Received: December 2022
DOI 10.17677/fn20714807.2022.06.02
Fluorine Notes, 2022, 145, 3-4
STUDY OF POLYFLUOROALKYL-CONTAINING PHENOLS AND THEIR SILYLATION PRODUCTS BY GAS CHROMATOGRAPHY-MASS SPECTROMETRY
E.A. Khakina, V.I. Dyachenko
A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 119991, Moscow, Vavilov St. 28
Fax: (499) 135 5085, e-mail: khakina90@ineos.ac.ru
Abstract: This report is devoted to analysis of polyfluoroalkyl-containing phenols 1a-e and their derivatization (silylation) products 2a-e by gas chromatography-mass spectrometry. It was shown that the intensities of molecular ions of compounds 1a-e depend on the position of substituents in the aromatic nucleus. It was established that the mechanism of fragmentation of molecular ions of silylation products of the studied phenols 2a-e depends on their structure.
Keywords: gas chromatography-mass spectrometry, derivatization, polyfluoroalkyl-containing phenols
Recently, the polyfluorinated compounds are increasingly used in production of various polymeric materials, hydrophobic coatings, and are also being studied as drugs with various spectrums of action [1-3]. Along with development of methods for synthesis of such compounds, the analytical methods for their determination and detection in various substances are being developed [4]. Gas chromatography-mass spectrometry with electron impact ionization is one of the most effective methods for analysis of volatile thermally stable compounds [5-7]. The NIST libraries, which summarize the spectral data for many compounds of various classes, make it possible to efficiently identify the components of mixtures. In addition, the active development of derivatization methods expands the area of applicability of gas chromatography-mass spectrometry. Functionalization of hydroxy-, amino-, carboxy- and other groups with commercially available derivatizing reagents results in conversion of low volatile compounds (polyalcohols, acids, etc.) into highly volatile ones, which can significantly improve the quality and signal-to-noise ratio in spectra [8].
However, after analyzing the NIST 17 MS database, it can be concluded that there is only limited amount of data for polyfluorinated compounds.
In this paper, we studied a number of polyfluoroalkyl-containing phenols (by gas chromatography-mass spectrometry with and without derivatization) to reveal the dependence of fragmentation patterns on its composition and structure. The objects studied were ortho- and para-substituted 2-(2,2,2-trifluoro-1-hydroxy-1-trifluoromethylethyl)-phenols 1а-е, differing in composition and position of substituents in aromatic nucleus (see Fig. 1).
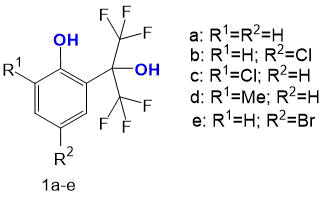
Figure 1. Ortho- and para-substituted 2-(2,2,2-trifluoro-1-hydroxy-1-trifluoromethylethyl)-phenols
These compounds were synthesized by authors using reaction of C-oxyalkylation of phenols with hexafluoroacetone according to previously described procedures [9-11], however the mass spectra of these compounds obtained by electron impact ionization with explanation of fragmentation could not be found in the literature.
Analysis of these compounds was performed via Shimadzu GCMS-QP2020 gas chromatography-mass spectrometer with SH-RTx-5MS column (30 m × 0.25 mm × 0.25 μm), using electron impact ionization method and single quadrupole detector in the mode of recording the total ion current of positive ions. Helium (99.9999%) was used as carrier gas. The analysis parameters are shown in Table 1. Registration was carried out in positive ion scanning mode within the range of 35–650 m/z. To prepare the samples, a 0.1 mg sample was dissolved in 1 ml of methylene chloride. The results obtained were analyzed using GCMSolution program.
