Received: February 2023
DOI 10.17677/fn20714807.2023.02.01
Fluorine Notes, 2023, 147, 1-2
DECAY SEQUENCES - ION SERIES OF MASS SPECTRA OF POLYOXAPERFLUOROALKANES AND POLYOXAPERFLUOROALKILS WITH TERMINAL HALIDE ATOMS
N.D. Kagramanov, S.R. Sterlin , A.A. Tyutyunov
A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences,
119991, GSP-1, Moscow, B-334, Vavilov St. 28
e-mail: ndkagram@gmail.com
Abstract: This report presents the ionic series of mass spectra of polyoxaperfluoroalkanes, as well as their derivatives with terminal halide atoms. In comparison with ionic series of linear perfluoroalkanes, as well as its derivatives with terminal halide atoms. Fragmentation changes due to oxygen atoms of the chain, as well as the structure of terminal groups, are discussed.
Keywords: ion series of mass spectra, regular and irregular fragment groups, OCF2 emission, rearrangements with formation of terminal fluorocarbonyl group, double series of rearranging ions, double series of fragmentation of molecular bromine-containing ions.
Experimental part
Samples of polyoxaperfluoroalkanes and polyoxaperfluoroalkils with terminal halide atoms, the spectra of which are not available in NIST libraries, were provided by NPO PiM-Invest CJSC. Electron ionization mass spectra were recorded via Finnigan Polaris Q chromatomass spectrometer (with ion trap, range 14-1000 Da, energy 70 eV and with Rtx-5MS capillary column (5% diphenyl/95% dimethyl polysiloxane 30 m long, 0.25mm-inner diameter, initial column temperature 30℃, isotherm 10 min; heating with a speed of 10°/min up to 250°C)). Since this report also contains spectra from NIST libraries obtained via magnetic or quadrupole apparatus, all presented mass spectra contain information about the type of apparatus on which this mass spectrum was recorded.
Introduction
In processes of electrosynthesis by Kolbe, the aliphatic products of radical dimerization were isolated and interpreted by NMR19F and chromatomass spectrometry methods [1]. For the first time, the homologues of a large number of polyoxaperfluoroalkanes and polyoxaperfluoroalkils with terminal halide atoms and with mass numbers from 500 to 1900 Da were synthesized and their mass spectra were recorded.
It was of interest to find out how oxygen chain links change fragmentation and ion series, in comparison with ion series of mass spectra of perfluoroalkanes and perfluoroalkyl halides. Fragmentation of linear n-alkanes and n-perfluoroalkanes always begins with terminal groups of CH3 and, respectively, with CF3. This is not only because two terminal groups with three hydrogen or fluorine atoms (compared to CH2 or CF2 groups in chain) are a more likely target for electron attack, but also because they are terminal groups.
There are two ion series in mass spectra of linear perfluoroalkanes [2,3]: the series of perfluoroalkyl ions [CnF2n +1]+ and the series of perfluoroalkenyl ions [CnF2n-2]+. The perfluoroalkyl series, terminating with +C3F7, +C2F5 and +CF3 ions, occurs as a result of primary detachment of fluorine atom and subsequent emissions of difluorocarbene. The series of perfluoroalkenyl ions [M‑3.F] begins with detachment of two fluorine atoms and formation of rearrangement cation radical with terminal perfluorovinyl group protecting one of the «flanks of the chain» from fragmentation. The excited rearranging cation radical emits the third fluorine atom. As a result, the opposite terminal group CF3 becomes the group +CF2 (1). Subsequent fragmentation with detachments of difluorocarbene is completed by a series of perfluoroallyl ions. Detachment of two fluorine atoms with formation of perfluorovinyl group, compared with detachment of one fluorine atom, may lead to a gain in energy required for detachment of third fluorine atom. The formation of two ion series can also be the result of difference in removal energies of one or three fluorine atoms. The reason for this difference in excitation energy +.M can be two variants of energy transfer that occur when an electron is removed - minimum and maximum. Along with values of radical detachment energies CF2=CF. and F. a certain “detachments symmetry” also plays a role in fragmentation processes, since the detachment of perfluorovinyl radical would completely exclude the possibility of perfluoroalkenyl series formation.
[M]+. -2 .F → CF3(CF2)n +CF-CF2. * -.F → +CF2 (CF2)nCF=CF2 (1)
Detachment of M-57 (M-3F) is usually accompanied by emission of CF2, so that instead of M-57 it corresponds to detachment of M-107. It is in this way occurs the formation of first fragment ion with m/z 931 0.5% (M-107) perfluoroeicosane C20F43 [4] MW 1038 NIST#: 239239 ID#: 36518 DB: mainlib.
Ionic series of mass spectra of α,ω-dihalogenperfluoroalkanes were also studied, in particular, 1,2-dibromo-1,1,2,2-tetrafluoroethane, 1,4-diiodperfluorobutane C4F8I2, 1,6-dichloro perfluorohexane C6Cl2F12 and 1,7-dibromoperfluoroheptan C7Br2F14 [4].
Mass spectra of α,ω-dihalogenperfluoroalkanes include four ionic series. These series terminate with following ions: (+CF3 m/z 69 - perfluoroalkyl series), (+C3F5 m/z 131 - perfluoroalkenyl), (+CF2Hal - halofluoroalkyl) and (+C3F4Hal - halofluoroalkenyl series).
The number of ion series probably corresponds to number of electrons being removed, as well as - to number of electrons whose detachments as a result of «optimal» excitation energy transfer options leads to increased energy +.M and their «additional» fragmentation detachments.
Perfluoroalkanes and perfluoroalkiles with terminal halide atoms contain regular fragment groups (CF2)n, which greatly simplifies the interpretation of their ion series. In contrast, the chains of polyoxaperfluoroalkanes molecules are heterogeneous because they contain fragments with different masses: O (m/z 16), CF2 (m/z 50) и CF(CF3) (m/z 100).
In mass spectra of polyoxaperfluoroalkanes with +.M ≥ 700 Da, the primary radical detachments occur, as a rule, for oxygen atom. In this case, the emitted radical may contain one or more oxygen atoms.
Despite the standard value of ionization energy, the mass spectra of polyoxaperfluoroalkanes obtained via mass spectrometer with mass analyzer («ion trap») differ from spectra taken under standard recording conditions - via magnetic and quadrupole devices.
The difference in mass spectra taken via ion trap is due not only to probability of protonation of molecular ions and possibility of ion-molecular reactions, but also - to distortion of peak intensities of molecular and fragment ions. The main reason for distortion of these spectra recorded via device with ion trap is ion separation time.
The time spent by ions and their separation in an ion trap is longer than in a magnetic sector or in a quadrupole filter. Due to increase in ion separation time, the fragmentation in ion trap occurs with a shift in intensity of peaks of successively fragmenting ions - from peaks with higher masses to peaks with lower masses. As a result, the peak intensities of molecular and primary fragment ions decrease, and the peak intensities of secondary and final ions increase (but the general nature of fragmentation does not change).
Fig. 1 shows the mass spectra of 1,1,1,2,3,4,4,4-octafluoro-2,3-bis(perfluoropropoxy)butane C10F22O2 (MW 570) obtained via magnetic equipment (VG 7070E) and ion trap (Polaris Q).
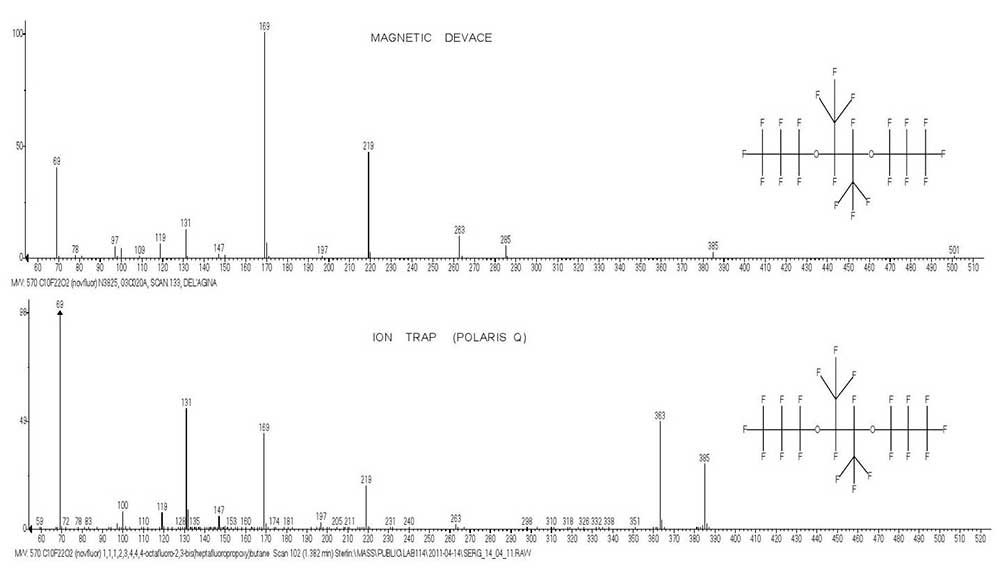
Figure 1. Mass spectra of 1,1,1,2,3,4,4,4-octafluoro-2,3-bis(heptafluoropropoxy) butane C10F22O2 MW:570, obtained via magnetic equipment (VG 7070E) and ion trap (Polaris Q).
The weak peak +.M-.CF3 m/z 501, which is present in magnetic spectrum, does not appear in spectrum recorded via equipment with ion trap.
These spectra differ in masses of base peaks: m/z 169 (VG) and 69 (Polaris Q). In addition, they also differ in that there is intense peak M -.C3F7 , -2.F (48%) for rearranging ion with m/z 363 in spectrum recorded via equipment with ion trap, which does not manifest itself in magnetic spectrum. The ion with m/Z 363 is the parent ion in series: 263, 197, 147, 97, 47, formed both in magnetic spectrum and spectrum recorded via equipment with ion trap.
