Received: December 2022
DOI 10.17677/fn20714807.2022.06.03
Fluorine Notes, 2022, 145, 5-6
REACTIONS OF 2-METHOXYTETRAFLUOROPROPANOIC ACID DERIVATIVES WITH AMINES
T.P. Vasilyeva*, V.I. Dyachenko, D.V. Vorobyeva
A.N. Nesmeyanov Institute of Organoelement Compounds RAS, Russian Federation, 119334, Moscow, Vavilov str. 28/1.
Fax: +7(499) 135 5085. E-mail: d-20@mail.ru
Abstract: The reactions of methyl ester and acid chloride of 2-methoxytetrafluoropropanoic acid with heterocyclic amines and 2-phenylethylamine have been studied. It has been shown that the best yields of aminolysis products are achieved using 2,3,3,3-tetrafluoro-2-methoxypropanoyl chloride. The interaction of methyl ester of same acid with heterocyclic amines is complicated by competing reaction of N-methylation of amines. As a result, a mixture of target amides and N‑methylammonium salts of 2-methoxytetrafluoropropanoic acid is formed, the ratio of which depends on structure of the amine used.
Keywords: amines, methyl 2,3,3,3-tetrafluoro-2-methoxypropionate, 2,3,3,3-tetrafluoro-2-methoxypropanoyl chloride, aminolysis, fluorine-containing 2-methoxypropanamides, N-methylammonium compounds.
The increased interest in chemistry of fluorine-containing O- or N-heterocycles in recent years is associated with their high physiological activity [1]. At the same time, it is known that derivatives of α-hydroxy-α-trifluoromethylcarboxylic acids also have unique pharmaceutical properties; more than 2800 compounds containing this structural unit have been described in papers on drug synthesis [2]. Therefore, the synthesis of heterocyclic derivatives of α-hydroxy-α-CF3-acids, which combine the advantages of both classes of compounds, is an urgent task. In previous papers, we developed a method for preparation of derivatives of α-hydroxy-α-CF3-carboxylic acids containing an α-aryl substituent [3], as well as a furan or 1,2,3-triazole heterocycle in the α- or β-position [4]. In continuation of these studies, in the present work, the reactivity of 2-methoxytetrafluoropropanoic acid derivatives with respect to heterocyclic amines and 2-phenylethylamine was studied. Aliphatic arylethylamides have been reported to have radioprotective activity; it was also noted that introduction of fluorine atoms into antiradiation agents increases their radioprotective effect and therapeutic index [5,6].
It is known that one of frequently used methods for preparation of amides of carboxylic acids is interaction of amines with esters by adding an amine to double C=O bond, followed by elimination of alcohol [7]. In this way, by the action of ammonium hydroxide on esters of 2‑alkoxytetrafluoropropionic acid, the corresponding amides were obtained with high yields [8, 9] (see Scheme 1).
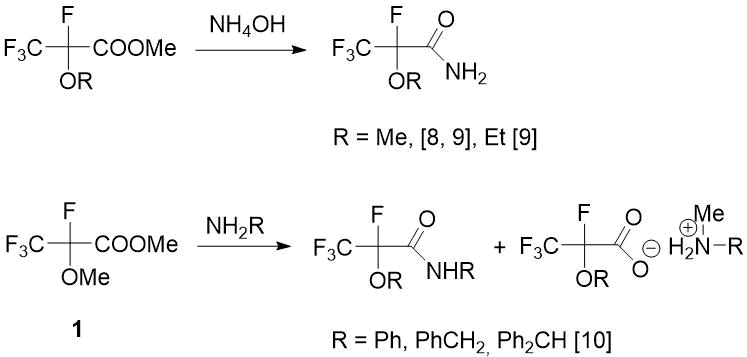
Scheme 1. Results of ammonolysis and aminolysis of 2-alkoxytetrafluoropropanoic acid esters [8-10].
Therefore, for synthesis of target amides of 2-methoxytetrafluoropropanoic acid, the readily available methyl ester 1 was chosen as a starting compound. However, later O. Paleta discovered for the first time that, unlike non-fluorinated analogs, as a result of reactions of ester 1 with primary amines (along with amides) methylation products of amines are formed, the ratio of which depends on reaction temperature and solvent [10] (see Scheme 1). According to other data, the action of such primary amine as (3-aminopropyl)trimethoxysilane on ether 1 gives the only product - the corresponding amide, which is used to obtain hydrophobic aerogels used as a insulators, catalyst carriers and optical materials [11] (see Scheme 2) .