Table 1. GC/MS analysis parameters.
|
Initial temperature and hold time |
50°C, 1 min |
|
Heating rate |
30°C/min up to 290°C |
|
Hold time at final temperature |
3 min |
|
Time of total analysis |
15 min |
|
Temperature of injector |
250°C |
|
Injector mode |
Split (1:10) |
|
Sample volume |
0.1 µl |
|
Helium flow rate through the column |
1 ml/min |
|
Ion source temperature |
200°C |
|
Interface temperature |
250°C |
|
Electron energy |
70 eV |
It was shown that in spectra of all initial phenols 1a-e under specified ionization conditions, rather intense signals of molecular ions are observed (see Fig. 2). The intensity of molecular ion signal noticeably increases in the presence of any substituent from CH3, Cl, Br series in aromatic nucleus. Moreover, the presence of substituent in para-position to phenol group leads to a greater increase in intensity of molecular ion signal (in comparison with its presence in ortho-position). The main route of molecular ions fragmentation of studied polyfluorinated phenols is initial elimination of fluorine atom followed by release of difluorocarbene, which is a common mechanism for fluorinated compounds fragmentation [12]. The subsequent fragmentation is accompanied by proton abstraction along with fluorine atom and difluorocarbene, which leads to formation of maximum intensity peak. Molecular ions of all studied compounds fragmented by the same mechanism, regardless of their structure.
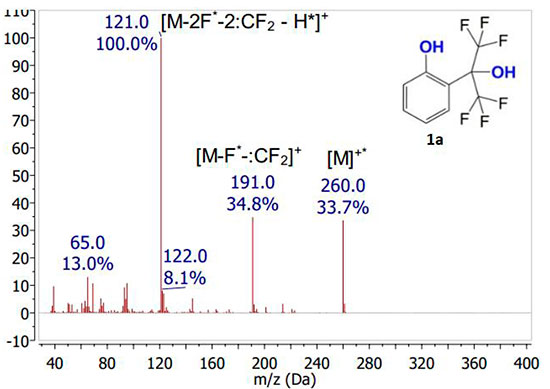
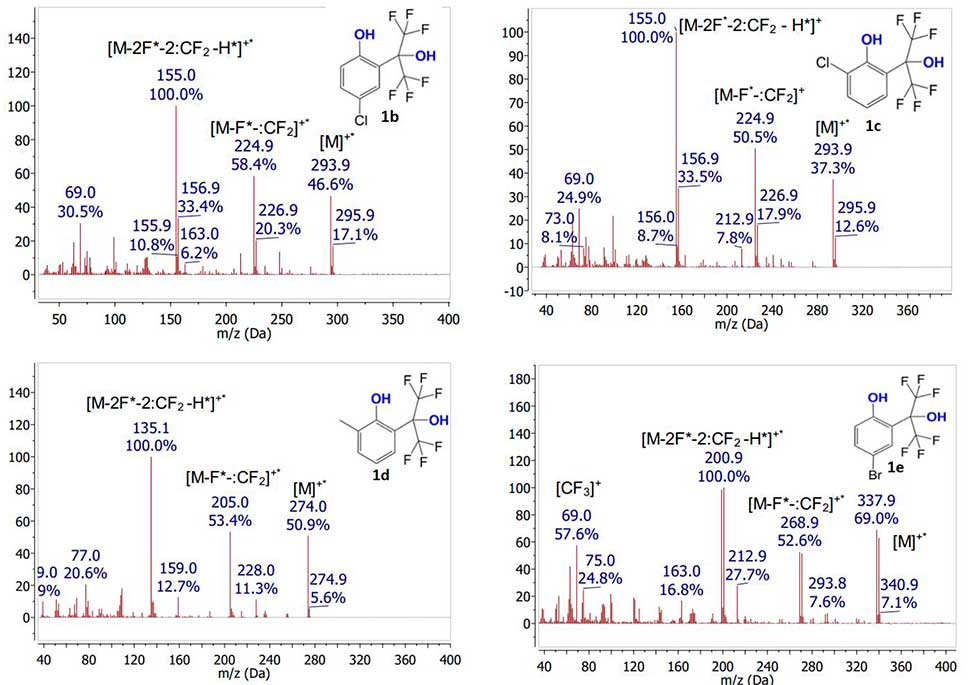
Figure 2. Mass spectra of compounds 1а-e obtained by electron impact ionization.
The intensity of molecular ions in mass spectra of compounds 1a-e depends on molecule structure and electronic properties of substituents. This intensity was calculated as a ratio of intensity of corresponding molecular ion to intensity of molecular ion in the spectrum of compound 1a. It should be noted that intensity of signal increases in the following order o-Cl < o-CH3 < p-Cl < p-Br (see Fig.3).