Ion with m/z 363 appears to have the structure +CF2(CF2)2O-CF(CF3)-CF2-FC=O. Another difference between compared mass spectra is that the peak of M/2 ion with m/z 285 (5.2%) appears only in spectrum recorded via equipment with ion trap (VG). Although the mass spectra recorded via equipment with ion trap do not correspond to standard conditions for record of spectra via magnetic and quadrupole equipment, they include all main fragment ions.
This makes it possible to establish their ion series and (then compared with magnetic spectra) obtain the additional information confirming the fragmentation pathways.
Ionic series of polyoxaperfluoroalkanes and general patterns of their fragmentation
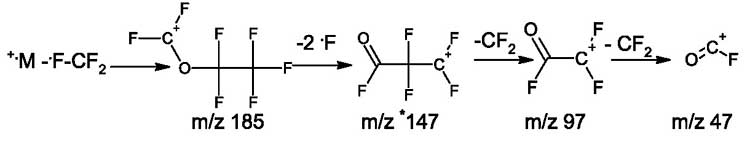
In mass spectra of polyoxaperfluoroalkanes, as well as of polyoxaperfluoroalkyl halides presented in this report, there are peaks of ions with m/z 147, m/z 97, m/z 47, the occurrence of which is due to simultaneous emission of two fluorine atoms and subsequent rearrangement leading to terminal fluorocarbonyl (acetylfluoride) group (see Fig. 2). When a fluorocarbonyl group occurs and one of terminal groups stabilizes, the detachment of radical F occurs from opposite terminal group and subsequent fragmentation leading to a series of ions with m/z 197, 147, 97.47.
Due to ease of primary detachment of radical from oxygen atom or with oxygen atom, the molecular ions do not appear in mass spectra of polyoxaperfluoroalkanes.
Example of mass spectrum of oxaperfluoroalkane with one oxygen atom is spectrum of 1,1‑oxybis(pentafluoroethane). Mass spectrum of compound (see Fig. 2) consists of two series: for oxaperfluoroalkyl, leading to formation of perfluoroalkyl ions with m/z 119 and 69 (marked in red), and for rearrangement series (marked in blue), terminating with peaks of ions with m/z 147, 97 and 47.
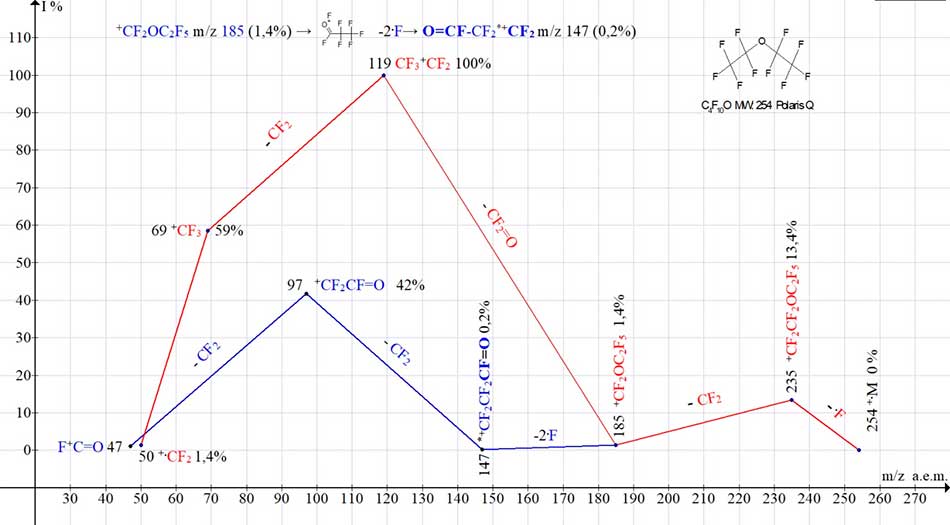
Figure 2. Two ion series of mass spectrum of 1,1`xybis(pentafluoroethane) С4F10 O MW:254
Ion Trap (Polaris Q).
When two fluorine atoms are detached from +CF2OC2F5 ion with m/z 185, the rearrangement ion (having m/z 147) with terminal fluorocarbonyl group occurs. It emits the difluorocarbene to form fragment ions with m/z 97 and 47 containing a fluorocarbonyl group.
Terminal perfluorooxaallyl group OCF2CF=CF2 stabilizes one of the flanks of +·M chain, excluding its fragmentation. It is thanks to it that molecular ion appears in mass spectrum. Both ion series of spectrum (see Fig.3): for oxaperfluoroalkyl (marked in red) and for oxaperfluoroallyl (marked in blue) begin with detachments of .OC3F6 and .OC3F7 radical from opposite terminal group OC3F7. Rearrangements occur in both ion series (rearrangement ions are marked in bold).
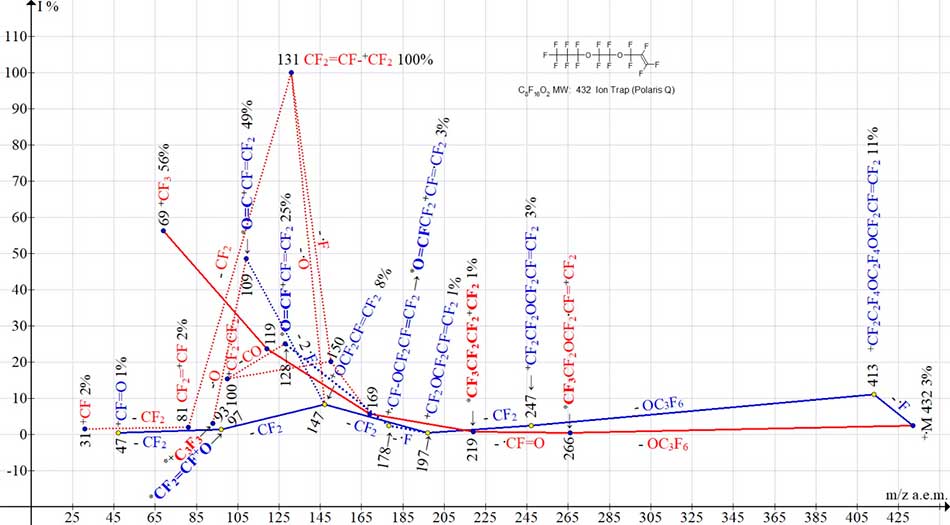
Figure 3. Two ion series of С8F16 O2 MW:432 mass spectrum:432 Ion Trap (Polaris Q).
When .CF=O radical is detaching from ion with m/z 266 (series marked in red), the rearrangement ion +C4F9 with m/z 219 arises, that fragmenting with formation of a series of perfluoroalkyl ions. As a result of successive detachments of .F, .OC3F6, CF2, CF2 the oxy-perfluorallyl ion +OCF2CF=CF2 with m/z 147 (series marked in blue) appears.
When oxygen atom is detached from ion with m/z 147 (or fluorine atom - from +C3F6 ion with m/z 150) (see Fig.3), the base ion of C8F16O2 spectrum becomes CF2=CF-+CF2 ion with m/z 131.
CF2=CF-CF2+O ion with m/z 147 does not contain any fluorocarbonyl group. When two fluorine atoms are detached from it and rearranged into CF2=CF-+C=O ion with m/z 109, any fluorocarbonyl group is also not formed. However, the final fragment ion +CF=O m/z 47 contains a fluorocarbonyl group. Detaching of oxygen atom from ion with m/z 109 is completed by rearrangement of +C3F3 ion with m/z 93.
Fig. 4 shows two ion series of mass spectrum C10F22O2 MW:570.
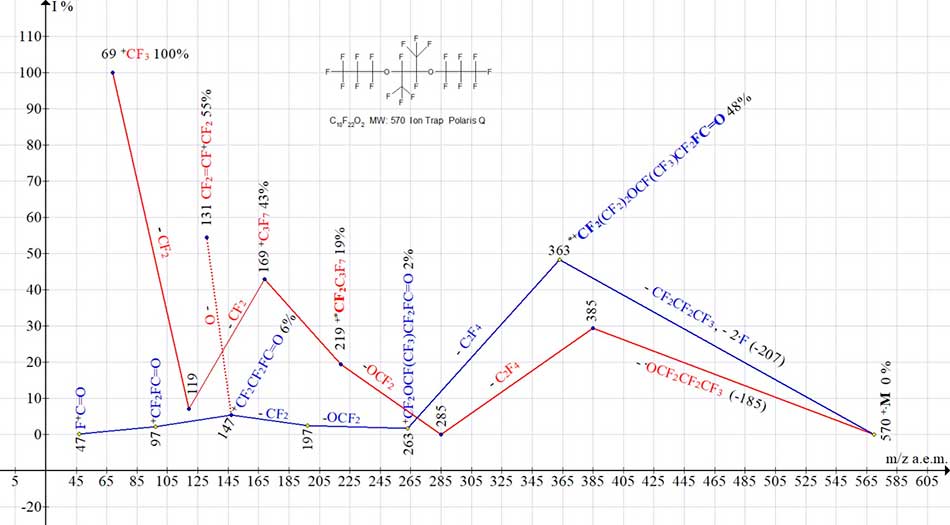
Figure 4. Two ion series of С10F22 O2 MW:570 mass spectrum:570 Ion Trap (Polaris Q).
Despite the fact that this compound is symmetrical, there is no decay of M/2.
Perfluoroalkyl ion series (marked in red) begins with detachment of .OC3F7 (-185) radical, then - with detaching of C2F4 molecule and subsequent emission of O=CF2 molecule, with formation of a rearrangement ion +CF2C3F7 with m/z 219, i. e. group that is not present in original molecule. The rearrangement series of ions (marked in blue) begins with detachment of .C3F7 radical (without oxygen) as well as - with emission of two fluorine atoms from two CF3 groups (the opposite terminal and one of central F3CCF groups (summary -207). As a result, there is the rearrangement ion with m/z 363 with terminal fluorocarbonyl group. Fragmentation of ion with m/z 363 results in a low-intensity series of fluorocarbonyl ions with m/z 197, 147, 97 and 47. Since there is no series of ions with terminal perfluorovinyl group in spectrum (see Fig. 4), the appearance of intense peak (55%) of perfluoroallyl ion CF2=CF-+CF2 with m/z 131 is probably the result of detaching of oxygen atom from the ion with m/z 147.
