Received: January2023
DOI 10.17677/fn20714807.2023.01.03
Fluorine Notes, 2023, 146, 5-6
QUANTUM-CHEMICAL STUDY OF STRUCTURE OF FLUOROALCOHOLATE ANIONS GENERATED FROM POLYFLUORINATED ALCOHOLS
S. V. Kudashev1, V. A. Babkin2, D. S. Andreev2, A. V. Ignatov2, N. V. Kuznetsova1, V. F. Zheltobryukhov1, E. A. Matushkin1, V. N. Arisova1, A. A. Kudasheva3
1Volgograd State Technical University,400005 Volgograd, Lenin av., 28.
e-mail: kudashev-sv@yandex.ru
2 Volgograd State Technical University (Sebryakovsky br.), 403343 Volgograd Region, Mikhailovka, Michurina st., 21.
3 Volgograd State Medical University, 400131 Pavshikh Bortsov sq., 1.
Annotation. Structure of H(CF2CF2)nCH2O– (n = 1-6) anions formed from corresponding polyfluorinated alcohols was studied by DFT-PBE0/6-311g** quantum-chemical method. The optimal geometric and electronic structure of fluoroalcoholate anions has been obtained. The conformations of studied particles, which are characterized by the lowest value of total energy, are determined. The contribution of proton-donor and proton-acceptor interactions to stabilization of anion particles is shown.
Keywords: polyfluorinated alcohols, alcoholate anions, fluoropolymers, quantum chemical calculation, DFT method, structure, intramolecular interactions.
Introduction
Modifying additives based on poly- and perfluorinated compounds contribute to improvement of complex of properties of polymers. Using of polyfluorinated alcohols H(CF2CF2)nCH2OH (n = 1-6) and montmorillonite clay makes it possible to increase the thermal, fire and light resistance of resulting polymer composite materials [1-3]. Using a complex of instrumental analytical methods, it was shown [4] that immobilization of polyfluorinated alcohols on indicated layered aluminosilicate contributes to the partial displacement of polysilicic and carbonic acids from salts, as well as the reaction of metal oxides included in clay mineral base with alcohols, which leads to formation of alcoholates [H(CF2CF2)nCH2O]yMx (М = Na+ (predominantly), K+, Ca2+, Al3+ , etc.).
The presence of metal fluoroalcoholates in composition of considered organoclays ensures their participation in mechanism of opening the ε-caprolactam ring during preparation of polycaproamide, increasing the molecular weight of product. In this connection, the establishment of electronic and geometric structure of H(CF2CF2)nCH2O– anions will allow us to further evaluate their reactivity in above mechanism and optimize the compositions of organoclays (ratio of alcohol and alcoholate phases) based on polyfluorinated alcohols and montmorillonite.
The aim of this paper is a quantum-chemical study (by DFT-PBE0/6-311g** method) of structure of fluoroalcoholate anions generated from polyfluorinated alcohols.
Results and discussion
The geometric and electronic structure of H(CF2CF2)nCH2O–anions (charge = -1, multiplicity = 1) was studied by semi-empirical quantum-chemical method DFT-PBE0/6-311g** (that built into Firefly program and partially based on GAMESS source code [5-7]) in approximation of isolated particle in gas phase and with geometry optimization in all parameters by standard gradient method. Structures of studied particles optimized in all parameters were obtained (see Fig. 1-6 and Table 1-6).
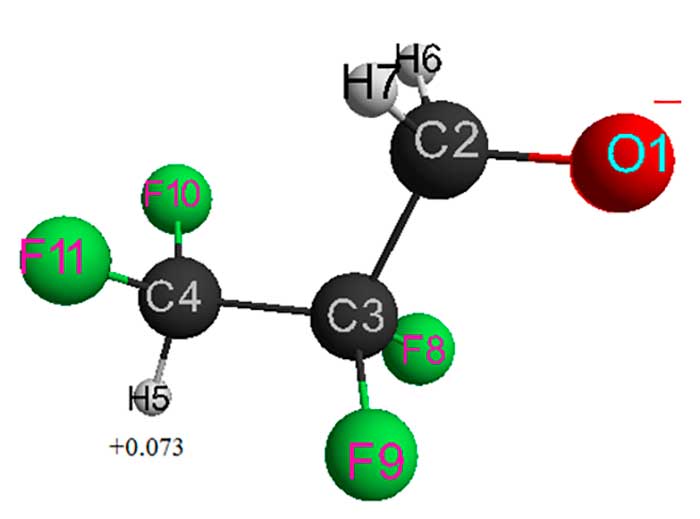
Figure 1. Geometrical and electronic structure of HCF2CF2CH2O–anion (total energy (Е0) = -1549485 kJ/mol).