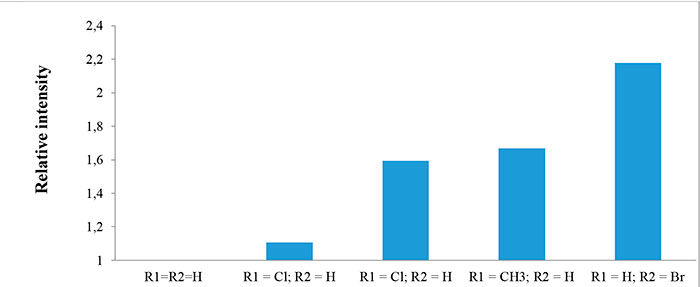
Figure 3. Relative intensity of molecular ions in the mass spectra of compounds 1а-e.
For derivatization, 1-(trimethylsilyl)imidazole was used (see Scheme 1). Ethyl acetate was used as a solvent during derivatization. To a solution of 0.1 mg of compound 1a-e in 1 ml of dry ethyl acetate in a vial for chromatographic analysis was added 3 μl of derivatizing agent. The resulting mixture was boiled for 30 s, then cooled to room temperature and analyzed under the same conditions as initial alcohols (see Table 2).
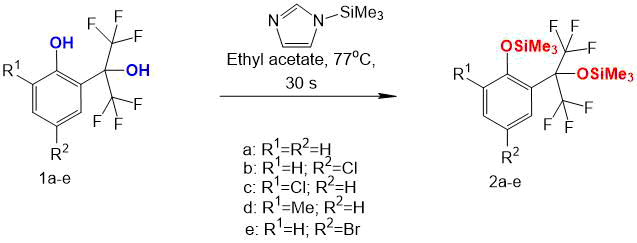
Scheme 1.
The reaction of studied phenols with derivatizing agent under specified conditions led to conversion of both alcohol groups into silyl ethers (despite steric hindrance), which was confirmed by GC-MS (see Fig. 4). At the same time, due to increase in molecular weight of silyl ethers in comparison with the mass of initial phenols; their retention time under these separation conditions also increased. Table 2 summarizes the retention times of starting phenols and their silyl ethers.
Table 2. Retention times (tR) of phenols 1a-e and their silyl ethers under GC-MS analysis
|
tR, min |
Compounds |
|||||||||
|
1а |
2a |
1b |
2b |
1c |
2c |
1d |
2d |
1e |
2e |
|
|
5,39 |
6,45 |
6,05 |
6,99 |
5,79 |
7,06 |
5,39 |
6,90 |
6,39 |
7,31 |
|
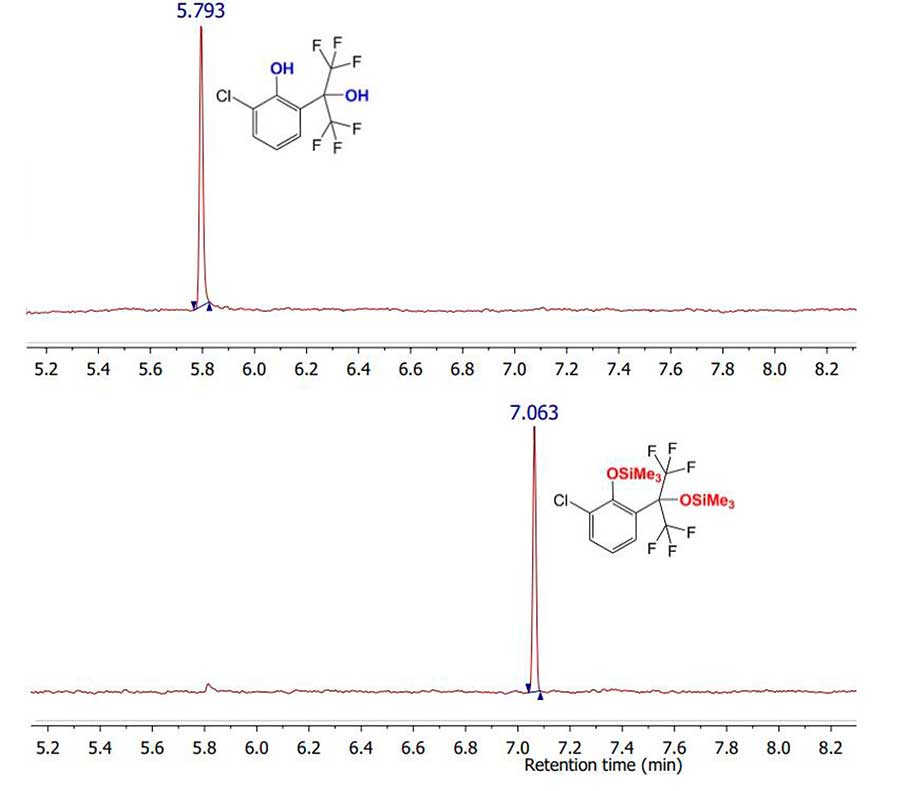
Figure 4. Comparison of total ionic current chromatograms for compounds 1c (top) and 2c (bottom).