Fig. 5 shows two ion series of C12F26O3 mass spectrum. Intense series of ions ending with perfluoroalkyl ions (marked in red), and its rearrangement series (marked in blue), terminates with ions of fluorocarbonyl group. In presented mass spectrum, the number of peaks of recorded ions is only thirteen. Probably due to insufficient amplification or low sensitivity of spectrometer, the low-intensity ion peaks did not appear in this spectrum (in particular, the peak of ion with m/z 147 (0%) and the peak of ion with m/z 47).
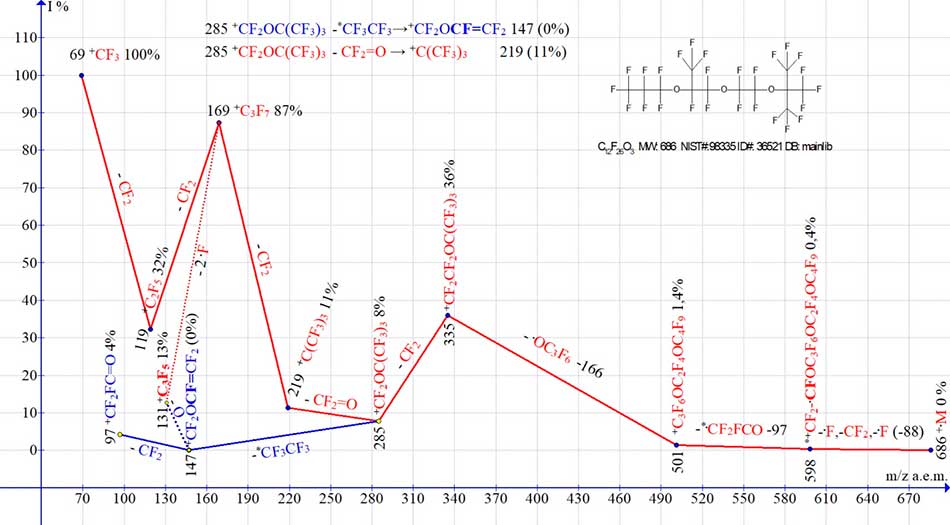
Figure 5. Two ion series of mass spectrum of perfluoro2,8-trimethyl-3,6,9-trioxadodecane С12F26 O3 MW:686 NIST#: 98335 ID#: 36521 DB: mainlib.
Of the two possible options for starting fragmentation [С12F26 O3]+., it does not start with .C(CF3)3 group (having m/z=219) with a tertiary carbon atom, and with perfluoropropyl group .C3F7 (having m/z=169). After detachment of trifluoromethyl radical (-.F, -CF2), another emission of fluorine atom occurs (with formation of rearranging cation radical with m/z 598).
Subsequent fragmentation, including detachment of rearrangement .CF2FC=O -97 radical, emission of OC3F6 -166 and detachment of CF2, leads to ion +CF2OC(CF3)3 with m/z 285. Before formation of ion with m/z 285, the group C(CF3)3 with tertiary carbon atom does not participate in fragmentation. +CF2OC(CF3)3 8% ion with m/z 285 fragments in two ways: with emission of CF2=O and formation of perfluoroalkyl ion +C(CF3)3 with m/z 219 (11%) (series in red), and with emission of hexafluoroethane molecule and formation of rearrangement ion *+CF2OCF=CF2 with m/z 147 (0%) and its fragmented ion +CF2FC=O with m/z 97 (4%) (series in blue).
Fig. 6 shows two ionic series of polyoxaperfluoroalkane C12F26O4 MW=702, the molecule of which has a plane with mirror symmetry, as well as a vertical axis of symmetry passing in the plane of symmetry (between two central groups F-C-CF3). There is no ion peak M/2 =351 in mass spectrum of this compound. Primary radical detachments occur at one of central oxygen atoms.
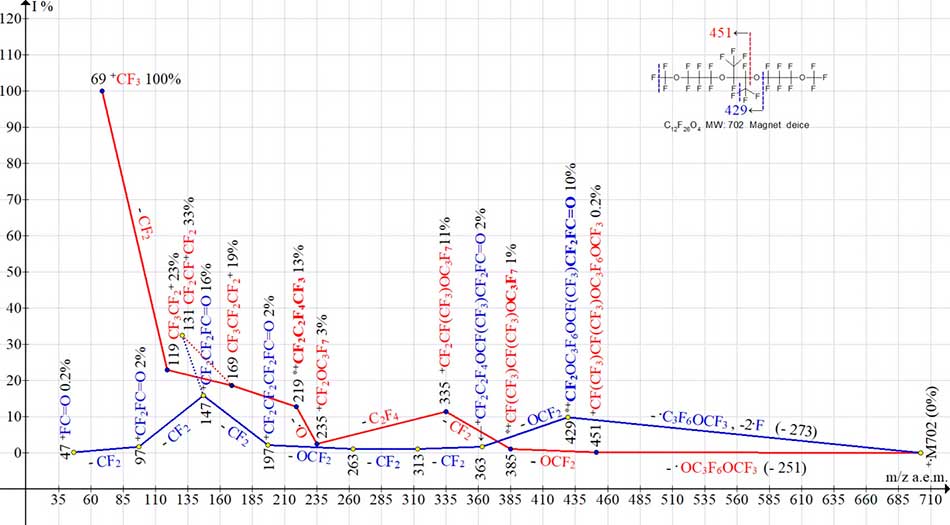
Figure 6. Two ion series of mass spectrum С12F26 O4 MW:702 (magnetic mass spectrometer VG-7070E)
Two ion series (see Fig. 6.) of mass spectrum (marked in red and blue) begin with rearranging ions {with m/z 385 and m/z 429). Ion with m/z 385, occurs when O=CF2 is detached from terminal group CF3O for ion with m/z 451. As a result, its terminal group becomes OC3F7 group. Ion with m/z 429 occurs after terminal group .C3F6OCF3 is emitted and two fluorine atoms are detached (marked in blue dotted line in structural formula). As a result, its terminal group becomes the fluorocarbonyl group FC=O.
The rearrangement series (marked in blue) terminates with ions having m/z 197, 147, 97 and 47 and containing a fluorocarbonyl group. Ion +CF2OC3F7 with m/z 235 (3%), occurs in this series (marked in red) and terminates with perfluoroalkyl ions. +CF2OC3F7 ion loses the oxygen atom to form +C4F9 ion with m/z 219 (13%), i. e. a group that is not present in original M+. The emission of oxygen atom confirms the energy benefits of formation of homogeneous perfluoroalkyl rearrangement ion +C4F9.
Fig. 7 shows two ionic series of polyoxaperfluoroalkane C17F34O5 with central carbonyl group. In series terminating with perftoalkyl ions (marked in red), the detachment of C3F7OCF(CF3)CF2OCF(CF3).C=O [- C9F17 O3 - m/z 479] is accompanied by emission of another oxygen atom. As a result of these two detachments (- m/z 495) the rearrangement ion C3F7OCF(CF3)CF2+CF(CF3) with m/z 435 arises. Its further fragmentation includes three CF2 detachments with formation of C3F7O+CF(CF3) ion having m/z 285.
Final emission of F2C=O molecule leads to rearrangement of perfluoroalkyl ion C3F7+CF2 with m/z 219 (i. e. the group that is not present in original compound).
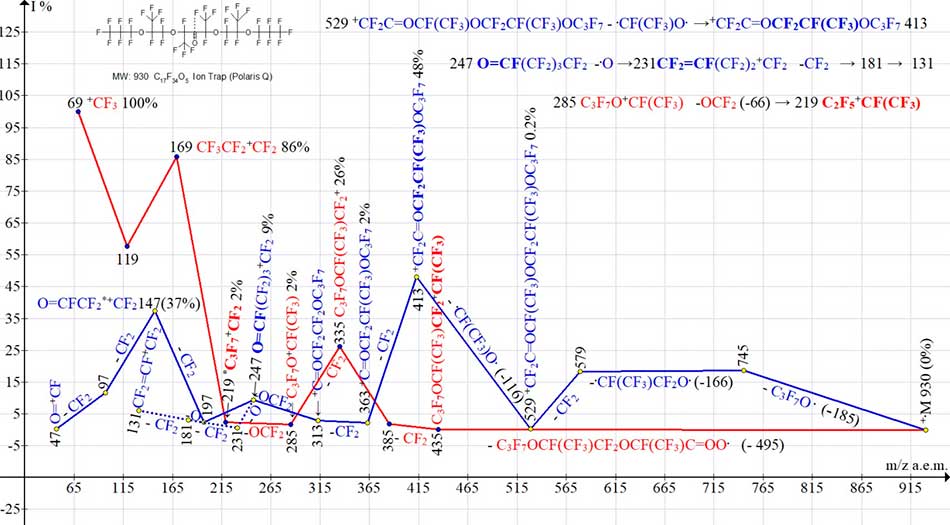
Figure 7. Two ion series of mass spectrum С17F34 O5 MW: 930 Ion Trap (Polaris Q).
In the absence of perfluoroallyl ion series, the appearance of weak peaks of ions with m/z 231 and 181 is the result of detachment of oxygen atom from the ion with m/z 247, as well as - from the ion with m/z 197.
Two ion series of mass spectrum of polyoxaperfluoroalkane C34F70O10 (MW 1898) are presented (see Fig.8).