Table 1 - Optimized bond lengths, bond anglesand atomic charges of HCF2CF2CH2O–anion.
|
Bond lengths |
R, Å |
Bond angles |
Deg. |
Atom |
Atomic charges |
|
C(2)-O(1) |
1,30 |
C(3)-C(2)-O(1) |
114 |
O(1) |
-0,653 |
|
C(3)-C(2) |
1,55 |
C(4)-C(3)-C(2) |
120 |
C(2) |
+0,078 |
|
C(4)-C(3) |
1,52 |
H(5)-C(4)-C(3) |
111 |
C(3) |
+0,321 |
|
H(5)-C(4) |
1,10 |
H(6)-C(2)-O(1) |
118 |
C(4) |
+0,332 |
|
H(6)-C(2) |
1,14 |
H(7)-C(2)-O(1) |
118 |
H(5) |
+0,073 |
|
H(7)-C(2) |
1,14 |
F(8)-C(3)-C(2) |
111 |
H(6) |
-0,046 |
|
F(8)-C(3) |
1,38 |
F(9)-C(3)-C(2) |
111 |
H(7) |
-0,047 |
|
F(9)-C(3) |
1,38 |
F(10)-C(4)-C(3) |
111 |
F(8) |
-0,280 |
|
F(10)-C(4) |
1.36 |
F(11)-C(4)-C(3) |
111 |
F(9) |
-0,280 |
|
F(11)-C(4) |
1,36 |
F(10) |
-0,250 |
||
|
F(11) |
-0,250 |
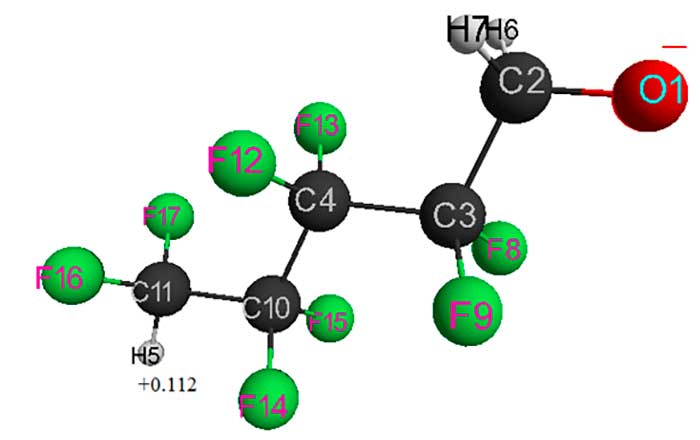
Figure 2. Geometric and electronic structure of HCF2CF2CH2O–– anion (Е0 = -2797037 kJ/mol).
Table 2. Optimized bond lengths, bond anglesand atomic charges of HCF2CF2CH2O–anion.