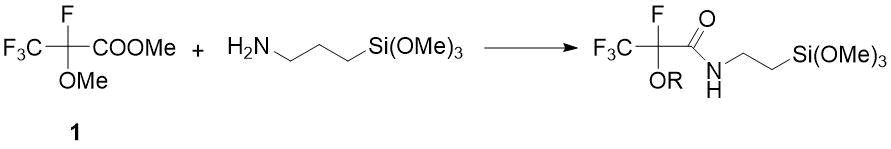
Scheme 2. Reaction of ester 1 with aminoalkylsilane [11].
Only (S)-(-)-1-phenethylamide--methoxytetrafluoropropanoic acid was obtained by traditional method - aminolysis of corresponding acid chloride (RFCOCl) [12,13], which is an intermediate in synthesis of volatile inhalation anesthetics of general formula CF3CF(OR)H, where R=Me, CF2H and C(Cl)(F)H [12-14].
Due to contradictory literature data relatively aminolysis of ester 1, it seemed interesting to find out the results of new reactions of 1 with heterocyclic amines. We chose as amines the 2‑furfurylamine, piperidine, morpholine and hexamethyleneimine. It turned out that these reactions, along with expected amides, also give N-methylammonium salts of 2-methoxytetrafluoropropanoic acid as a result of competitive attack of carbonyl carbon atom and methyl group by amine. In this case, the relative amounts of products depend on structure of taken substrate. With primary amine, 2-furfurylamine, a mixture (2:1) of corresponding amide 2, and methylation product 6 is formed in the form of ammonium salt. The action of heterocyclic secondary amines (piperidine or morpholine) on ester 1 gives approximately equal amounts of amides 3 or 4 and N‑methylammonium salts 7 or 8. The reaction of sterically hindered hexamethyleneimine with 1 lead to predominant formation of N-methylation product 9 - the ratio of amide 5 and salt 9 is 1:3.2 (see Scheme 3).
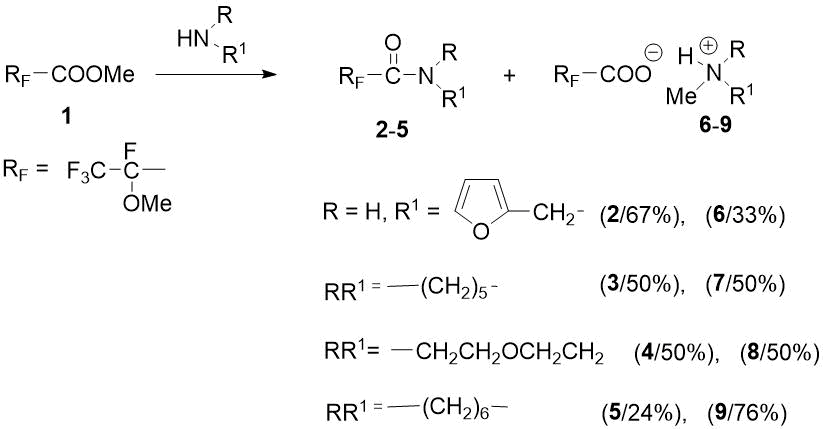
Scheme 3. Ratio of reaction products of methyl ester 1 with heterocyclic amines.
The resulting amides 2-5 were purified by vacuum distillation. Salts 6-9 cannot be purified (about this were also reported in [10] for analogous salts), and it were characterized by 1H and 19F NMR spectra. The RF values for amides 2-5 in TLC (in acetone-CCl4 = 1:3 system) are in the range of 0.58-0.73, while salts 6-9 remain at the start (RF = 0-0.18).
Since the aminolysis of ester 1 gave low yields of fluorine-containing amides after isolation (21–62%), we obtained another derivative of 2-methoxytetrafluoropropanoic acid - acid chloride 10, by saponification of ester 1 to acid [8] and subsequent reaction with phthaloyl dichloride [12] (see Scheme 4). The reactions of acid chloride 10 with morpholine, hexamethyleneimine and phenylethylamine gave the corresponding amides 4, 5, 11 in high yields (71–91%), and we used an additional 1 eq. amine or triethylamine (see Scheme 4).
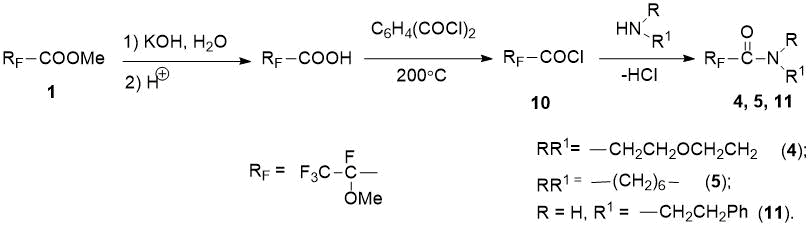
Scheme 4. Synthesis of fluorine-containing amides 4, 5, 11 from acid chloride 10.