In spectrum, recorded within the range of 1000 Da, the first recorded peak is the peak of ion with mass of 667. The peak-free zone for mass spectrum within the range 1898 - 667 at 1231 Da is the result of instability of molecular cation radical with m/z 1898. Decay of +.M can occur at detachment of radical M/2 (m/z 949) + CF(CF3)OCF2CF(CF3)O (m/z 282) with formation of the stable ion [C12F25O3]+ C3F7OCF(CF3)CF2OCF(CF3)CF2OCF(CF3)+CF2 (9%) with m/z 667. As a result, the mass of detached radical (1231 Da) exceeds the mass of resulting ion by 564 Da Given that C34F70O10 molecule is symmetric, there is reason to believe that the decay of M+. it can also occur with emission of neutral OCF(CF3) molecule OCF(CF3)CF2OCF(CF3)-(CF3)CFOCF2(CF3)CFO with a mass of 564 Da from central part of molecular cation-radical (with formation of symmetric cation- radical [C24F50O6]+. , having m/z 1334. The symmetric cation-radical further decays into ion and radical with the same masses equal to 667Da In this case, the mass of resulting ion [C12F25O3]+ with m/z 667 is greater than the mass of detached neutral molecule C10F20O4 by 103 Da. Both pathways of fragmentation of cation +C12F25O3 m/z 667 (9%) formed from С34F70O10 (Polaris Q, Ion Trap) (see Fig. 8) lead to the same ion with m/z 285.
One pathway is the path of four consecutive, low–intensity detachments, and other way - two detachments with a total mass of -382.
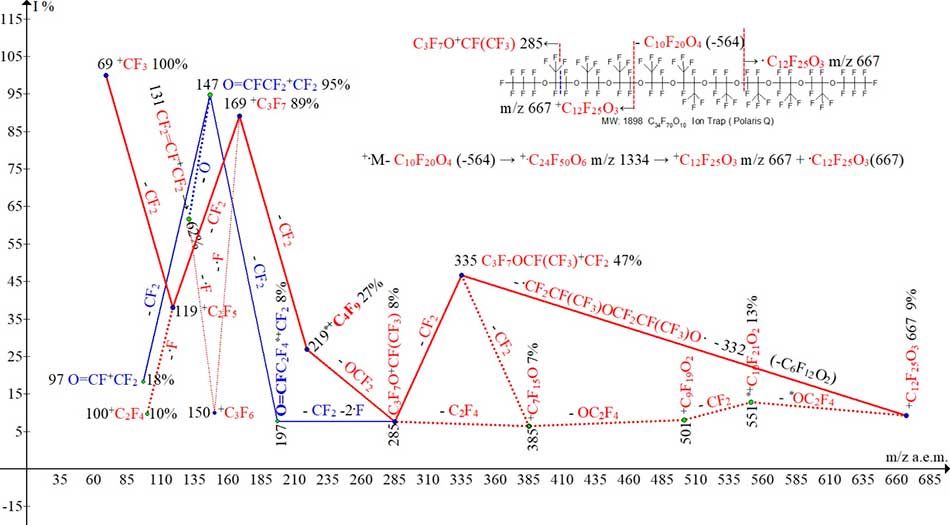
Figure 8. Two ion series of mass spectrum (C17F35O5)2 MW:1898 Ion Trap (Polaris Q).
Emission of 285 - 66 [CF2O] = 219 (series in red) leads to rearrangement of *+С4F9 ion with m/z 219 (27%) – to a group that is not present in original molecule. Detachment of difluorocarbene and two fluorine atoms 285 - 50, -38 = 197 (8%) (series in blue) to rearrangement ion O=CFC2F4+CF2 with m/z 197 (8%) to fluorocarbonyl group. Ion with m/z 197 fragments to form the intense peak of ion with m/z 147 (95%) and its fragmented ion 97 (18%). Despite the absence of perfluoroallyl ion series in spectrum, the intensity of peak for CF2=CF-+CF2 ion with m/z 131 is high (62%). Possible pathways to occurrence of ion with m/z 131 can be either detachment of oxygen atom from ion O=CFCF2+CF2 with m/z 147 (blue dotted line) or detachment of fluorine atom from ion +C3F6 (red dotted line).
Ionic series of polyoxaperfluoroalkyl with a terminal chlorine atom
Fig. 9 shows the ion series of mass spectrum of 1-(2-chloro-1,1,2,2-tetrafluoroethoxy)-pentadecaftorgeptan - compounds with one oxygen atom.
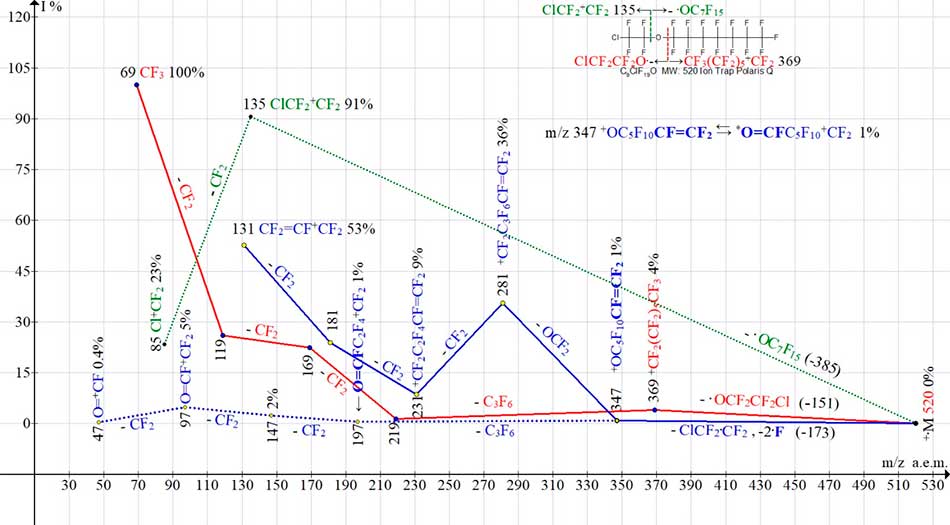
Figure 9. Four ion series of mass spectrum С9ClF19O MW:520 Ion Trap (Polaris Q).
Decays of molecular cation radicals with formation of perfluoroalkyl series (in Fig. 9 marked in red) and also - chloroperfluoroalkyl series (marked in green), occur for oxygen atom - the only «heterogeneity» of chain. After detachments of oxygen - containing radicals .OC7F15 (series in green) and .OC2F4Cl (series in red) generate ClCF2+CF2 with m/z 135 and CF3(CF2)5+CF2 with m/z 369 ions.
Detachment of .CF2CF2Cl radical without oxygen atom is accompanied by additional emission of two more fluorine atoms (series in blue), detached from opposite terminal pentafluoroethyl group.
As a result of these detachments, the rearrangement ion CF2=CF(CF2)5O+ (m/z 347 (1%)), with terminal perfluorovinyl group, is formed. It emits O=CF2 molecule, forming a series of intense peaks of ions with m/z 281, 231, 181, culminating in formation of a perfluoroallyl ion with m/z 131(53%) (marked in blue). Another pathway for ion fragmentation with m/z 347 terminates with a low-intensity series of ions with a fluorocarbonyl group (marked with blue dotted line). Formation of series for ions with m/z 197, 147, 97 and 47 confirms that the ion with m/z 347 is also rearranged to form a terminal fluorocarbonyl group (see Fig. 9).
Three ion series of С9СlF19O2 mass spectrum are shown in Fig.10.
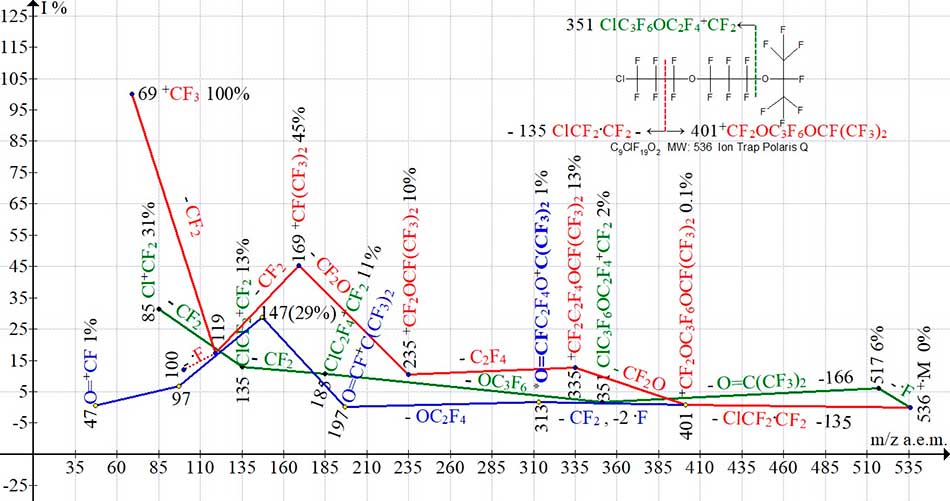
Figure 10. Three ion series of mass spectrum С9ClF19O2 MW: 536 Ion Trap (Polaris Q).
Replacement of terminal trifluoromethyl group С9ClF19O (see Fig.9.) with heptafluoropropyl group CF(CF3)2 (see Fig. 10), with tertiary carbon atom and increase in a number of oxygen atoms in chain by another atom leads to a significant change in fragmentation of С9ClF19O2. The series of allyl ions with m/z 281, 231, 181 is not formed. Compared with trifluoromethyl group (see Fig. 9), the heptafluoropropyl group with m/z 219 (see Fig.10) fragments with detachment of fluorine atom, and then - with emission of hexafluoroacetone. This reduces the excess excitation energy of sequentially fragmenting chlorine-containing ions (marked in green), the number of which in series, compared with the spectrum of C9ClF19O (see Fig.9) increases from two to five (see Fig.10). The presence of two oxygen atoms in chain increases the likelihood of a series of ions containing a fluorocarbonyl group and eliminates the possibility of formation of perfluoroallyl series terminating with ion CF2=CF-+CF2 having m/z 131. Intensity of ion peak with m/z 147 increases to 29% and intensity of ion peak with m/z 131 in spectrum of С9СlF19O (in Fig.10 not represented) is only 3%. Given the high intensity of ion peak with m/z 147, the ion +C3F5 with m/z131 may be formed by detachment from ion O=CFCF2+CF2 with m/z 147.