In contrast to spectra of initial phenols 1a-e, in the spectrum of their silyl ethers 2a-e the intensity of molecular ions signals is rather low. It should be noted that fragmentation mechanism of silyl ethers (in contrast to fragmentation of phenols) depends on the location of substituent in the aromatic nucleus. In the presence of any substituent in para-position to Me3SiO group, the first stage of fragmentation of molecular ion is fluorine atom removal, followed by release of difluorocarbene by analogy with initial phenols (see Fig. 5).
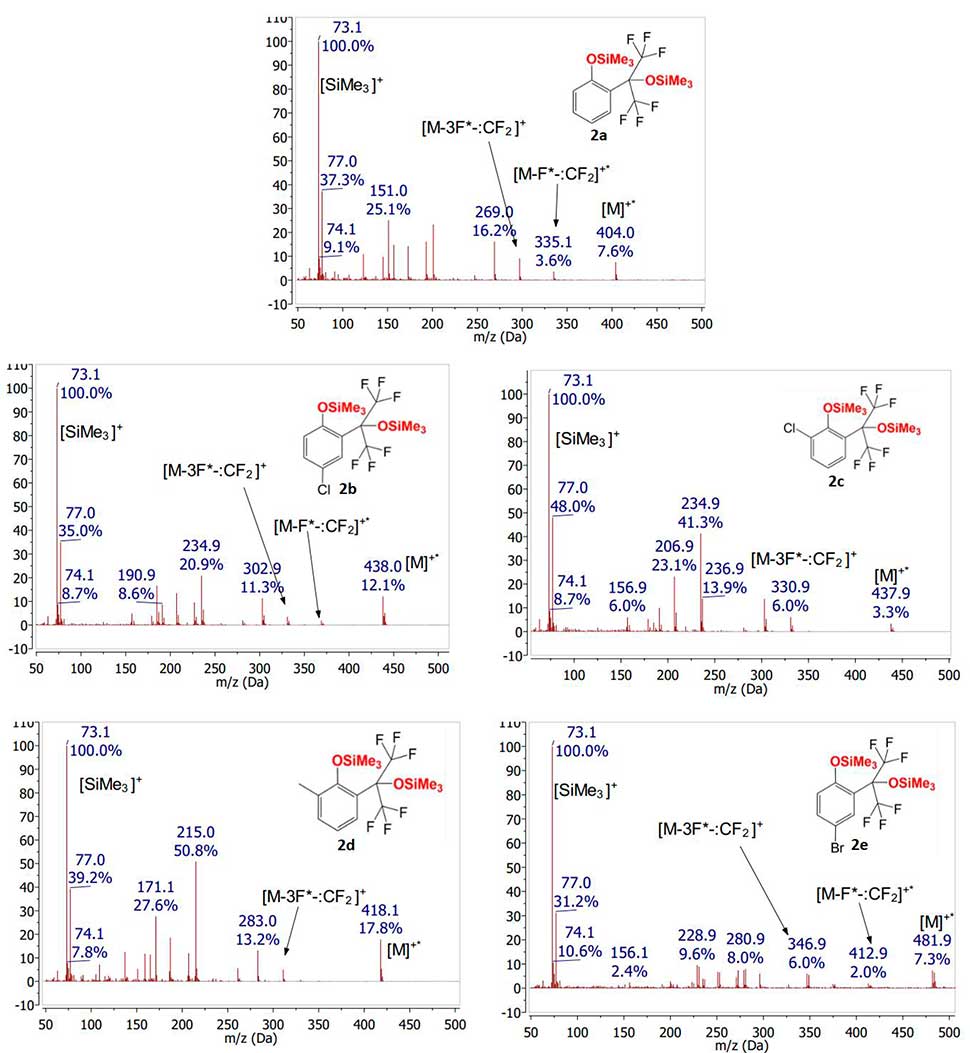
Figure 5. Mass spectra of compounds 2a-e obtained by electron impact ionization.