According to 19F NMR spectrum, hexamethyleneimide 5 obtained from RFCOCl 10 exists as a 6:1 mixture of two stereoisomers. The fluorine-substituted alkylamides 2-5 and 11 synthesized by us are new compounds, the composition and structure of which have been proven by 1H and 19F NMR data and elemental analysis. In 19F NMR spectra of amides, the signal of CF group appears in the region of -49.93÷ -58.07 ppm, while the signal of CF3 group appears at -2.15÷ -4.90 ppm.
Thus, we have shown that the results of aminolysis of ester 1 depend on structure of amine. According to our and published data [10, 11], the preparation of amides with preparative yields is possible only when using sterically unhindered primary methyleneamines with general formula R‑CH2NH2. With respect to sterically hindered secondary heterocyclic amines, the methyl ester 1 exhibits a dual reactivity, and as a result, a mixture of corresponding amides and N‑methylammonium salts of 2-methoxytetrafluoropropanoic acid is formed. In these cases, the acid chloride RFCOCl 10 should be used instead of ester 1 to obtain amides. Since many drugs contain the amide function [15, 16], the synthesis of fluorine-containing amides is an urgent problem.
Experimental part
NMR spectra were recorded via Bruker Avance-200 and Bruker Avance-300 spectrometers at operating frequencies of 200 MHz and 300 MHz (internal standard SiMe4) for 1H NMR and 188 MHz and 282 MHz - for 19F NMR (internal standard CF3COOH), respectively. Elemental analysis was performed at Laboratory of Microanalysis of A. N. Nesmeyanov Institute of Organoelement Compounds of Russian Academy of Sciences. The reaction progress was monitored by TLC method at Merck plates (silica gel 60 F254, 0.25 mm). The RF values of obtained compounds were determined in the acetone-СCl4 = 1:3 system. The starting methyl ester 1 was synthesized by reaction of hexafluoropropene epoxide with methanol [8]. 2,3,3,3-tetrafluoro-2-methoxypropanoyl chloride 10 was obtained from corresponding acid [8] and phthaloyl dichloride as described in [12, 13]. All solvents used were purified according to standard procedures.
Aminolysis of methyl-2,3,3,3-tetrafluoro-2-methoxypropionate (1).
Reaction 1 with 2-furfurylamine.
A mixture of 3.5 g (18.4 mmol) of ester 1 and 1.0 g (10.3 mmol) of 2-furfurylamine was heated with stirring at 50-60°C until this reaction was complete (9 h). The temperature was brought to room temperature, the reaction mixture was evaporated in vacuo, and 3.05 g of oil was obtained, containing according to TLC analysis and NMR spectra (1H, 19F), a mixture (2:1) of target amide 2 and N-(furan-2-ylmethyl)-N-methyl-O-(2,3,3,3-tetrafluoro-2-methoxypropanoyl)hydroxyl ammonium 6. To remove the salt 6, the resulting oil was dissolved in diethyl ether, washed with water, dilutes hydrochloric acid and again washed with water. The ethereal layer was separated, evaporated in vacuo, CCl4 was added to residue, and prepared solution was filtered through a small layer of silica gel (5 mm). Removal of this solvent in vacuo gave 1.64 g (62%) of orange oil (after treatment with cold petroleum ether it was a light-yellow powder). 2,3,3,3-tetrafluoro-N-(furan-2-ylmethyl)-2-methoxypropanamide 2, m.p. 30°C, RF = 0.66 (solution in CCl4). 1H NMR spectrum (200 MHz, CD3OD, δ, ppm): 3.51 (s, 3H, OCH3); 4.43 (s, 2H, CH2N); 4.84 (s, 1H, NH); 6.19-6.25 (m, 1H, furan); 6.30-6.36 (m, 1H, furan); 7.41 (s, 1H, furan). 19F NMR spectrum (188 MHz, CD3OD, δ, ppm): -4.90 (br.s, 3F, CF3); -53.26 (br.s, 1F, СF). Founded (%): C, 42.21; H, 3.62; N, 5.33. C9H9F4NO3. Calculated (%): C, 42.35; H, 3.53; N, 5.49.
Reaction 1 with piperidine.
