Received: November 2022
DOI 10.17677/fn20714807.2023.01.01
Fluorine Notes, 2023, 146, 1-2
COPOLYMERS OF PERFLUORO-2,2-DIMETHYL-1,3-DIOXOL AND PERFLUORONYLVINYL ETHER FOR PRODUCING OF LIGHT-GUIDING COATINGS
V.I. Sokolov1, O.Yu. Gordeeva1, I.O. Goryachuk1, S.I. Molchanova1, E.V. Polunin2
1Federal Scientific Research Center "Crystallography and Photonics" RAS,119333, Leninsky ave. 59, Moscow, Russia
2N. D. Zelinsky Institute of Organic Chemistry Russian Academy of Sciences, 119991, Leninsky ave. 47, Moscow, Russia
Annotation: Amorphous copolymers of perfluoro-2,2-dimethyl-1,3-dioxol and perfluoronylvinyl ether with different molar content of ether units x in the macromolecule have been synthesized by ultrahigh pressure method. These copolymers, that have high optical transparency in visible and near-IR spectral regions and also - low refractive index n = 1.305-1.325 (depending on x), are capable of film formation and so can be used as light-guiding coatings for various applications in integrated optics and photonics.
Keywords: perfluoro-2,2-dimethyl-1,3-dioxol, perfluoronyl vinyl ether, amorphous perfluorinated polymers, ultrahigh pressure polymerization, light-guiding polymer coatings, highly transparent polymeric materials.
Introduction
Amorphous perfluorinated polymers have a high optical transparency, low refractive index n, low material dispersion dn/dλ in "telecommunication" wavelength ranges near λ= 0.85, 1.3 and 1.55 µm, and therefore are promising for creating various waveguide elements of integrated optical devices [1-4]. In this case, the complete replacement of hydrogen atoms by fluorine atoms leads to a low absorption coefficient and a low refractive index in a polymer, and amorphism leads to low light scattering. Additionally, these perfluorinated polymers are hydrophobic, have high chemical resistance and are resistant to effect of environment conditions (primarily – to temperature and humidity).
The range of commercially available amorphous perfluoropolymers is currently very limited. To the best of our knowledge, such polymers are commercially produced only by DuPont (TeflonAF 1600 and TeflonAF 2400 perfluoropolymers), Asachi Glass (Cytop), Solvay (Hyflon AD) and Chromis Technologies (CyclAFlor) [5, 6]. Because of this, the synthesis of new amorphous perfluorinated polymeric materials with improved set of useful properties is the urgent task.
Amorphous perfluoropolymers are obtained, as a rule, by radical homo- or copolymerization of two or more monomers in solutions or in emulsions. At the same time, many perfluorinated monomers (for example, perfluorostyrene [7] and perfluoroisopropyl vinyl ether [8]) enter into reaction of radical polymerization with great difficulty.
Because of this, the copolymerization of these monomers is often carried out at elevated pressure (10-20 ths. atm.) and at temperature of 100-160°C [7–10]. This paper reports on synthesis of copolymers of perfluoro-2,2-dimethyl-1,3-dioxol and perfluoronylvinyl ether by ultrahigh pressure method and analyzes their optical properties from the point of view of suitability for creating of light-guiding coatings in various photonic devices.
Synthesis of amorphous perfluorinated copolymers at ultrahigh pressure
To obtain copolymers, the monomers perfluoro-2,2-dimethyl-1,3-dioxol D1 and perfluoronylvinyl ether E6 produced by "P&M-Invest" were used, which are transparent colorless liquids (see Fig. 1). Prior to synthesis, D1 monomer was purified from stabilizer by distillation. Synthesis of copolymers was carried out in Teflon ampoules with a volume of 2–3 ml in press molds of cylinder–piston type at a pressure of 14-15 ths. atm. and temperature 130-150°C for 72-96 h. In this case, no radical polymerization initiators were used. The product obtained after opening the ampoule was, as a rule, a highly viscous uncolored substance containing, in addition to the linear copolymer, highly volatile components (unreacted monomers, dimers, etc.). To remove these components, these copolymers were evacuated to constant weight at 140°C.