Fig. 11 shows three ionic series of mass spectrum of polyoxaperfluoroalkyl chloride С12ClF25O3 MW:702. Compared with ion series of mass spectrum of polyoxaperfluoroalkane С12F26 O4 (with the same molecular weight MW:702) (see Fig. 6), during fragmentation of which the primary detachment of .OC3F6OCF3 and .C3F6OCF3 + 2.F radicals occurs, with masses of -251 and -273 Da, in spectrum of polyoxperfluoroalkyl chloride (see Fig. 11) the mass of primary radical emissions: atom .F -19 and .OC3F6Cl -201 Da are significantly smaller.
The terminal chlorine atom (with mass of 35/37) stabilizes the perfluoropolyxaalkyl chloride molecule, resulting in a minimum detachment of fluorine atom by mass, but with subsequent emission of .C(CF3)2OC3F6O. radical with mass of 332, there is a chlorine-containing ion ClC3F6OC2F4+CF2 with m/z 351 (2%) and mass of M/2.
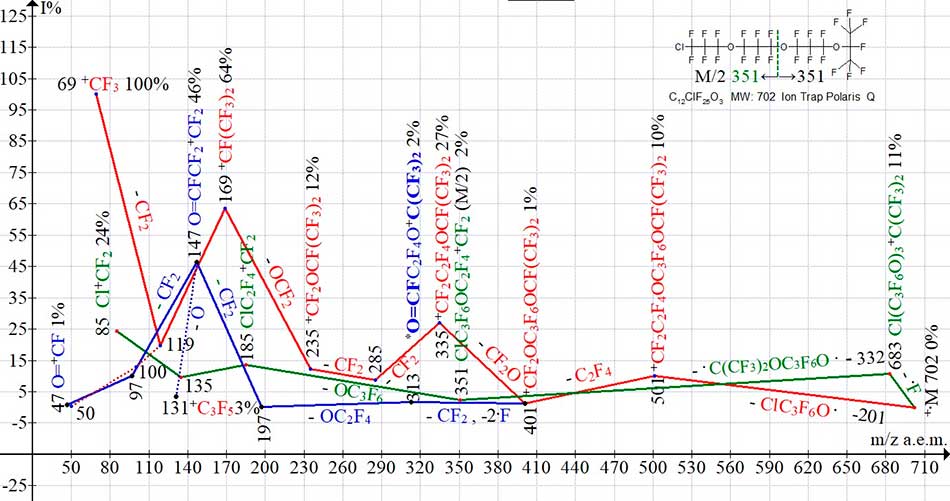
Figure 11. Three ion series of mass spectrum С12ClF25O3 MW:702 Ion Trap (Polaris Q).
When increasing the number of oxygen atoms in the chain of polyoxaperfluoroalkyl chlorides, there is a noticeable increasing in intensity of peaks containing the fluorocarbonyl group (197, 147, 97, 47). Appearance of series of perfluoroallyl ions becomes impossible, however a weak peak of perfluoroallyl ion with m/z 131 (3%) is formed when detachment of oxygen atom from the ion with m/z 147 (see Fig. 11).
In mass spectra of α,ω-dichloropolyoxaperfluoroalkanes: [Cl(C3F6O)2CFCF3]2 MW:934, [Cl(C3F6O)3CFCF3]2 MW:1266, [Cl(C3F6O)4CFCF3]2 MW:1598 large peak-free zones occur (up to M/2). This is probably due to the fact that stabilizing effect of two terminal chlorine atoms is directed in opposite direction. In С16Cl2F32O4 - С28Cl2F56O8 mass spectra the peaks of primary ions have the same or similar m/z values and similar intensity values. Thus, the first chlorine-containing ions of their mass spectra are the following:
С16Cl2F32O4 MW:934 - (M/2) + C2F4 = +[C10ClF20O2] m/z 567 (11%);
С22Cl2F44O6 MW:1266 - (M/2) - OCF2 = +[C10ClF20O2] m/z 567 (6%);
С28Cl2F56O8 MW:1598 - (M/2) - OCF(CF3) = +[C12ClF24O3] m/z 683 (8%);
In mass spectra of these three compounds (with two terminal chlorine atoms) the intensities of perfluoroallyl ion CF2CFCF2 with m/z 131 and the ion with fluorocarbonyl group +СF2CF2FC=O with m/z 147 are quite high (about 50-80%). However, if the sequence of a series of ions with fluorocarbonyl group 197, 147, 97 and 47 is well manifested in a spectra, then the intense perfluorallyl ion (46%) is not the allyl series ion, but is formed from +СF2CF2FC=O ion with m/z 147 when oxygen atom or +.С3F6 ion is separated from it (when the fluorine atom is detached from it).
Fig. 12 shows three ion series of С16Cl2F32O4 mass spectrum. The presence of two terminal chlorine atoms in С16Cl2F32O4 molecule does not lead to appearance of molecular ion in spectrum. The mass of primary radical, detached from chlorine-containing series (marked in green), is 367 Da.
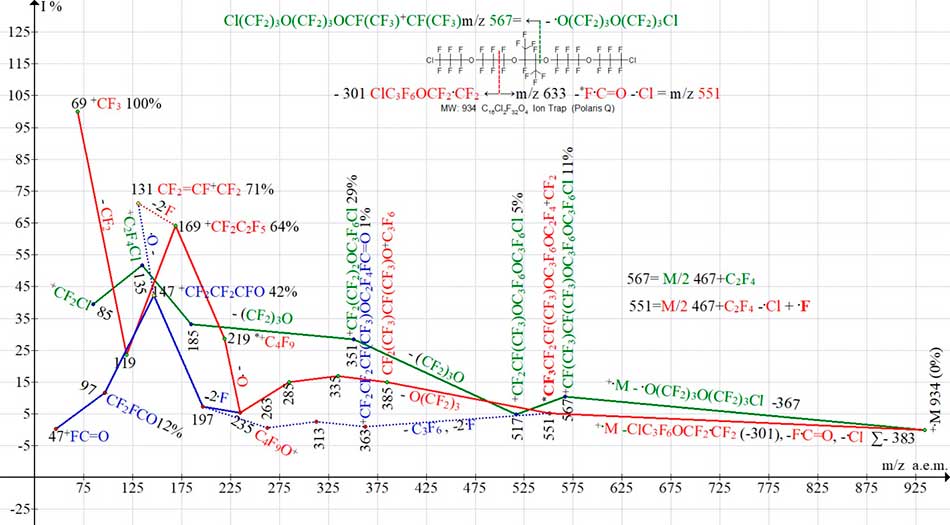
Figure 12. Three ion series of mass spectrum С16Cl2F32O4 MW: 934 Ion Trap (Polaris Q).
Compared with ionic series of monochloride (see Fig.11) in dichloride series (see Fig.12), the peak-free zones increase to 367 and 383 Da.
The first rearrangement ion *CF3CF2CF(CF3)OC3F6OC2F4+CF2 with m/z 551 Da (series marked in red in Fig. 12) occurs after detachment of radicals ClС3F6OCF2.CF2 (- 301) (- 301) and F.C=O (-47), as well as after emission of second chlorine atom. Ion with m/z 551 contains the terminal rearrangement group C4F9, which is not present in original molecule.
Fragmentation of this series (marked in red) leads to perfluoroalkyl ions with m/z 219, 169, 119 and 69. Rearrangement ion with m/z 551 also fragments with emission of C3F6 molecule and two fluorine atoms, forming a series of ions with fluorocarbonyl terminal group (marked in blue dotted and solid line) with m/z 197, 147, 97 and 47.
The intense peak (71%) of perfluoroallyl ion CF2=CF-+CF2 with m/z 131 is not allyl series ion, but is formed when the oxygen atom detaches from ion +СF2CF2FC=O (m/z 147), or the fluorine atom - from ion +.С3F6 (m/z 150).
Ionic series of polyoxaperfluoroalkyl with terminal bromine atom
Fig. 13 shows four ion series of С7BrF15O2 mass spectrum.
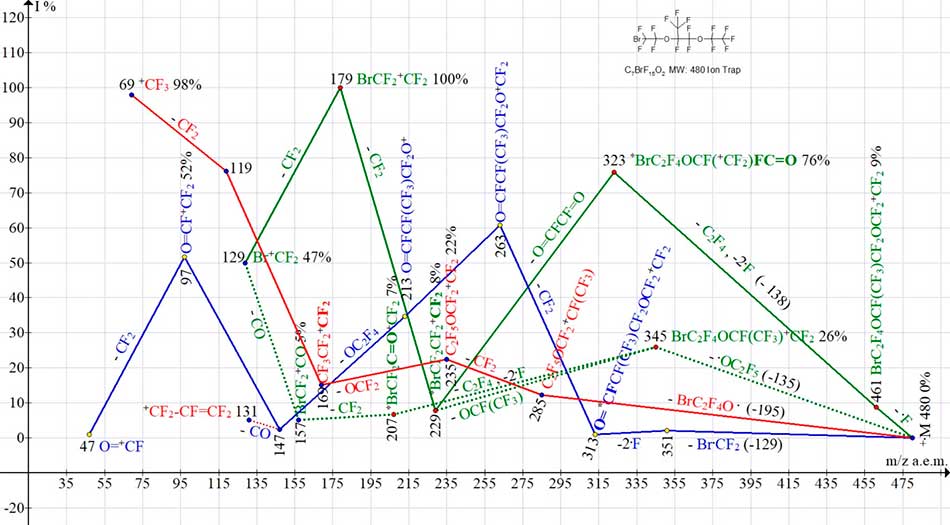
Figure 13. Four ion series of mass spectrum С7 BrF15O2 MW:480 Ion Trap (Polaris Q).
