.33; N, 9.92.
(4с) 2-amino-4-(perfluoropropyl)-4,5-dihydropyrano[3,2-b]indole-3-carbonitrile,
C15H8F7N3O, MM: 379.
Yield 85 % (0.1 g), light beige powder, Rf= 0.4 (PE+EA, 8+2).
1Н NMR (DMSO-d6, δ, ppm., J/Hz): 5.07 (1H, t, 10.8 Hz, CHCF2); 7.08 (1H, t, 7.3 Hz, Ar); 7.19 (1H, t, 7.4 Hz, Ar); 7.43 (1H, d, 8.2 Hz, Ar); 7.47 (1H, d, 7.9 Hz, Ar); 7.51 (2H, br. s, NH2); 11.24 (1H, s, NH).
13С NMR (DMSO-d6, δ, ppm., J/Hz): 37.60 (t, 24.9 Hz, СНCF2); 46.10 (t, 5.1 Hz); 107-118 (multiplets family of C3F7); 109.69 (CN); 112.35; 115.19; 115.89; 119.67; 120.34; 122.88; 131.20; 133.92; 164.75.
19F NMR (DMSO-d6, δ, ppm., J/Hz): -80.40 (3F, t, 10.4 Hz, CF3); -112.27 (2F, s, CF2); -124.94 (2F, s, CF2).
MS (EI, 70 eV), m/z (Irel (%)): 379 [М]+ (16.21); 210 [M-С3F7]+ (100).
Calculated: C, 47.51; H, 2.13; N, 11.08. Found: C, 47.47; H, 2.18, N, 11.04.
(1d) 5-acetyl-2-amino-4-(perfluorobutyl)-4,5-dihydropyrano[3,2-b]indole-3-carbonitrile,
C18H10F9N3O2, MM:471.
Yield 76 % (0.36 g), light beige powder, Rf= 0.2 (PE+EA, 8+2).
1Н NMR (DMSO-d6, δ, ppm., J/Hz): 2.82 (3H, s, CH3); 5.53 (1H, dd, 4.2 Hz, 18.7 Hz, CHCF2); 7.41 (1H, t, 7.4 Hz, Ar); 7.50 (1H, t, 7.9 Hz, Ar); 7.59 (1H, d, 7.7 Hz, Ar); 7.83 (2H, br. s, NH2); 7.97 (1H, d, 8.5 Hz, Ar).
13С NMR (DMSO-d6, δ, ppm., J/Hz): 26.93 (СH3); 38.08 (t, 23.0 Hz, СНCF2); 47.44 (t, 8.5 Hz); 111.02 (CN); 112.07-119.46 (multiplets family of C4F9); 115.58; 116.94; 118.77; 119.61 123.71; 126.59; 133.68; 138.57; 164.74; 170.40.
19F NMR (DMSO-d6, δ, ppm., J/Hz): -80.45 (3F, s, CF3); -113.44 (1F, d, 272.2 Hz); -117.47 (1F, dd, 273.3 Hz, 15.1 Hz, CF2); -121.68 (2F, s, CF2); -124.76 (1F, dd, 21.2 Hz, 291.1 Hz); ‑126.52 (1F, dt, 14.9 Hz, 30.3 Hz).
MS (EI, 70 eV), m/z (Irel (%)): 471 [М]+ (0.81); 428 [M-Ac]+ (1.15); 252 [M-С4F9]+ (65.66); 210 [M-С4F9-Ac]+ (79.57).
Calculated: C, 45.87; H, 2.14; N, 8.92. Found: C, 45.92; H, 2.09; N, 8.88.
(4d) 2-amino-4-(perfluorobutyl)-4,5-dihydropyrano[3,2-b]indole-3-carbonitrile,
C16H8F9N3O, MM:429.
Yield 88 % (0.12 g), light beige powder, Rf= 0.3 (PE+EA, 8+2).
1Н NMR (DMSO-d6, δ, ppm., J/Hz): 5.07 (1H, t, 11.1 Hz, CHCF2); 7.08 (1H, t, 7.5 Hz, Ar); 7.18 (1H, t, 7.6 Hz, Ar); 7.41 (1H, d, 8.3 Hz, Ar); 7.45 (1H, d, 8.1 Hz, Ar); 7.48 (2H, br. s, NH2); 11.22 (1H, s, NH).
13С NMR (DMSO-d6, δ, ppm., J/Hz): 37.84 (t, 25.3 Hz, СНCF2); 46.15; 109.76 (CN); 111.71-118.34 (multiplets family of C4F9); 112.38; 115.21; 115.90; 119.69; 120.37; 122.89; 131.26; 133.93; 164.81.