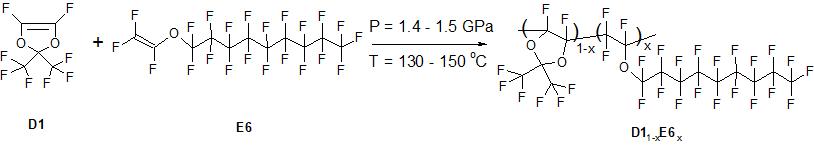
Figure 1. Synthesis scheme of copolymers of perfluoro-2,2-dimethyl-1,3-dioxol D1 and perfluoronylvinyl ether E6 by radical polymerization at ultrahigh pressure (without using of initiators), where x is molar concentration of ether units in D11-xE6x copolymer macromolecule.
Optimization of synthesis time at ultrahigh pressure is important for obtaining copolymers with desired characteristics. With a short reaction time, the resulting linear copolymer has a low molecular weight, while the yield of final product is also low. With a long duration of synthesis, the probability of obtaining a partially cross-linked polymer is high. The crosslinking may be due to opening of dioxolane ring in D1 units, or due to dissociation of aliphatic radicals Rf = -(CF2)8-CF3 in the E6 ether units embedded in the polymer chain. The conducted studies have shown that the optimal time for synthesis of amorphous perfluorinated copolymers Rf = -(CF2)8-CF3 at a rate temperature 130-150°С and pressure 14-15 ths. atm. (without using of initiators) is 72-96 h. In this case, the yield of linear polymer lies in the range of 70–80%.
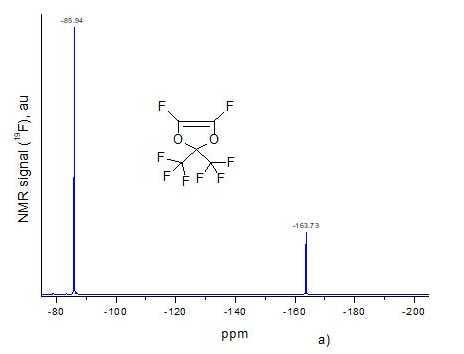
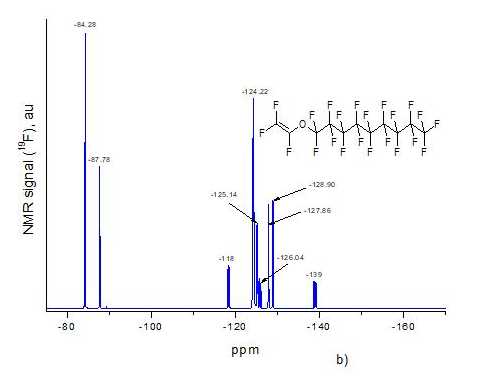
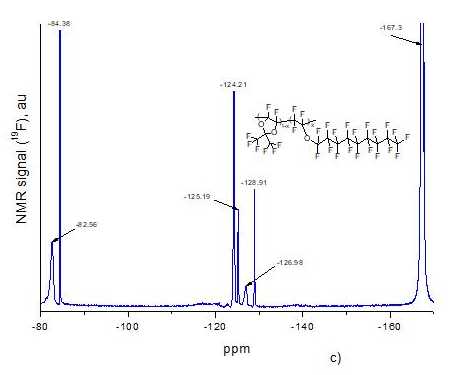
Using the ultrahigh pressure method, we synthesized D11-xE6x copolymers with different molar content x of ether units in macromolecule. This content was determined using 19F NMR spectra of resulting copolymers. Fig. 2a and Fig. 2b shows NMR spectra of monomers D1 and E6, respectively, and Fig. 2c –spectrum of D11-xE6x copolymer with x = 0.5. All spectra were obtained via Bruker AM-300 spectrometer (282.40 MHz), and spectrum of copolymer was measured in hexafluorobenzene.
|
|
|
Figure 2. 19F NMR spectra of monomer D1 (a), monomer E6 (b) and copolymer D11-xE6x with x = 0.5 (c), measured via Bruker AM-300 spectrometer (282.40 MHz).
Signal at -85.94 ppm in Fig. 2a corresponds to six fluorine atoms in two trifluoromethyl groups CF3 of monomer D1, and signal at -163.73 ppm – two F atoms at double C=C bond in this monomer. Signal at -84.28 ppm in Fig. 2b corresponds to trifluoromethyl group CF3 of monomer E6, and signal at -87.78 ppm - CF2O group. Signal at -118 ppm refers to =CFO group, signal at -124.22 ppm - to four CF2 groups in the middle of aliphatic chain, and signals at -125.14, -127.86 and -128.90 ppm – to other three CF2 groups in this chain. The multiplets near -126 ppm and -139 ppm refer to terminal F atoms at C=C double bond in E6 monomer.