Two bromine-containing ion series is marked in green. One series of perfluoroalkyl is marked in red and one series of ions containing a fluorocarbonyl group - is marked in blue.
In bromine-containing series of ions with more intense ion peaks, after emission of fluorine atom, the detachment of C2F4 and two atoms .F occurs with formation of rearrangement ion BrC2F4OCF(+CF2)FC=O with m/z 323.
Ion with m/z 323 emits the dicarbonyl difluoroethane molecule O=CF-CF=O, turning into ion BrC2F4+CF2 with m/z 229. In a less intense bromine-containing series (after detachment of .OC2F5 and formation of BrC2F4OCF(CF3)+CF2 ion with m/z 345), the emission of C2F4 and two fluorine atoms results in rearrangement ion BrCF2C=O+CF2 with m/z 207.
The subsequent detachment of CF2 with formation of BrCF2+C=O ion with m/z 157 (5%) and emission of CO (-28) completes Br+CF2 ion with m/z 129 (47%).
A series starting with emission of Br.CF2 and detachment of two fluorine atoms (marked in blue), terminates with ions having m/z 197,147, 97 and 47 and containing a fluorocarbonyl group.
A series in which the detachment of BrC2F4O. with maximum mass occurs (- 195 Da) (marked in red) terminates with perfluoroalkyl ions. All four series of ions (see Fig.13) are rearranging. In three series, additional, simultaneous detachments of two fluorine atoms occurs, and in one series (marked in red) - emission of OCF2 molecule from the middle of chain.
Fig. 14 shows three ion series of С11BrF23O3 mass spectrum.
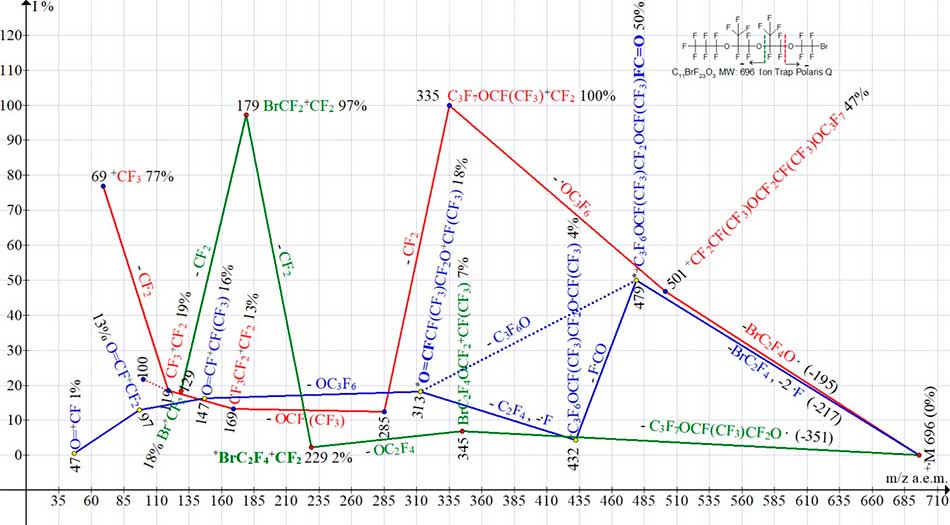
Figure 14. Three ion series of mass spectrum С11BrF23O3 MW:696 Ion Trap (Polaris Q).
Of the three ionic series of С11BrF23O3 spectrum, presented in Fig.14, in two series of detachments of bromine-containing radicals occurs: in BrC2F4O. series marked in red and in BrC2F4 + 2.F series marked in blue. The series marked in red terminates with perfluoroalkyl ions. The first fragment ion in blue series with m/z 479 is a rearrangement ion with terminal fluorocarbonyl group. As a result of detachments from it - C3F6O (marked by blue dotted line), or two detachments (- F.C=O и -C2F4, - .F) (marked by two solid blue lines), the ion with m/z 313 arises. It fragments to form a series of ions: 147, 97 and 47 with terminal fluorocarbonyl group.
In bromine-containing series (marked in green), the first bromine-containing ion +С3F6OC2F4Br with a mass of m/z 345 (7%) fragments with emission of OC2F4 and formation of rearrangement ion *BrC2F4+CF2 with m/z 229 (2%) - a group that is not present in original molecule. Perfluoroallyl ion, whose intensity does not exceed 6%, is not indicated in Fig. 14.
Ionic series of two structural isomers С11BrF23O3 with different terminal groups (see Fig.14 and Fig.15) differ in intensities of most peaks, as well as in number of arising bromine-containing ions.
Detachment of M-F from isomer with terminal group CF3CF2CF2O (see Fig.14) does not occur. In its bromine-containing series (see Fig. 14) the number of bromine-containing ions is less than in bromine-containing series of isomer with terminal group CF3)2FC (see Fig. 15). In particular, there are no ions with m/z 677, 561 and 395.
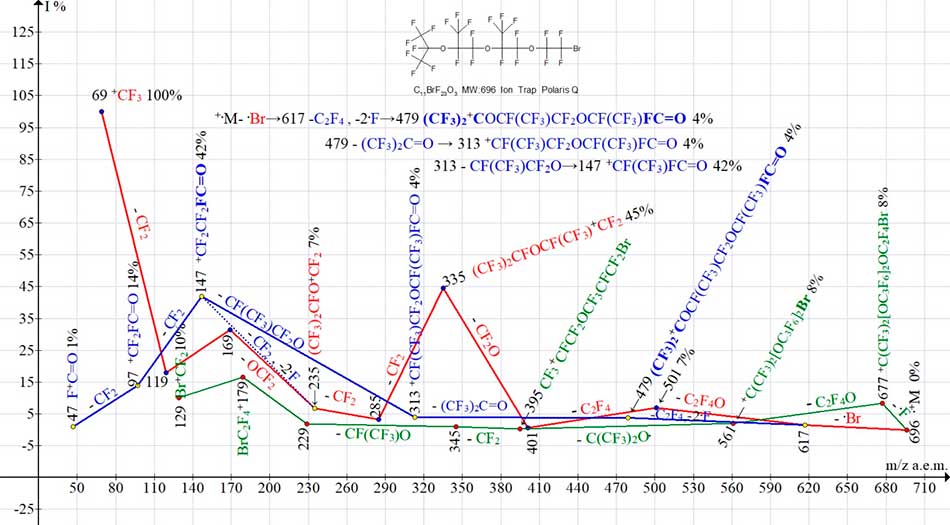
Figure 15. Three ion series of mass spectrum С11BrF23O3 MW:696 Ion Trap (Polaris Q).
In another isomer the fluorine atom is detached from terminal group (CF3)2FCO (see Fig. 15). The sequence of detachments in this bromine-containing series (marked in green) allows us to conclude that next detachment - .OC2F4 comes from opposite terminal group OC2F4Br. As a result, a rearrangement ion with terminal group OCF(CF3)CF2Br with m/z 561 arises (see Fig. 15).
In spectrum of isomer with terminal group CF3CF2CF2O (see Fig. 14) the intensity of ion peaks with m/z 285 and 335 (see Fig.14) doubled, and peak with m/z 501 is 9 times larger than that for isomer with terminal group (CF3)2FC (see Fig. 15). Intensity of peak BrCF2+CF2 with m/z 179 in spectrum of isomer with terminal group CF3CF2CF2O (see Fig. 14), 6 times greater than for isomer with group (CF3)2FCO (see Fig. 15).
Compared with terminal group CF3CF2CF2O (see Fig. 14), the terminal group (CF3)2FCO (see Fig. 15) apparently, it is the main reason for decrease in intensity of most fragment ions of this isomer.
Fig. 16 shows three ion series of С16BrF33O5 mass spectrum.
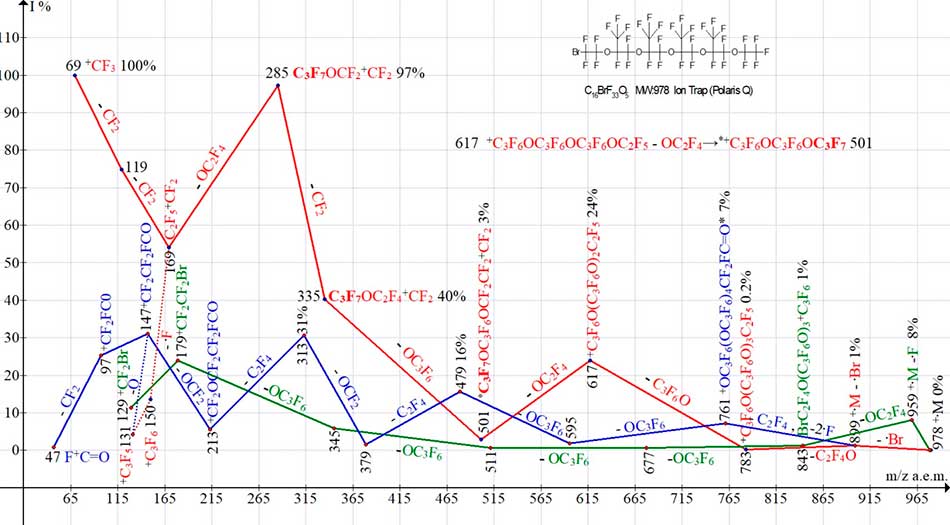
Figure 16. Three ion series of mass spectrum С16BrF33O5 MW:978 Ion Trap (Polaris Q).