|
Bond lengths |
R, Å |
Bond angles |
Deg. |
Atom |
Atomic charges |
|
C(2)-O(1) |
1,30 |
C(3)-C(2)-O(1) |
113 |
O(1) |
-0,638 |
|
C(3)-C(2) |
1,56 |
C(4)-C(3)-C(2) |
117 |
C(2) |
+0,095 |
|
C(4)-C(3) |
1,54 |
H(5)-C(11)-C(10) |
108 |
C(3) |
+0,376 |
|
H(5)-C(11) |
1,10 |
H(6)-C(2)-O(1) |
118 |
C(4) |
+0,342 |
|
H(6)-C(2) |
1,14 |
H(7)-C(2)-O(1) |
118 |
H(5) |
+0,112 |
|
H(7)-C(2) |
1,14 |
F(8)-C(3)-C(2) |
111 |
H(6) |
-0,027 |
|
F(8)-C(3) |
1,37 |
F(9)-C(3)-C(2) |
111 |
H(7) |
-0,046 |
|
F(9)-C(3) |
1,36 |
C(10)-C(4)-C(3) |
117 |
F(8) |
-0,276 |
|
C(10)-C(4) |
1,55 |
C(11)-C(10)-C(4) |
117 |
F(9) |
-0,259 |
|
C(11)-C(10) |
1,54 |
F(12)-C(4)-C(3) |
109 |
C(10) |
+0,335 |
|
F(12)-C(4) |
1,36 |
F(13)-C(4)-C(3) |
110 |
C(11) |
+0,364 |
|
F(13)-C(4) |
1,36 |
F(14)-C(10)-C(4) |
111 |
F(12) |
-0,241 |
|
F(14)-C(10) |
1,35 |
F(15)-C(10)-C(4) |
109 |
F(13) |
-0,237 |
|
F(15)-C(10) |
1,36 |
F(16)-C(11)-C(10) |
112 |
F(14) |
-0,225 |
|
F(16)-C(11) |
1,35 |
F(17)-C(11)-C(10) |
111 |
F(15) |
-0,232 |
|
F(17)-C(11) |
1,35 |
F(16) |
-0,220 |
||
|
F(17) |
-0,224 |
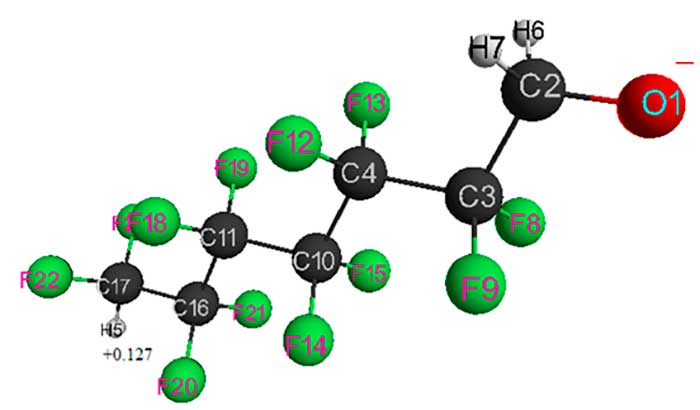
Figure 3. Geometrical and electronic structure of HCF2CF2CH2O–anion (Е0 = -4044571 kJ/mol).
Table 3. Optimized bond lengths, bond angles and atomic charges of HCF2CF2CH2O–anion.
|
Bond lengths |
R, Å |
Bond angles |
Deg. |
Atom |
Atomic charges |
|
C(2)-O(1) |
1,30 |
C(3)-C(2)-O(1) |
112 |
O(1) |
-0,633 |
|
C(3)-C(2) |
1,57 |
C(4)-C(3)-C(2) |
116 |
C(2) |
+0,097 |
|
C(4)-C(3) |
1,54 |
H(5)-C(17)-C(16) |
108 |
C(3) |
+0,382 |
|
H(5)-C(17) |
1,10 |
H(6)-C(2)-O(1) |
118 |
C(4) |
+0,360 |
|
H(6)-C(2) |
1,14 |
H(7)-C(2)-O(1) |
118 |
H(5) |
+0,127 |
|
H(7)-C(2) |
1,14 |
F(8)-C(3)-C(2) |
111 |
H(6) |
-0,024 |
|
F(8)-C(3) |
1,37 |
F(9)-C(3)-C(2) |
111 |
H(7) |
-0,044 |
|
F(9)-C(3) |
1,36 |
C(10)-C(4)-C(3) |
117 |
F(8) |
-0,274 |
|
C(10)-C(4) |
1,56 |