19F NMR (DMSO-d6, δ, ppm., J/Hz): -80.38 (3F, s, CF3); -112.03 (2F, s, CF2); -121.67 (2F, s, CF2); -125.83 (2F, d, 11.1 Hz).
MS (EI, 70 eV), m/z (Irel (%)): 429 [М]+ (15.64); 210 [M-С4F9-Ac]+ (100).
Calculated: C, 44.77; H, 1.88; N, 9.79. Found: C, 44.81; H, 1.82; N, 9.73.
(1e) 5-acetyl-2-amino-4-(1,1,2,2,3,3,4,4,5,5,6,6-dodecafluorohexyl)-
4,5-dihydropyrano[3,2-b]indole-3-carbonitrile,
C20H11F12N3O2, MМ: 553.
Yield 71 % (0.39 g), light beige powder, Rf= 0.2 (PE+EA, 8+2).
1Н NMR (DMSO-d6, δ, ppm., J/Hz): 2.80 (3H, s, CH3); 5.67 (1H, dd, 4.6 Hz, 17.2 Hz, CHCF2); 6.03 (1H, tt, 5.2 Hz, 51.9 Hz, CF2H); 7.37 (1H, t, 7.6 Hz, Ar); 7.47 (1H, t, 7.8 Hz, Ar); 7.65 (1H, d, 7.6 Hz, Ar); 7.76 (1H, d, 8.4 Hz, Ar); 7.91 (2H, br. s, NH2).
13С NMR (DMSO-d6, δ, ppm., J/Hz): 26.73; 38.12 (t, 25.2 Hz, CHCF2); 46.26 (t, 4.5 Hz); 108.07 (t, 30.9 Hz, CHF2); 109.47 (t, 3.7 Hz, CN); 110.21-119.35 (multiplets family of С6F12); 112.79, 116.78, 116.54; 119.46; 120.49, 122.17; 131.95; 133.56; 164.63; 170.37.
19F NMR (DMSO-d6, δ, ppm., J/Hz): -111.87 (2F, s, CF2); -120.75 (2F, s, CF2); -121.92 (2F, s, CF2); -123.17 (2F, s, CF2); -129.05 (2F, s, CF2); -138.44 (2F, s, CF2).
MS (EI, 70 eV), m/z (Irel (%)): 553 [М]+ (0.97); 511 [M-Ac]+ (5.41); 252 [M-С6F12H]+ (85.04); 210 [M-С6F12H-Ac]+ (100).
Calculated: C, 43.41; H, 2.00; N, 7.59. Found: C, 43.46, H, 1.96, N, 7.53.
(4e) 2-amino-4-(1,1,2,2,3,3,4,4,5,5,6,6-dodecafluorohexyl)-
4,5-dihydropyrano [3,2-b]indole-3-carbonitrile,
C18H9F12N3O, MМ: 511.
Yield 87 % (0.28 g), light beige powder, Rf= 0.3 (PE+EA, 8+2).
1Н NMR (DMSO-d6, δ, ppm., J/Hz): 5.05 (1H, t, 11.0 Hz, CHCF2); 7.08 (1H, t, 7.5 Hz, Ar); 7.15 (1H, tt, 5.3 Hz, 51.7 Hz, CF2H, signals overlay); 7.19 (1H, t, 7.4 Hz, Ar); 7.40-7.48 (4H, Ar, NH2, signals overlay); 11.22 (1H, br. s, NH).
13С NMR (DMSO-d6, δ, ppm., J/Hz): 38.04 (t, 25.1 Hz, CHCF2); 46.38 (t, 4.8 Hz); 108.00 (t, 30.8 Hz, CHF2); 109.95 (t, 3.9 Hz, CN); 110.18-119.05 (multiplets family of С6F12); 112.41, 115.28, 115.94; 119.70; 120.45, 122.89; 131.30; 133.97; 164.87.
19F NMR (DMSO-d6, δ, ppm., J/Hz): -111.94 (2F, s, CF2); -120.77 (2F, s, CF2); -121.98 (2F, s, CF2); -123.23 (2F, s, CF2); -129.19 (2F, s, CF2); -138.52 (2F, d, 50.3 Hz, CF2H).
MS (EI, 70 eV), m/z (Irel (%)): 511 [М]+ (2.80); 210 [M-С6F12H]+ (100).
Calculated: C, 42.29; H, 1.77; N, 8.22. Found: C, 42.34, H, 1.73, N, 8.18.
Acknowledgments
This work was supported by the Ministry of Science and Higher Education of the Russian Federation (Contract/agreement No. 075-00697-22-00 and was performed employing the equipment of «Center for molecular composition studies» of INEOS RAS. To perfume this work, fluorocarbonyl compounds were provided by P&M Invest.