The presence of substituent in ortho-position to trimethylsiloxy group of silyl ethers 2а-е, trimethylsiloxy signal with m/z ratio corresponding to [M-F*-:CF2]+ is not observed. In this case, an ion signal is observed, probably corresponding to the composition [M-3F*-:CF2]+.
Apparently, the founded patterns of mass spectral fragmentation of fluoroalkyl-containing phenols 1а–е and their trimethylsilyl ethers 2а–е can also be characteristic of other analogs containing other alkyl and alkoxy groups, aryl substituents, halogen atoms, etc. in aromatic nucleus.
Conclusions
2-(2,2,2-Trifluoro-1-hydroxy-1-trifluoromethylethyl)-phenols 1a-e and their di-(trimethylsilyl) ethers 2a-e were studied by gas chromatography-mass spectrometry. It has been shown that derivatization leads to addition of two silyl groups (despite the potential steric hindrance caused by CF3 groups).
The dependence of molecular ion intensity for phenols 1а-е on their structure was demonstrated. It was found that fragmentation of phenol molecular ions 1а-е and their derivatization products 2а-е proceeds according to different mechanisms and depends on location of substituent in the aromatic nucleus.
This can be a weighty argument in the identification of these isomers by their mass spectral characteristics.
Acknowledgements
The work was carried out within the framework of State Assignment No. 075-00697-22-03 of the Ministry of Science and Higher Education of the Russian Federation using the scientific equipment of the Center for Research on the Structure of Molecules of the INEOS RAS.
References
- H. Amii, Perfluoroalkyl Substances: Synthesis, Applications, Challenges and Regulations, ed. by Bruno Ameduri, Royal Society of Chemistry, 2022, 66-112.
- Robertson JF. R, Come SE, Jones SE, Beex F, Kaufmann M, Makris A, Nortier JW. R, Possinger K, Rutqvist L.-E., Eur. J. Cancer, 2005, 41, 346.
- Tong C.-L, Xu X.-H, Qing F.-L., Angew. Chem. Int. Ed., 2021, 60, 22915.
- Gao K, Chen Yu., Xue Q., FuJ., Fu K., Fu J., Zhang A., CaiZ., Jiang G., Trends in Analytical Chemistry, 2020, 133, 116114.
- Krüsemann E. J. Z., PenningsJ. L. A., Cremers J. W. J. M., Bakker F., Boesveldt S., Talhout R., Journal of Pharmaceutical and Biomedical Analysis, 2020, 188, 113364.
- Bisceglia K.J., Kroening G., Bikram S., ACS Symposium Series, 2019, 1319, 51.
- Mojiri A., Zhou J. L.,Ohashi A., Ozaki N., Kindaichi T., Science of the Total Environment, 2019, 696, 133971.
- Harvey D. J., Vouros P., Mass Spectrometry Reviews, 2020, 39, 105.
- Dyachenko V.I., Galakhov M.V., Kolomiets A.F., Fokin A.V., Chemistry of Heterocyclic Compounds, 1989, 25, 1200.
- Dyachenko V.I., Kolomets A.F., Fokin A.V., Russ. Chem. Bull., 1988, 36, 2646.
- Dyachenko V.I., Galakhov M.V., Kolomiets A.F., Fokin A.V., Russ. Chem. Bull., 1989, 38, 831.
- Kagramanov N.D., Fluorine notes, 2020, 1(128), 3-4.
ARTICLE INFO
Received 02 December 2022
Accepted 15 December 2022
Available online December 2022
Recommended for publication by PhD M. Manaenkova
eLIBRARY Document Number (EDN) PRLAUZ

Fluorine Notes, 2022, 145, 3-4