Let us analyze 19F NMR spectrum of D11-xE6x copolymer with x = 0.5 (see Fig. 2c). First of all, we note that signal at -167.3 ppm in this figure is associated with solvent (hexafluorobenzene) in which this copolymer was dissolved. Signal at -82.56 ppm corresponds to two methyl groups of CF3 ring in dioxolane D1 units in copolymer macromolecule, and signal at -84.38 ppm - to terminal CF3 group of E6 alkyl ether (this signal is present in the spectrum of E6 monomer, see Fig. 2b). Signal at -124.21 ppm refers to four CF2 groups in the middle of alkyl chain (available in the spectrum of E6 monomer, see Fig. 2b), the signals at -125.19 ppm, -126.98 ppm. and -128.91 ppm – to other CF2 groups (these signals are also present in the spectrum of E6 monomer). From analysis of Fig. 2c, we can conclude that the ratio of dioxolane and ether units in this copolymer is D1:E6 1:1 (x = 0.5). Note that D1 monomer is more active in the process of radical polymerization than E6 monomer.
Because of this, the molar concentration of perfluoronylvinyl ether units in copolymer may be lower than in initial mixture. In this case, a strong difference in polymerization activity of D1 and E1 monomers can lead to formation of partially block copolymers.
To estimate the molecular weight of obtained copolymers, the average hydrodynamic diameter of macromolecular globules in perfluorooctane was measured. The measurements were performed by dynamic light scattering method via 90Plus_Zeta instrument (Brookhaven Instruments Corp., USA) under illumination by laser beam with a wavelength of 640 nm. Fig. 3 shows a typical histogram of globule size distribution. It can be seen that average diameter of globules is <D> = 25 nm. Thus, D11-xE6x copolymers can be attributed to the class of macromolecular substances.
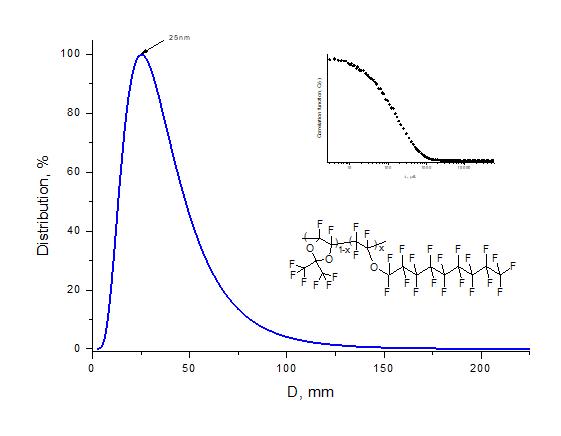
Figure 3. Size distribution of macromolecular globules of D11-xE6x copolymer with x = 0.5 measured by dynamic light scattering method in perfluorooctane, where D is globule diameter.
The insets show the form of autocorrelation function and fragment of copolymer structure.
The synthesized copolymers do not dissolve in acetone, hexane, octane, benzene, toluene, but dissolve at room temperature in perfluorinated solvents such as perfluorohexane, perfluorooctane, hexafluorobenzene, perfluoro-(1,3-dimethyl)-cyclohexane (carbogal), perfluorodecalin.
Structural diagnostics of perfluorinated copolymers
When synthesizing of perfluorinated copolymers of dioxol D1 and ether E6, there are several factors that lead to disordering of macromolecular chain. First, D1 and E6 monomers can bind to each other in different sequences. Secondly, the articulation of E6 monomer units with each other can be of the "head-to-tail" or "head-to-head" type. For these reasons, the resulting copolymer should exhibit the properties of amorphous substance.