C(11)-C(10)-C(4) |
114 |
F(9) |
-0,257 |
|
C(11)-C(10) |
1,56 |
F(12)-C(4)-C(3) |
109 |
C(10) |
+0,396 |
|
F(12)-C(4) |
1,36 |
F(13)-C(4)-C(3) |
110 |
C(11) |
+0,375 |
|
F(13)-C(4) |
1,36 |
F(14)-C(10)-C(4) |
110 |
F(12) |
-0,243 |
|
F(14)-C(10) |
1,34 |
F(15)-C(10)-C(4) |
109 |
F(13) |
-0,235 |
|
F(15)-C(10) |
1,35 |
C(16)-C(11)-C(10) |
114 |
F(14) |
-0,214 |
|
C(16)-C(11) |
1,55 |
C(17)-C(16)-C(11) |
116 |
F(15) |
-0,227 |
|
C(17)-C(16) |
1,54 |
F(18)-C(11)-C(10) |
109 |
C(16) |
+0,333 |
|
F(18)-C(11) |
1,35 |
F(19)-C(11)-C(10) |
110 |
C(17) |
+0,376 |
|
F(19)-C(11) |
1,34 |
F(20)-C(16)-C(11) |
110 |
F(18) |
-0,215 |
|
F(20)-C(16) |
1,35 |
F(21)-C(16)-C(11) |
109 |
F(19) |
-0,215 |
|
F(21)-C(16) |
1,35 |
F(22)-C(17)-C(16) |
111 |
F(20) |
-0,219 |
|
F(22)-C(17) |
1,35 |
F(23)-C(17)-C(16) |
111 |
F(21) |
-0,219 |
|
F(23)-C(17) |
1,35 |
F(22) |
-0,211 |
||
|
F(23) |
-0,216 |
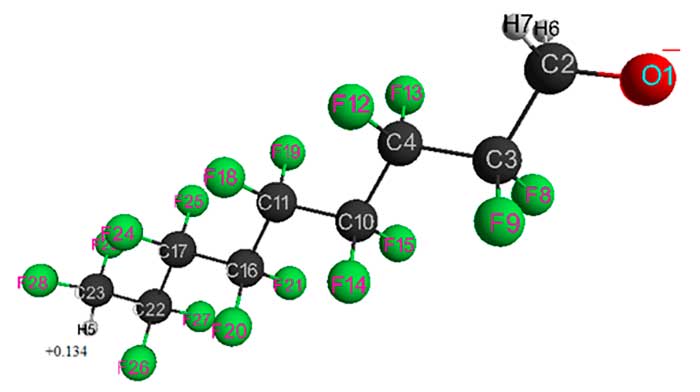
Figure4. Geometrical and electronic structure of H(CF2CF2)4CH2O–– anion (Е0 = -5292102 kJ/mol).
Table 4. Optimized bond lengths, bond anglesand atomic charges of H(CF2CF2)4CH2O–anion.
|
Bond lengths |
R, Å |
Bond angles |
Deg. |
Atom |
Atomic charges |
|
C(2)-O(1) |
1,30 |
C(3)-C(2)-O(1) |
112 |
O(1) |
-0,630 |
|
C(3)-C(2) |
1,57 |
C(4)-C(3)-C(2) |
116 |
C(2) |
+0,097 |
|
C(4)-C(3) |
1,54 |
H(5)-C(23)-C(22) |
108 |
C(3) |
+0,382 |
|
H(5)-C(23) |
1,10 |
H(6)-C(2)-O(1) |
118 |
C(4) |
+0,362 |
|
H(6)-C(2) |
1,14 |
H(7)-C(2)-O(1) |
118 |
H(5) |
+0,134 |
|
H(7)-C(2) |
1,14 |
F(8)-C(3)-C(2) |
111 |
H(6) |
-0,022 |
|
F(8)-C(3) |
1,37 |
F(9)-C(3)-C(2) |
111 |
H(7) |
-0,043 |
|
F(9)-C(3) |
1,36 |
C(10)-C(4)-C(3) |
116 |
F(8) |
-0,274 |
|
C(10)-C(4) |
1,56 |
C(11)-C(10)-C(4) |
114 |
F(9) |
-0,256 |
|
C(11)-C(10) |
1,56 |
F(12)-C(4)-C(3) |
110 |
C(10) |
+0,402 |
|
F(12)-C(4) |
1,36 |
F(13)-C(4)-C(3) |
110 |
C(11) |
+0,395 |
|