The synthesis was carried out from 1.71 g (9.0 mmol) of ester 1 and 0.81 g (9.5 mmol) of piperidine similarly, but at 72°С for 5.5 h. 2.1 g of oil containing according to TLC analysis and NMR spectra (1H, 19F), a mixture (1:1) of target 2,3,3,3-tetrafluoro-2-methoxy-1-(piperidin-1-yl)propan-1-on 3 ( RF = 0.58) and 1-methyl-1-((2,3,3,3-tetrafluoro-2-methoxy-propanoyl)oxy)piperidin-1-ium 7 (RF = 0.18)was obtained. Amide 3 was isolated from this mixture by distillation with yield of 0.77 g (35%) as a colorless oil, b.p. 72°С/3 mmHg. 1H NMR spectrum (200 MHz, C6D6,δ, ppm): 1.07-1.27 (m, 6H, 3CH2); 3.28 (s, 3H, OCH3); 3.22-3.54 (m, 4H, СН2NCH2). 19F NMR spectrum (188 MHz, C6D6, , ppm): -2.15 (s, 3F, CF3); -49.93 (s, 1F, CF). Founded (%): C, 44.29; H, 5.44; N, 5.68. C9H13F4NO2. Calculated (%): C, 44.44; H, 5.35; N, 5.76.
Reaction 1 with morpholine.
A mixture of 0.51 g (2.7 mmol) of ester 1 and 0.23 g (2.7 mmol) of morpholine was stirred at 20°C until the reaction was completed (7 days). After the reaction mixture was evacuated, 0.5 g of a low-melting product was obtained, containing, according to TLC analysis and NMR spectrum (19F), the mixture (1:1) of target amide 4 and 4-methyl-4-((2,3,3,3-tetrafluoro-2-methoxy-propanoyl)oxy)morpholine-4-ium 8.
19F NMR spectrum (188 MHz, acetone-d6, δ, ppm): -1.50 (s, 3F, CF3 for 8); -3.00 (s, 3F, CF3 for 4); -48.42 (s, 1F, CF for 8); -51.25 (s, 1F, CF for 4).
Reaction of 2,3,3,3-tetrafluoro-2-methoxy-1-morpholino-propan-1-on (4).
To a solution of 1.52 g (7.8 mmol) of RFCOCl 10 in 10 ml of diethyl ether was added 1.39 g (16 mmol) of morpholine in 14 ml of diethyl ether while cooling with cold water. The next day, the formed hydrochloride precipitate was filtered off, the mother liquor was evaporated in vacuo and residue was distilled. 1.6 g (84%) of amide 4 was obtained as a colorless oil, b.p. 61°С/0.045 mm Hg, nD = 1.4209 (20.5°С). 1H NMR spectrum (200 MHz, C6D6, , ppm): 3.02 (s, 3H, OCH3); 3.04-3.28 (m, 8H, 4CH2). 19F NMR spectrum (188 MHz, C6D6, δ, ppm, J/Hz): -2.18 (d, 3F, CF3, J = 4.0); -50.32 (s, 1F, CF). Founded (%): C, 39.05; H, 4.57; N, 5.65. C8H11F4NO3. Calculated (%): C, 39.18; H, 4.49; N, 5.71.
Reaction 1 with hexamethyleneimine.
This synthesis was carried out from 1.06 g (5.6 mmol) of ether 1 and 0.59 g (6.0 mmol) of hexamethyleneimine, similarly to reaction of 1 with morpholine. 1.55 g of oil was obtained containing, according to TLC analysis and NMR spectra (1H, 19F), a mixture (1:3.2) of target amide 5 and 1-methyl-1-((2,3,3,3-tetrafluoro-2-methoxy-propanoyl)oxy)azepan-1-ium 9.
19F NMR spectrum (188 MHz, CD3OD ,δ, ppm): -2.00 and -4.70 (both s, 3F, CF3 for 5); -4.10 (s, 3F, CF3 for 9); -50.10 and -59.40 (both s, 1F, CF for 5); -53.60 (s, 1F, CF for 9). Amide 5 was isolated from mixture by distillation with a yield of 0.30 g (21%) as a light-yellow oil, b.p. 81-83°С/3 mm Hg.
Reaction of 1-(azepan-1-yl)-2,3,3,3-tetrafluoro-2-methoxypropan-1-on (5).
To a solution of 1,6 g (8,2 mmol) RFCOCl 10 in 8 ml of diethyl while cooling with cold water was added a solution 1.0 g (10.1 mmol) of hexamethyleneimine and 0.83 g (8.2 mmol) of triethylamine in 12 ml a solution of diethyl ether with stirring. The next day, the formed hydrochloride precipitate was filtered off, the mother liquor was evaporated in vacuo, and the residue was distilled. 1.49 g (71%) of amide 5 was obtained as a colorless oil, b.p. 58-60С/0.05 mm Hg, nD = 1.4271 (20.5°С) and RF = 0.73 (solution in benzene). 1H NMR spectrum (200 MHz, C6D6,δ, ppm): 1.00-1.46 (m, 8H, 4CH2); 3.10 (s, 3H, OCH3); 2.86-3.36 (m, 4H, 2CH2). 19F NMR spectrum (188 MHz, C6D6,δ, ppm): -2.17 and -4.87 (s, 3F, CF3); -50.52 and -58.00 (s, 1F, CF). Founded (%): C, 46.54; H, 5.98; N, 5.31. C10H15F4NO2. Calculated (%): C, 46.69; H, 5.84; N, 5.45.