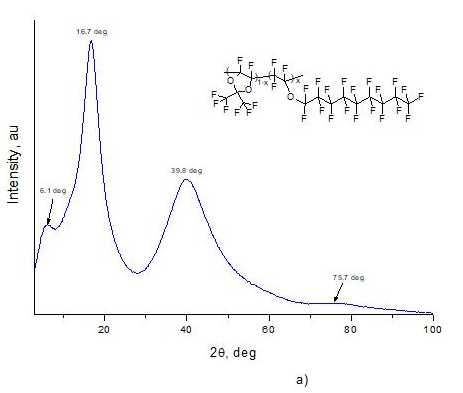
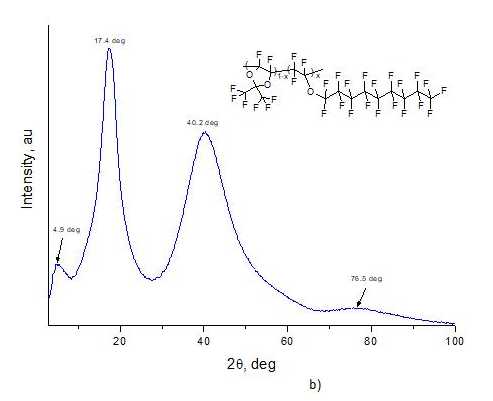
Structure of copolymers was studied via Rigaku Miniflex600 wide-angle X-ray diffractometer (Cu, λ= 1.54184 Å). Fig. 4a and Fig. 4b shows X-ray diffraction patterns of films made from D11-xE6x copolymers with x = 0.5 and x = 0.9, respectively. As can be seen from these figures, there are no sharp peaks in diffraction patterns and only a few wide "halos" are observed, which indicates the amorphism of these materials. Note that angular positions of these «halo» maxima for copolymers with x = 0.5 (2θ = 6.1, 16.7, 39.8, 75.7 deg., see Fig. 4a) and x = 0.9 (2θ= 4.9, 17.4, 40.2, 76.5 deg., see Fig. 4b) are close. The diffraction patterns of D11-xE6x copolymers with other values of molar concentration x of ether units in macromolecule had a similar form.
|
|
Figure 4. X-ray diffraction patterns of D11-xE6x copolymers with (a) x = 0.5 and (b) x = 0.9 obtained via Rigaku Miniflex600 wide-angle X-ray diffractometer.
Investigation of IR absorption spectra of perfluorinated copolymers
Investigation of transparency of D11-xE6x copolymers in IR spectral region was carried out via Shimadzu8400s IR-Fourier spectrometer. To do this, films of these copolymers were formed onto transparent KBr plates. Solutions of copolymers in perfluorooctane were used, which were deposited onto substrate by centrifugation and then dried at 60°C to completely evaporate the solvent. The transmission spectra of clean KBr plate and polymer-coated substrate were measured. The absorption spectrum of polymeric material was obtained as a logarithm of their ratio and is shown in Fig. 5.
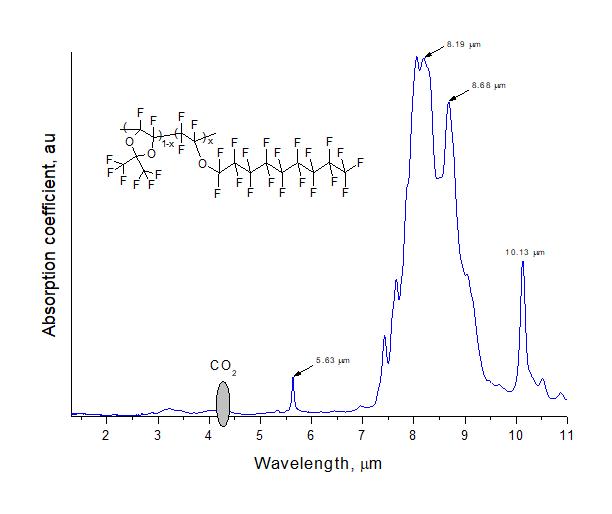
Figure 5. IR absorption spectrum of amorphous perfluoropolymer D11-xE6x with x = 0.5.
Fig. 5 shows that intense absorption bands associated with stretching vibrations in CF, CF2 and CF3 groups of the copolymer lie in the range of 7–11 μm. In telecommunication C-wavelength range (1530–1565 nm) the considered copolymer has a high degree of transparency.