F(13)-C(4) |
1,36 |
F(14)-C(10)-C(4) |
111 |
F(12) |
-0,243 |
|
F(14)-C(10) |
1,34 |
F(15)-C(10)-C(4) |
109 |
F(13) |
-0,234 |
|
F(15)-C(10) |
1,35 |
C(16)-C(11)-C(10) |
113 |
F(14) |
-0,212 |
|
C(16)-C(11) |
1,56 |
C(17)-C(16)-C(11) |
113 |
F(15) |
-0,226 |
|
C(17)-C(16) |
1,56 |
F(18)-C(11)-C(10) |
109 |
C(16) |
+0,393 |
|
F(18)-C(11) |
1,35 |
F(19)-C(11)-C(10) |
110 |
C(17) |
+0,381 |
|
F(19)-C(11) |
1,34 |
F(20)-C(16)-C(11) |
110 |
F(18) |
-0,217 |
|
F(20)-C(16) |
1,34 |
F(21)-C(16)-C(11) |
109 |
F(19) |
-0,212 |
|
F(21)-C(16) |
1,35 |
C(22)-C(17)-C(16) |
113 |
F(20) |
-0,208 |
|
C(22)-C(17) |
1,55 |
C(23)-C(22)-C(17) |
116 |
F(21) |
-0,214 |
|
C(23)-C(22) |
1,54 |
F(24)-C(17)-C(16) |
109 |
C(22) |
+0,334 |
|
F(24)-C(17) |
1,34 |
F(25)-C(17)-C(16) |
110 |
C(23) |
+0,380 |
|
F(25)-C(17) |
1,34 |
F(26)-C(22)-C(17) |
110 |
F(24) |
-0,207 |
|
F(26)-C(22) |
1,35 |
F(27)-C(22)-C(17) |
109 |
F(25) |
-0,209 |
|
F(27)-C(22) |
1,35 |
F(28)-C(23)-C(22) |
111 |
F(26) |
-0,217 |
|
F(28)-C(23) |
1,34 |
F(29)-C(23)-C(22) |
110 |
F(27) |
-0,213 |
|
F(29)-C(23) |
1,35 |
F(28) |
-0,207 |
||
|
F(29) |
-0,213 |
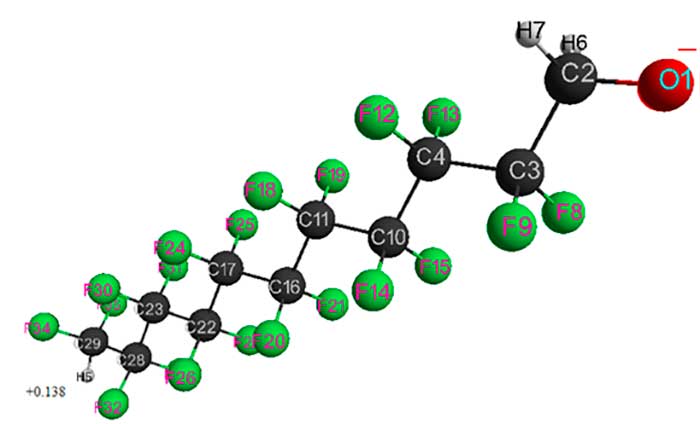
Figure 5. Geometrical and electronic structure of HCF2CF2CH2O–anion (Е0 = -6539623 kJ/mol).
Table 5. Optimized bond lengths, bond angles and atomic charges of HCF2CF2CH2O–anion.
|
Bond lengths |
R, Å |
Bond angles |
Deg. |
Atom |
Atomic charges |
|
C(2)-O(1) |
1,30 |
C(3)-C(2)-O(1) |
112 |
O(1) |
-0,629 |
|
C(3)-C(2) |
1,57 |
C(4)-C(3)-C(2) |
116 |
C(2) |
+0,097 |
|
C(4)-C(3) |
1,54 |
H(5)-C(29)-C(28) |
108 |
C(3) |
+0,381 |
|
H(5)-C(29) |
1,10 |
H(6)-C(2)-O(1) |
118 |
C(4) |
+0,361 |
|
H(6)-C(2) |
1,14 |
H(7)-C(2)-O(1) |
118 |
H(5) |
+0,138 |
|
H(7)-C(2) |
1,14 |
F(8)-C(3)-C(2) |
111 |
H(6) |
-0,022 |
|
F(8)-C(3) |
1,37 |
F(9)-C(3)-C(2) |
111 |
H(7) |
-0,042 |
|
F(9)-C(3) |
1,36 |
C(10)-C(4)-C(3) |