Reaction of 2,3,3,3-tetrafluoro-2-methoxy-N-phenethylpropanamide (11).
To a solution of 1.33 g (11.0 mmol) of 2-phenylethylamine in 13 ml of benzene was added 1.07 g (5.5 mmol) of RFCOCl 10 in 5 ml of benzene while cooling with cold water. The next day, the precipitated hydrochloride was filtered off, the mother liquor was evaporated in vacuo, and the residue was a yellow crystallizing oil. Obtained 1.4 g (91%) of amide 11 in the form of light-yellow crystals with m.p. 62-64°С, RF = 0.71 (solution in benzene). 1H NMR spectrum (300 MHz, C6D6, δ, ppm, J/Hz): 2.34-2.51 (m, 2H, CH2Ph); 3.09 (s, 3H, OCH3); 3.13-3.28 (m, 2H, CH2N); 5.95 (br.s, 1H, NH); 6.98 (d, 2H, Ph, J = 7.0); 7.09-7.21 (m, 3H, Ph). 19F NMR spectrum (282 MHz, C6D6, , ppm): -4.7 (s, 3F, CF3); -58.07 (s, 1F, CF). Founded (%): C, 51.48; H, 4.74; N, 4.95. C12H13F4NO2. Calculated (%): C, 51.61; H, 4.66; N, 5.02.
Acknowledgments
This work was supported by the Ministry of Science and Higher Education of the Russian Federation (Contract/agreement No. 075-00697-22-03) and was performed employing the equipment of Center for molecular composition studies of INEOS RAS.
References
- Saloutin V.I., Saloutina L.V., Zapevalov A.Ya., Chupakhin O.N., Russ. Chem. Bull. (Int. Ed.), 2016, 65, 2163.
- Wang P., Feng L.-W., Wang L., Li J.-F., Liao S., Tang Y., J. Am. Chem. Soc., 2015, 137, 4626.
- Vasilyeva T.P., Vorobyeva D.V., Russ. Chem. Bull., 2018, 67, 1426.
- Vasilyeva T.P., Vorobyeva D.V., Russ. Chem. Bull., 2020, 69, 300.
- Vasilyeva T.P., Znamensky V.V., Pharm. Chem. J. (Engl. transl.), 1994, 28, 47.
- Vasilyeva T.P., Lisina N.I., Znamensky V.V., Pharm. Chem. J. (Engl. transl.), 1997, 31, 258.
- Titze L., Iher Т., Preparative organic chemistry, (Deutch transl.) Ed. Alekseeva U.E. М.:Mir, 2009, 162.
- Sianesi D., Pasetti A., Tarli F., J. Org. Chem., 1966, 31, 2312.
- Pasetti A., Tarli F., Sianesi D., Gazz.Chim. Ital., 1968, 98, 277.
- Dolensky B., Kvičala J., Paleta O., J. Fluorine Chem., 2016, 185, 31.
- Lermontov S., Sipyagina N., Malkova A., Yarkov A., Vasil’ev S., Simonenko N., Baranchikov A., Ivanov V., RSC Advances, 2016, 6(84), 80766.
- Rozov L., Rafalko P., Evans S., Brockunier L., Ramig K., J. Org. Chem., 1995, 60, 1319.
- Rozov L., Ramig K., Tetrahedron Lett., 1994, 35, 4501.
- Ramig K., Brockunier L., Rafalko P., Rozov L., Angew. Chem., Int., Ed., 1995, 34, 222.
- Nicholson W., Barreteau F., Leitch J., Payne R., Priestley I., Godineau E., Battilocchio C., Browne D., Angew. Chem. Int. Ed., 2021, 60, 21868.
- Ning X., Lou S., Mao Y., Xu Z., Xu D., Org. Lett. 2018, 20, 2445.
ARTICLE INFO
Received 09 December 2022
Accepted 19 December 2022
Available online December 2022
Recommended for publication by PhD Olga Bryzgalova
eLIBRARY Document Number (EDN) MVJUQS

Fluorine Notes, 2022, 145, 5-6