References
- J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok, H. Liu, The importance of fluorine in the life science industry, Chem. Rev., 2013, 114, 2432-2506. doi: 10.1021/cr4002879.
- E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnely, N. A. Meanwell, Fluorine in Pharmaceuticals: Looking Beyond Intuition, J. Med. Chem., 2015, 58, 8315-8359. doi: 10.1021/acs.jmedchem.5b00258.
- J.-P. Bégué, D. Bonnet-Delpon, Recent advances (1995–2005) in fluorinated pharmaceuticals based on natural products, J. Fluorine Chem., 2006, 127, 992-1012. doi.org/10.1016/j.jfluchem.2006.05.006.
- O. Yu. Fedorovskii, A. Yu. Volkonskii, A. S. Golubev, Yu. Ya. Spiridonov, N. D. Chkanikov, Synthesis of ethyl α-nitro-β-trifluoromethyl acrylate and β-trifluoromethyl-substituted tryptophan analogs and their plant growth regulating activity, Russian Chemical Bulletin, 2017, 66, 6, 1116‑1121. doi.org/10.1007/s11172-017-1863-z.
- N.D. Chkanikov, Yu.Ya. Spiridonov, O.Yu. Fedorovskii, S.S. Khalikov, A.M. Muzafarov, Ethyl ether 2-{4-[3-(4-chlorphenyl)-1-methylureido]phenyl}-2-hydroxy-3,3,3-trifluoropropionic acid: application as an antidote of herbicides and method of preparation. #2666732, 12 September. (in Russian)
- Multicomponent reactions, ed. by J. Zhu, H, Bienayme, Wiley-VCH Verlag Gmbh & Co. KGaA, 2006.
- Ruijter Eelco, V.A. Orru Romano, Multicomponent reactions – opportunities for the pharmaceutical industry., Drug Discovery Today: Technologies, 2013, 10(1), e15-e20. doi:10.1016/j.ddtec.2012.10.012.
- Trost B. M., Atom Economy. A Challenge for Organic Synthesis. Angew. Chem. Int. Ed. Engl., 1995, 34 (3), p. 259–281. doi.org/10.1002/anie.199502591.
- O. Yu. Fedorovskii, N. D. Chkanikov, Three-component one-step synthesis of new nitriles – 3-(1H-indole-3-yl)-2-cyano-4,4,4-trifluorobutanoic acid, Fluorine Notes, 2021, 1(134), 3-4. doi: 10.17677/fn20714807.2021.01.02.
- O. Yu. Fedorovskii, N. D. Chkanikov, C2-Alkylation in a three-component reaction of fluorocarbonyl compounds, pyrrole derivatives and malononitrile in competition with C2‑oxyalkylation, Fluorine Notes, 2022, 1(140), 3-4. doi: 10.17677/fn20714807.2022.01.02.
- W. J. Middleton, 1,1-Dicyano-2,2-bis(trifluoromethyl)ethylene, J. Org. Chem., 1965, 30, 1402‑1407. doi.org/10.1021/jo01016a013.
- V. S. Velezheva, D. E. Gedz’, D. V. Gusev, A. S. Peregudov, B. V. Lokshin, Z. S. Klemenkova, 2-Arylidene-3-indolinones in Synthesis of Indole Heterocycles and 4-Quinolones. In Nitrogen-Containing Heterocycles and Alkaloids, V. G. Kartsev, G. A. Tolstikov, Eds.; Iridium Press: Moscow, Russia, 2001; vol. 1, 247-253.
- A. M. Shestopalov, O. A. Naumov, and V. N. Nesterov., One-step synthesis of 5-acetyl-2-amino-4-aryl-3-cyano-4H-pyrano[3,2-b]indoles. Molecular and crystal structure of 5-acetyl-2-amino-4-(4-chloro-3´-nitrophenyl)-3-cyano-4H-pyrano[3,2-b]indole., Russian Chemical Bulletin, Int. Ed., 2003, 52(1), 179-186. doi.org/10.1023/A:1022468903806.
- V.I. Galkin, R.D. Sayahov, R.A. Cherkasov, Steric effects: the problem of their quantitative assessment and manifestation in the reactivity of hetero-organic compounds, Russian Chemical Reviews, 1991, 60(8), 815-829. doi.org/10.1070/RC1991v060n08ABEH001113.
ARTICLE INFO
Received 14 February 2023
Accepted 27 February 2023
Available online April 2023
Recommended for publication by PhD V. Don
eLIBRARY Document Number (EDN) KKYAZH

Fluorine Notes, 2023, 147, 3-4