117 |
F(8) |
-0,273 |
|
C(10)-C(4) |
1,56 |
C(11)-C(10)-C(4) |
114 |
F(9) |
-0,256 |
|
C(11)-C(10) |
1,56 |
F(12)-C(4)-C(3) |
109 |
C(10) |
+0,405 |
|
F(12)-C(4) |
1,36 |
F(13)-C(4)-C(3) |
110 |
C(11) |
+0,402 |
|
F(13)-C(4) |
1,36 |
F(14)-C(10)-C(4) |
111 |
F(12) |
-0,243 |
|
F(14)-C(10) |
1,34 |
F(15)-C(10)-C(4) |
109 |
F(13) |
-0,234 |
|
F(15)-C(10) |
1,35 |
C(16)-C(11)-C(10) |
113 |
F(14) |
-0,213 |
|
C(16)-C(11) |
1,56 |
C(17)-C(16)-C(11) |
114 |
F(15) |
-0,225 |
|
C(17)-C(16) |
1,56 |
F(18)-C(11)-C(10) |
109 |
C(16) |
+0,400 |
|
F(18)-C(11) |
1,34 |
F(19)-C(11)-C(10) |
110 |
C(17) |
+0,388 |
|
F(19)-C(11) |
1,34 |
F(20)-C(16)-C(11) |
110 |
F(18) |
-0,215 |
|
F(20)-C(16) |
1,34 |
F(21)-C(16)-C(11) |
108 |
F(19) |
-0,214 |
|
F(21)-C(16) |
1,34 |
C(22)-C(17)-C(16) |
115 |
F(20) |
-0,219 |
|
C(22)-C(17) |
1,56 |
C(23)-C(22)-C(17) |
113 |
F(21) |
-0,206 |
|
C(23)-C(22) |
1,56 |
F(24)-C(17)-C(16) |
109 |
C(22) |
+0,405 |
|
F(24)-C(17) |
1,34 |
F(25)-C(17)-C(16) |
109 |
C(23) |
+0,383 |
|
F(25)-C(17) |
1,35 |
F(26)-C(22)-C(17) |
108 |
F(24) |
-0,200 |
|
F(26)-C(22) |
1,34 |
F(27)-C(22)-C(17) |
111 |
F(25) |
-0,219 |
|
F(27)-C(22) |
1,34 |
C(28)-C(23)-C(22) |
113 |
F(26) |
-0,214 |
|
C(28)-C(23) |
1,55 |
C(29)-C(28)-C(23) |
115 |
F(27) |
-0,202 |
|
C(29)-C(28) |
1,54 |
F(30)-C(23)-C(22) |
110 |
C(28) |
+0,335 |
|
F(30)-C(23) |
1,34 |
F(31)-C(23)-C(22) |
108 |
C(29) |
+0,382 |
|
F(31)-C(23) |
1,34 |
F(32)-C(28)-C(23) |
109 |
F(30) |
-0,205 |
|
F(32)-C(28) |
1,35 |
F(33)-C(28)-C(23) |
110 |
F(31) |
-0,205 |
|
F(33)-C(28) |
1,35 |
F(34)-C(29)-C(28) |
110 |
F(32) |
-0,211 |
|
F(34)-C(29) |
1,35 |
F(35)-C(29)-C(28) |
111 |
F(33) |
-0,215 |
|
F(35)-C(29) |
1,34 |
F(34) |
-0,211 |
||
|
F(35) |
-0,206 |
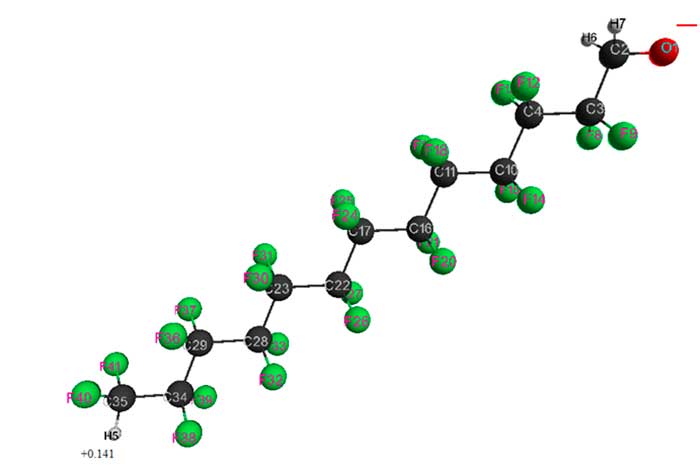
Figure 6. Geometrical and electronic structure of HCF2CF2CH2O– anion (Е0 = -7787149 kJ/mol).