Measurement of refractive index of perfluorinated copolymers
To measure the refractive index of synthesized copolymers at He-Ne laser wavelength (λ= 632.8 nm) Metricon2010/M prism contact device (Metricon corp., USA) was used. By centrifugation via SPIN1200-T instrument (MIDAS Inc., South Korea) light-guiding films 1-10 µm thick were fabricated at glass substrates. The value of refractive index was determined from angular position of m-lines in dependences of intensity of reflected beam on the angle of incidence. The measurements were carried out at TE and TM polarizations of laser beam, which made it possible to determine the refractive indices nTE и nTM in directions along and across the film, respectively. It was found that the values of nTE и nTM differ little from each other, i.e. these polymer films are isotropic. The dependence of average refractive index of these films n = (nTE + nTM)/2 on molar concentration x of E6 perfluorinated ether units in macromolecule is shown in Fig. 6. As follows from this figure, the refractive index of D11-xE6x copolymers lies in the range n = 1.305-1.325 and decreases with decreasing x. Note also that copolymers with small values x are glassy, while those with large values x are rubbery. This indicates a decrease in glass transition temperature of copolymers with increasing x.
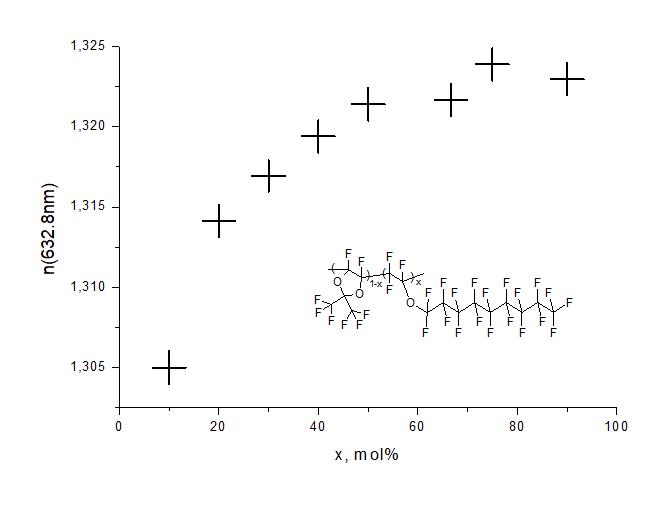
Figure 6. Dependence of average refractive index n = (nTE + nTM)/2 of D11-xE6x copolymers at a wavelength of 632.8 nm on molar concentration x of ether units in macromolecule.
The question of what is the minimum refractive index n of organic materials has long attracted the attention of researchers. This is due to ever-increasing requirements to reduce the attenuation of light signals during their propagation through polymer waveguide or fiber and the need to increase the speed of information flow transmission. Decrease in n leads to decrease in attenuation coefficient due to light scattering on optical inhomogeneities n of the material, as well as to decrease in its material dispersion dn/d, which determines the degree of spreading of light pulses during their propagation along waveguide and thereby limits the data transfer rate. Previously, on the basis of theoretical estimates, it was predicted that amorphous polymers in which all hydrogen atoms are completely replaced by fluorine atoms have the minimum refractive index n 1.29 ± 0.005 [2]. Among polymers of this type currently known, the lowest refractive index n 1.295 is, as far as we know, Teflon AF2400 developed by Du Pont company. It is a copolymer of perfluoro-2,2-dimethyl-1,3-dioxol and tetrafluoroethylene with molar concentration of latter units in macromolecule x = 13 mol% [11].
A further decrease in refractive index and material dispersion of amorphous perfluoropolymers can apparently be achieved by optimization their chemical structure, as well as by increasing in free volume associated with the presence in these polymers the pores system of with a size much smaller than wavelength of optical radiation.
The latter leads to decrease in material density and, as a consequence, to decrease in n. It is believed that increase in free volume can be achieved, for example, by introducing bulky side groups attached directly (or through spacers) to main chains and hindering the close packing of these chains [12, 13]. However, our results do not support this concept. Thus, the introduction of perfluorocyclopentylethyl [14] or perfluorononyl ether units into copolymer composition even leads to some increase in refractive index (see Fig. 6). Apparently, the theoretical concepts of relationship between refractive index of perfluoropolymers and their structure and free volume need to be refined.
Conclusion
Amorphous copolymers of perfluoro-2,2-dimethyl-1,3-dioxol and perfluoronylvinyl ether have been synthesized by ultrahigh pressure method. These copolymers, that have a high optical transparency at the telecommunication wavelength ranges near 0.85, 1.3 and 1.5 µm and low refractive index n = 1.305-1.325, are capable of film formation and are promising for creation of various waveguide elements for integrated optical devices. This expands the class of amorphous perfluorinated polymers for various applications in photonics. The synthesized copolymers can be used, for example, as a light-guiding coatings and cladding for waveguide amplifiers in telecommunications C-wavelength range (1530–1565 nm) based on polymers with embedded erbium-doped fluoride nanocrystals, as well as for creation of optical fiber cladding.