Of the three ion series, shown in Fig. 16, only non-arrangement series is the bromine-containing series (marked in green). It includes seven bromine-containing ions. In most intense polyoxaperfluoroalkyl ion series (marked in red), there are consecutive detachments .Br, C2F4O and C3F6O are terminated by ion with m/z 617. Detachment of OC2F4 from opposite terminal group OCF2CF3 leads to a rearrangement ion with m/z 501, with terminal group CF3CF(CF3)O, which is not present in original molecule. A series of fluorocarbonyl ions with m/z 147, 97, 47 (marked in blue) begins with detachment of C2F4 and two fluorine atoms. There is a rearrangement ion (m/z 761) with terminal fluorocarbonyl group. It fragments with detachment of two radicals .OC3F6, emissions of - C2F4, -OCF2, - C2F4, -OCF2, loss of fluorocarbonyl group and its formation again (ions with m/z 147.97, 47).
Fig. 17 shows the ion series of C14Br2F28O4 mass spectrum.
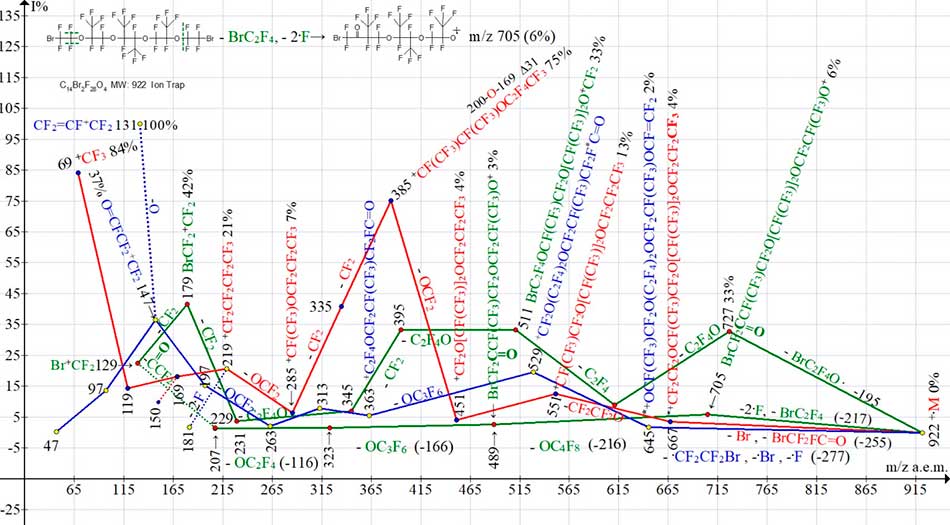
Figure 17. Four ion series of mass spectrum С14Br2F28O4 MW:922 Ion Trap (Polaris Q).
During fragmentation of dibromide C14Br2F28O4 (see Fig. 17), as a result of two different primary detachments of bromine-containing radicals (as well as result of additional emission of two fluorine atoms in one of series) two «parallel» series of bromine-containing ions are formed (marked in green). The primary detachments in these two C14Br2F28O4 series differ in that in series with a more intense peaks the detachment of -BrC2F4 (-195) occurs. and in series with a less intense series - detachments of -BrC2F4 and - 2.F (-217) from opposite terminal group BrCF2CF2O. In a less intense series as a result of primary detachments, the rearrangement ion with m/z 705 containing BrCF2C=O group is formed.
The next, «extraordinary release» -OС4F8 in this series comes from the middle of chain. The presence of carbonyl group in ions with m/z 705, 489 and 207 confirms the detachment of molecule O=CCF2 (-78) from ion with m/z 229, leading to formation of Br+CF2 ion.
Two different fragmentation pathways +.M (C14Br2F28O4 with formation of two series of bromine-containing ions are either the result of two different excitation energies +.M, or the result of removal of two electrons from molecule that are topologically different with respect to bromine atom.
The series of polyoxaperfluoroalkyl ions (marked in red) begins with emission of bromine atom, as well as with detachment of rearrangement molecule BrCF2FC=O to form a rearrangement ion with terminal group CF3 having m/z 667. After a series of detachments of oxygen-containing fragments, the ion with m/z 219 arises, that fragments to form a series of perfluoroalkyl ions.
Another series (marked in blue) begins with formation of rearrangement ion (m/z 646) with terminal FC=CF2 group. This ion series terminates with formation of ions with fluorocarbonyl group (m/z 147, 97 and 47).
Ionic series of polyoxaperfluoroalkyl with terminal iodine atoms
In mass spectra of polyoxaperfluoroalkils with terminal chlorine or bromine atom the molecular cation radicals do not appear due to their instability. In some cases, the first fragment ion in their spectra is M-F ion. Compared with other halides, the terminal iodine atom contributes to maximum stabilization of molecular ion, especially if the linear chain of compound is not very large.
In all mass spectra of polyoxaperfluoroalkyliodides presented in this report, there are molecular ions. However, their intensity decreases as molecular weight increases and proportion of iodine atom mass to mass of chain decreases.
Ion series of С5F11IO mass spectrum are shown in Fig. 18.
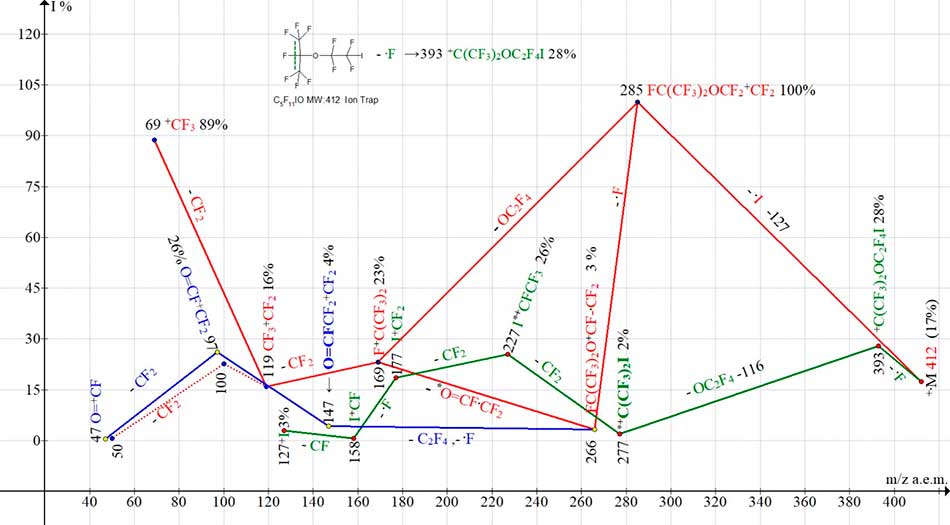
Figure 18. Three ion series of mass spectrum С5F11IO MW:412Ion Trap (Polaris Q).
Intensity of peak of molecular ion С5F11IO with m/z 412 is 17% (see Fig. 18). Using examples of mass spectra of perfluoroalkyl homologues with terminal iodine atom, it was found that when the iodine atom is detached, a base ion [M-I]+ arises if the ratio of stabilized chain mass to iodine mass is 2:1 or 3:1[4]. This rule seems to be (see Fig. 18). It is also preserved for lower homologues of perfluorooxaalkyl iodides.
Since the chain mass, stabilized by iodine atom, is 285 Da (412-127 =285) and the peak mass ratio 285:127=2.24 for ion +.M-.I with m/z =285 is the base.
In iodine-containing series (marked in green), the detachment and emission from the middle of chain of group -OC2F4 with formation of rearrangement ion I+C(CF3)2 (with m/z 277) deserves attention.
Ion series of С8F17IO2 MW:578 mass spectrum are shown in Fig. 19.
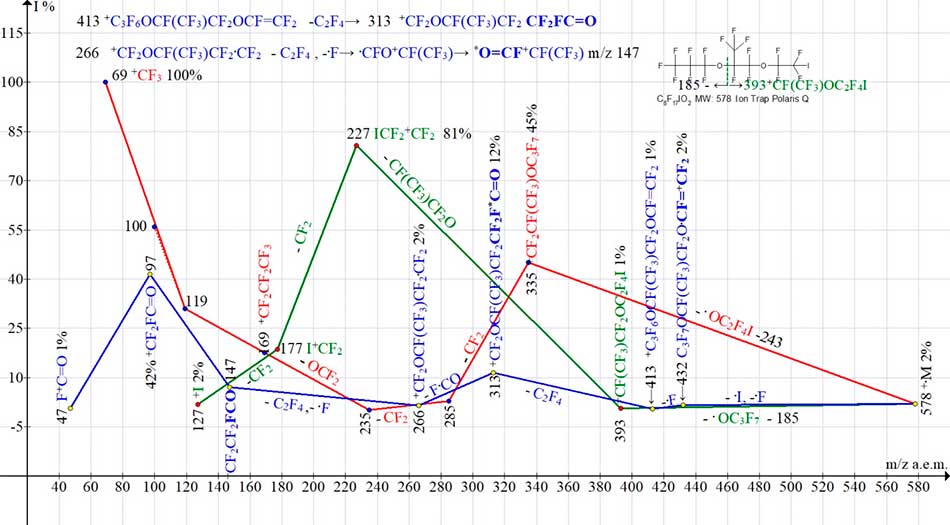
Figure 19. Three ion series of mass spectrum C8F17IO2 MW:578 Ion Trap (Polaris Q).
As a result of increase in mass of iodine-stabilized chain by 166 Da, the intensity of molecular ion peak with m/z 578 (see Fig. 19) compared with the peak intensity of molecular ion with m/z 412 (see Fig. 18) decreases from 17% to 2%. Emission of one iodine atom in spectrum does not manifest itself. Iodine is either emitted together with fluorine atom (with formation of terminal oxaperfluorovinyl group) (series marked by blue), or as part of terminal group .OC2F4I (series marked in red).
In «blue series» the rearrangements occur with formation of ions (m/z 313 and 147) with terminal fluorocarbonyl groups. When the ion with m/z 313 occurs, the oxaperfluorovinyl group of ion with m/z 413 is rearranged into a fluorocarbonyl group. Subsequent detachment of fluorocarbonyl group (ion with m/z 266), emissions of C2F4 and atom .F, again culminate in formation of ions with fluorocarbonyl groups having m/z 147, 97 and 47.