Table 6. Optimized bond lengths, bond angles and atomic charges of HCF2CF2CH2O–anion.
|
Bond lengths |
R, Å |
Bond angles |
Deg. |
Atom |
Atomic charges |
|
C(2)-O(1) |
1,30 |
C(3)-C(2)-O(1) |
112 |
O(1) |
-0,628 |
|
C(3)-C(2) |
1,57 |
C(4)-C(3)-C(2) |
116 |
C(2) |
+0,097 |
|
C(4)-C(3) |
1,54 |
H(5)-C(35)-C(34) |
108 |
C(3) |
+0,381 |
|
H(5)-C(35) |
1,10 |
H(6)-C(2)-O(1) |
118 |
C(4) |
+0,361 |
|
H(6)-C(2) |
1,14 |
H(7)-C(2)-O(1) |
118 |
H(5) |
+0,141 |
|
H(7)-C(2) |
1,14 |
F(8)-C(3)-C(2) |
111 |
H(6) |
-0,021 |
|
F(8)-C(3) |
1,37 |
F(9)-C(3)-C(2) |
111 |
H(7) |
-0,042 |
|
F(9)-C(3) |
1,36 |
C(10)-C(4)-C(3) |
117 |
F(8) |
-0,273 |
|
C(10)-C(4) |
1,56 |
C(11)-C(10)-C(4) |
114 |
F(9) |
-0,256 |
|
C(11)-C(10) |
1,56 |
F(12)-C(4)-C(3) |
109 |
C(10) |
+0,405 |
|
F(12)-C(4) |
1,36 |
F(13)-C(4)-C(3) |
110 |
C(11) |
+0,403 |
|
F(13)-C(4) |
1,36 |
F(14)-C(10)-C(4) |
111 |
F(12) |
-0,243 |
|
F(14)-C(10) |
1,34 |
F(15)-C(10)-C(4) |
109 |
F(13) |
-0,234 |
|
F(15)-C(10) |
1,35 |
C(16)-C(11)-C(10) |
113 |
F(14) |
-0,213 |
|
C(16)-C(11) |
1,56 |
C(17)-C(16)-C(11) |
114 |
F(15) |
-0,225 |
|
C(17)-C(16) |
1,56 |
F(18)-C(11)-C(10) |
109 |
C(16) |
+0,397 |
|
F(18)-C(11) |
1,34 |
F(19)-C(11)-C(10) |
110 |
C(17) |
+0,391 |
|
F(19)-C(11) |
1,34 |
F(20)-C(16)-C(11) |
110 |
F(18) |
-0,214 |
|
F(20)-C(16) |
1,34 |
F(21)-C(16)-C(11) |
108 |
F(19) |
-0,215 |
|
F(21)-C(16) |
1,34 |
C(22)-C(17)-C(16) |
115 |
F(20) |
-0,219 |
|
C(22)-C(17) |
1,56 |
C(23)-C(22)-C(17) |
113 |
F(21) |
-0,204 |
|
C(23)-C(22) |
1,56 |
F(24)-C(17)-C(16) |
109 |
C(22) |
+0,412 |
|
F(24)-C(17) |
1,34 |
F(25)-C(17)-C(16) |
109 |
C(23) |
+0,401 |
|
F(25)-C(17) |
1,35 |
F(26)-C(22)-C(17) |
108 |
F(24) |
-0,199 |
|
F(26)-C(22) |
1,34 |
F(27)-C(22)-C(17) |
111 |
F(25) |
-0,220 |
|
F(27)-C(22) |
1,34 |
C(28)-C(23)-C(22) |
113 |
F(26) |
-0,212 |
|
C(28)-C(23) |
1,56 |
C(29)-C(28)-C(23) |
113 |
F(27) |
-0,201 |
|
C(29)-C(28) |
1,56 |
F(30)-C(23)-C(22) |
110 |
C(28) |
+0,396 |
|
F(30)-C(23) |
1,34 |
F(31)-C(23)-C(22) |
108 |
C(29) |
+0,382 |
|
F(31)-C(23) |
1,34 |
F(32)-C(28)-C(23) |
108 |
F(30) |
-0,204 |
|
F(32)-C(28) |
1,34 |
F(33)-C(28)-C(23) |
110 |
F(31) |
-0,207 |
|
F(33)-C(28) |
1,34 |
C(34)-C(29)-C(28) |
113 |
F(32) |
-0,207 |
|
C(34)-C(29) |
1,55 |
C(35)-C(34)-C(29) |
115 |
F(33) |
-0,204 |
|
C(35)-C(34) |
1,54 |
F(36)-C(29)-C(28) |
109 |
C(34) |
+0,336 |
|
F(36)-C(29) |
1,34 |
F(37)-C(29)-C(28) |
109 |
C(35) |
+0,384 |
|
F(37)-C(29) |
1,34 |
F(38)-C(34)-C(29) |
109 |
F(36) |
-0,206 |
|
F(38)-C(34) |
1,35 |
F(39)-C(34)-C(29) |
110 |
F(37) |
-0,202 |
|
F(39)-C(34) |
1,35 |
F(40)-C(35)-C(34) |
110 |
F(38) |
-0,209 |
|
F(40)-C(35) |
1,35 |
F(41)-C(35)-C(34) |
111 |
F(39) |
-0,215 |
|
F(41)-C(35) |
1,34 |
F(40) |
-0,210 |
||
|
F(41) |
-0,204 |
As can be seen, the chemical structure of HCF2CF2CH2O–anions have a decisive influence on their electronic and geometric structure. An increase in number of -CF2CF2- groups leads to a change in total energy of anions (decreases from 1549485 kJ mol 1 to 7787149 kJ mol-1 when passing from n = 1 to n = 6), in negative oxygen atomic charges (increases from -0.653 to -0.628) and in positive hydrogen atomic charges of HCF2 group (increases from 0.073 to 0.141). Stabilization of fluoroalcoholate anions is promoted by proton-donor and proton-acceptor interactions CF2H+δ···–O–CH2 and CH2···F2C, as well as interactions CF2H···●O–CH2 and CH2···F2C for radical species H(CF2CF2)nCH2O● described in [4, 8, 9].