Acknowledgment
This work was financially supported by the Ministry of Science and Higher Education of the Russian Federation within the framework of the state task of the Federal Research Center "Crystallography and Photonics" of the Russian Academy of Sciences. The equipment of the Center for Collective Use of the Federal Research Center «Crystallography and Photonics» of the Russian Academy of Sciences was used in the work.
.
References
- W. Groh, Overtone absorption in macromolecules for polymer optical fibers, Makromol. Chem., 1988, 189, 2861-2874.
- W. Groh, A. Zimmermann, What is the lowest refractive index of organic polymers? Macromolecules, 1991, 24, 6660-6663.
- M. Zhou, Low-loss polymeric materials for passive waveguide components in fiber optical telecommunication, Opt. Eng., 2002, 41(7), 1631-1643.
- A. Vitale, R. Bongiovanni, B. Ameduri, Fluorinated Oligomers and Polymers in Photopolymerization, Chemical Reviews, 2015, 115(16), 8835-8866,
- B. Ameduri, The Promising Future of Fluoropolymers. Macromolecular Chemistry and Physics, Wiley-VCH Verlag, 2020, 221(8), 1900573.
- M.A. El-Okazy, L. Liu, C.P. Junk, E. Kathmann, W. White, S.E. Kentish. Gas separation performance of copolymers of perfluoro(butenyl vinyl ether) and perfluoro(2,2-dimethyl-1,3-dioxole), Journal of Membrane Science, 2021, 634, 119401.
- L.A. Wall, D.W. Brown, High pressure polymerization of perfluorostyrene, Journal of Fluorine Chemistry, 1972, 2(1), 73-85.
- Polunin E.V., Molchanova S.I., Pogodina J. E., Sokolov V.I., Zavarzin I. V., Homo- and co-polymerisation of perfluoroisopropylvinyl ether under high pressure, Fluorine Notes, 2017, 5(114), 5-6.
- Sokolov V.I., Boyko V.E., Goryachuk I.O., Igumnov S.M., Molchanova S.I., Pogodina Yu.E., Polunin E.V., Synthesis and optical properties of copolymers of perfluoro-2,2-dimethyl-1,3-dioxole and perfluoropropyl vinyl ether, Russian Chemical Bulletin, International Edition, 2017, 7, 1284-1289.
- Sokolov V.I., Goriachuk I.O., Zavarzin I.V., Molchanova S.I., Pogodina Yu.E., Polunin E.V., Yarosch A.A., New copolymers of perfluoro-2-ethyl-2-methyl-1,3-dioxole and perfl uorovinyl ether with low non-monotonic refractive index, Russian Chemical Bulletin, International Edition, 2019, 68(3), 569-564.
- M.K. Yang, R.H. French, E.W. Tokarsky, Optical properties of Teflon® AF amorphous fluoropolymers, Journal of Micro/Nanolithography, MEMS, and MOEMS, 2008, 7(3), 033010.
- Tokarev A.V., Bondarenko G.N., Yampolskii Yu.P., Chain structure and stiffness of Teflon AF glassy amorphous fluoropolymers, Polym. Sci., Ser. A, 2007, 49(8), 1510-1523.
- Yampolskii Yu.P., Amorphous perfluorinated membrane materials: Structure, properties and application, Russian Journal of General Chemistry, 2009, 79(3), pp. 657-665.
- Sokolov V. I., Goryachuk I. O., Molchanova S. I., Polunin E. V., Investigation of optical properties of amorphous copolymers of perfluoro-2,2-dimethyl-1,3-dioxol and perfluoro-(2-cyclopentyl) ethylvinyl ether obtained at ultrahigh pressure, Fluorine Notes, Fluorine Notes, 2022, 5(144), 3-4.
ARTICLE INFO
Received 09 November 2022
Accepted 24 November 2022
Available online February 2023
Recommended for publication by PhD V. Don
eLIBRARY Document Number (EDN) RAWARM

Fluorine Notes, 2023, 146, 1-2