Fig. 20 shows the ion series of mass spectrum С8F17IO2 MW:578. The intensity of molecular ion peak, whose mass is 910 Da, is only 1%.
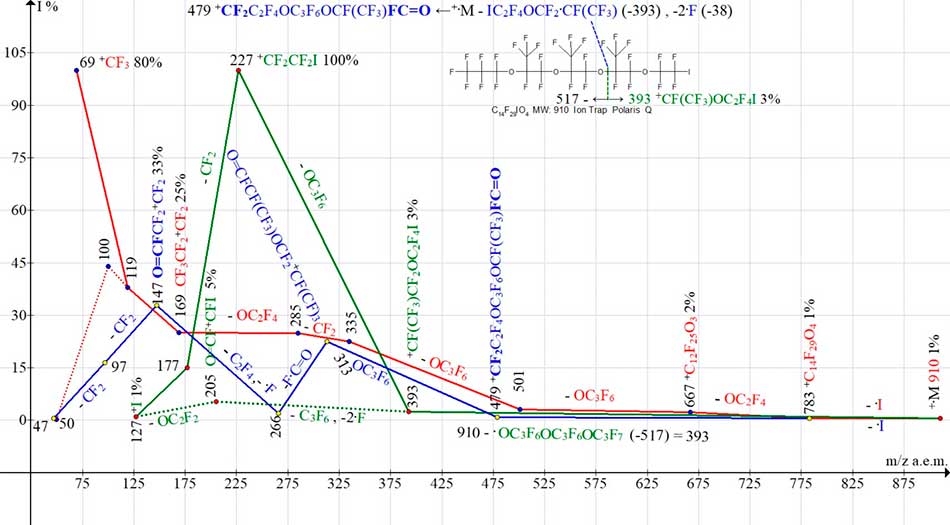
Figure 20. Three ion series of mass spectrum С14F29IO4 MW:910 Ion Trap (Polaris Q).
When the iodine atom is detached, the intensity of M-I ion peak (with m/z 783) is 1%. A decrease in intensity of M-I peak with increasing in molecular weight of M is the result of increasing in mass ratio (M -I:I) 783:127 = 6.2. M-I ion with m/z 783 either fragments with emissions of OC2F4, OC3F6 and CF2, forming a series of perfluoroalkyl ions (series marked in red), or (after emission of CF(CF3)CF(CF3)CF2OC2F4 group and detachment of two fluorine atoms) forms a rearrangement ion (m/z 479) with a terminal fluorocarbonyl group (series marked in blue). During fragmentation, the fluorocarbonyl group is lost (ion with m/z 266), and then, after emission of C2F4 and fluorine atom, the fluorocarbonyl group is re-formed (ion with m/z 147).
Chain breaks of +.M-517 (with formation of ion IC2F4OCF2+CF(CF3) with m/z 393 (series marked in green) and +.M-393 with additional emission of two fluorine atoms and formation of ion +C3F6OCF(CF3)CF2OCF(CF3)FC=O with m/z 479 (series marked in blue) occur in the same chain link (see Fig. 20). The first fragmented iodine-containing ion IC2F4OCF2+CF(CF3) with m/z 393 fragments in two ways: both with detachment of OC3F6 and formation of base peak of ICF2+CF2 ion, and with emission of C3F6 and two fluorine atoms with formation of “parallel” series of iodine-containing ions.
Conclusion
Mass spectrum of compound, presented in the form of its ion series (with corresponding peak intensities, ion structures and fragmentation sequences) allows us to solve the same problems as analysis of peaks of metastable ions. When analyzing metastable ions, a summary fragmentation scheme is usually considered, without isolating ion series and discussing their structures [5]. The series of ion fragmentation chains, presented in this report, correspond to mass spectra of compounds (with real intensities of their peaks). Consideration of primary ions and finite fragment ions compositions, as a rule, allows us to determine the number of ion series of mass spectrum. The ion series will branch if the resulting ion cannot be formed as a result of detachment of regular fragment group. When the ion is fragmented, along with regular detachment, a parallel detachment may also occur, which differs from regular one by a regular fragment group, unless this of course contradicts the composition and structure of this ion. As a result, this ion can move from one ion series to another.
Molecular ions do not appear in mass spectra of polyoxaperfluoroalkanes, regardless of their molecular weight. During fragmentation of symmetric molecules with MW 570-702 Da, the detachments of terminal radicals (with or without an oxygen atom) occurs in two central groups: O(CFCF3)2O. Fragmentation of polyoxaperfluoroalkane with two different terminal groups CF3CF2CF2 and C(CF3)3 begins with CF3CF2CF2 group and terminates with C(CF3)3 group.
In mass spectrum of symmetric polyoxaperfluoroalkane C34F70O10 (MW 1898) the first fixed peak is the peak of ion with a mass of 667. Since C34F70O10 molecule is symmetric, there is reason to believe that decay of M+. occurs with emission of neutral molecule [OCF(CF3)CF2OCF(CF3)-]2 with a mass of 564 Da from central part of molecular radical cation. The resulting symmetric radical cation [C24F50O6]+. with m/z 1334 decays into ion and radical with the same masses equal to 667Da.
In mass spectra of polyoxaperfluoroalkanes with number of oxygen atoms of 2 or more, as a rule, there are two ion series. One of them leads to fluoroalkyl ions, and the other - to ions with fluorocarbonyl group.
In mass spectrum of oxaperfluoroalkyl chloride with one oxygen atom ClC2F4O(CF2)6CF3 (see Fig. 9), the additional (third) ionic series of perfluoroallyl ions appears. After primary detachment of ClCF2CF2 radical, as well as two fluorine atoms from opposite terminal group, the ion +OC5F10CF=CF2 with m/z 347 is formed. It fragments in two ways, both with formation of series of perfluoroallyl ions (with m/z 281,231,181 and 131), and ions with fluorocarbonyl group (with 197, 147, 97 and 47). The occurrence of two series of fragmentation of ion +CF2CF2FC=O with m/z 347 is a result of its rearrangement into O=CFCF2C4F8+CF2 ion.
In mass spectra of polyoxaperfluoroalkanes and polyoxaperfluoroalkils with terminal halide atoms, due to presence of oxygen atoms in the chain, the formation of series of perfluoroallyl ions is impossible.
However, CF2=CF-+CF2 ion with m/z 131 is formed. It occurs as a result of oxygen atom detachment from ion +CF2CF2FC=O with m/z 147, or as a result of oxygen atom detachment from ion +C3F6.
In spectrum of С14F29IO4 the rearrangement of iodine-containing ion with m/z 393 leads to formation of second iodine-containing series of ions.
In spectra of polyoxaperfluoroalkyl bromidesthe primary detachments of two radicals with different masses lead to formation of two series of bromine-containing ions.
Two fragmentation pathways for +.M (C14Br2F28O4), leading to formation of two series of bromine-containing ions, are either the result of difference in excitation energies +.M, or the result of removal of two electrons from molecule that are topologically different with respect to bromine atom.
Presented examples of ionic series of polyoxaperfluoroalkanes and polyoxaperfluoroalkyl with terminal halide atoms allow us to conclude about a certain «foreignness» of oxygen atoms in perfluoroalkyl chain. During fragmentation, the oxygen atom either moves to terminal fluorocarbonyl group, or detaches as part of terminal group, or is emitted from the middle of chain (in the form of atom O, molecule O=CF2, OCF2CF2 with formation of perfluoroalkyl and haloperfluoroalkyl ions. As a result of such detachments, the fragmented ions +CnF2n+1 are formed, with a number of fluorine atoms greater than in original molecule.
When fragmenting [(CF3)2CFOC2F4I]+. (see Fig. 18) the extraordinary emission of OC2F4 with formation of rearrangement ion (CF3)2+CFI deserves attention.
In mass spectra of polyoxaperfluoroalkyl chlorides and bromides, the primary peak is M-F peak, and the peaks of molecular cation radicals do not appear. Compared with other halides, the terminal iodine atom in mass spectra of polyoxaalkyl iodides contributes to maximum stability of molecular ion, especially if the linear chain of compound is not very large. As the molecular weight increases with increasing in chain mass by more than 3 times (compared to mass of terminal iodine atom), the intensity of peaks of molecular iodide ions decreases.
Acknowledgement
This work was supported by the Ministry of Science and Higher Education of the Russian Federation and was performed employing the equipment of «Center for molecular composition studies» of INEOS RAS.
References
- Grinberg V.A., Tyutyunov A.A., Kagramanov N.D., Igumnov S.M., Mayorov N.A., Mayevskiy E.I., Sterlin S.R., The peculiarities of electrochemical behavior of ω-bromopolyoxaperfluorocarboxylic acids, Fluorine notes, 2017, 2(111), 1-2.
- Kagramanov N.D., Algorithms for fragmentation of n-alkanes and n-perfluoroalkanes, 2020, 1(128), 3-4.
- Kagramanov N.D., A Series of Fragment Ions of Cycloalkanes, Perfluorocyclohexane, Perfluoropolycycloalkanes, Fluorine notes, 2021, 3(136), 3-4.
- Kagramanov N.D., Fragmentation sequences - ion mass spectra series n-alkylhalgenides, α,ω-dihaloalkanes, α,ω-dihaloperfluoroalkanes, Fluorine notes, 2022, 1(140), 5-6.
- Gomeri, A., Beshenei, I., Kagramanov, N.D. et al, Mass spectrometric study of the vacuum pyrolysis of perfluorohexene-2 and determination of the ionization potential of the perfluoro-2-buten-4-yl radical, Russ. Chem. Bull., 1991, 40, 1098-1101.
ARTICLE INFO
Received 02 February 2023
Accepted 16 February 2023
Available online April 2023
Recommended for publication by Prof. S. M. Igumnov.
eLIBRARY Document Number (EDN) IONUHD

Fluorine Notes, 2023, 147, 1-2