Conclusion
Using DFT-PBE0/6-311g** method, the electronic and geometric structure of HCF2CF2CH2O–– fluoroalcoholate anions was calculated, and optimized bond lengths, bond angles and atomic charges were determined. An increase in number of -CF2CF2-groups, as well as the proton-donor and proton-acceptor interactions between atoms in anion together ensure the stabilization of its structure.
References
- Kudashev S. V., Methods for introducing poly- and perfluorinated fragments into macromolecular systems (Review), Fluorine notes, 2020, 3(130), 3-4.
- Kudashev S. V., Daniel Z. M., Dauda F., Arisova V. N., Bogdanov A. I., Zheltobryukhov V. F., Fofonova A. A., Matushkin E. A., Preparation of organoclay based on 1Н,1Н,13Н-trihydroperfluorotridecan-1-ol and montmorillonite, Fluorine notes, 2022, 3(142), 1-2.
- Kudashev S. V., Daniel Z. M., Dauda F., Arisova V. N., Bogdanov A. I., Zheltobryukhov V. F., Fofonova A. A., Matushkin E. A., X-ray study of montmorillonite modified with tetrafluorophthalic acid and its anhydride, Fluorine notes, 2022, 2(141), 1-2.
- KudashevS. V., Modification of a number of heterochain polymers with compositions based on polyfluorinated alcohols and montmorillonite, Abstract of PhD thesis (in candidacy for a degree of PhD in Chemistry by specialty 02.00.06, VolgGTU, Volgograd, 2020, 48 p. (in Russian)
- Granovsky A. A., Firefly version 8, 2013, Access mode: http://classic.chem.msu.su/gran/firefly/index.html.
- Schmidt M. W., Baldridge K. K., Boatz J. A., Elbert S. T., Gordon M. S., Jensen J. H., Koseki S., Matsunaga N., Nguyen K. A., Su S. J., Windus T. L, Dupuis M., Montgomery J. A., General atomic and molecular electronic structure system, Journal of Computational Chemistry, 1993, 14, 1347-1363.
- Bode B. M., Gordon M. S., MacMolPlt: A graphical user interface for GAMESS, Journal of Molecular Graphics and Modeling, 1998, 16(3), 133-138.
- Kudashev S. V., Babkin V. A., Andreev D. S., Ignatov A. V., Kuznetsova N. V., Kosogorina M. D., Structure of paramagnetic centers generated from polyfluorinated alcohols, Fluorine Notes, 2020, 6(133), 1-2.
- Kudashev S. V., Kondrasenko A. A., Matsulev A. N., Babkin V. A., Belousova V. S. Andreev., D. S, Zheltobryukhov V. F., Kuznetsova N. V., Modification of polycaproamide with a composition based on 1H,1H,13H-trihydroperfluorotridecan-1-ol and montmorillonite, Chemical fibers, 2021, 5, 3-7 (in Russian).
ARTICLE INFO
Received 30 January 2023
Accepted 13 February 2023
Available online February 2023
Recommended for publication by PhD O.V. Bryzgalova
eLIBRARY Document Number (EDN) WROKYV

Fluorine Notes, 2023, 146, 5-6
