Received: December 2022
DOI 10.17677/fn20714807.2022.06.01
Fluorine Notes, 2022, 145, 1-2
FLUORINE-CONTAINING ACRYLIC POLYMERS WITH DR1 ELECTRO-OPTICAL CHROMOPHORE IN THE SIDE CHAIN
V.I. Sokolov1, I.O. Goryachuk1, N.K. Davydova2, S.I. Molchanova1, E.V. Polunin3, V.N. Sergeev2
1Federal Scientific Research Center "Crystallography and Photonics" RAS, 119333, Leninsky ave. 59, Moscow, Russia
2A.N. Nesmeyanov Institute of Organoelement Compounds of the RAS, 119991, Vavilova St. 28, Moscow, Russia
3N. D. Zelinsky Institute of Organic Chemistry RAS, 119991, Leninsky ave. 47, Moscow, Russia
Abstract: A new fluorine-containing acrylic polymer with a covalently attached electro-optical chromophore 2-(ethyl(4-((4-nitrophenyl)diazenyl)phenyl)amino)ethyl (DR1) in the side chain was synthesized by the method of ultrahigh pressure (without using of initiators). The resulting polymer is capable of film formation and can be used to create light-guiding films and optical waveguides. It has been established that under the action of visible radiation an irreversible photobleaching of polymer occurs, which is accompanied by a decrease in its refractive index and makes it possible to form channel waveguides in a polymer film. The fluorine-containing electro-optical polymer has increased optical transparency in telecommunication C-wavelength range and is promising for creation of optical modulators operating in near-IR spectral region.
Keywords: electrooptical chromophores, fluorine-containing acrylic polymers, light-guiding films, photobleaching, polymeric waveguides.
Introduction
Electro-optical (EO) polymer materials are widely used upon creation of high-speed integrated-optical modulators and switches operating in "telecommunication" C-wavelength range of 1530-1565 nm [1-8]. To create such materials, the chromophores are used that can change their refractive index under the action of applied electric field. An EO polymer can be produced either by introducing a chromophore molecules into passive matrix (guest-host system) [4-6] or by covalently incorporating them into the side chains of polymer macromolecules (side-chain system) [7-9]. The latter approach is more promising, since it prevents the agglomeration of chromophores, which leads to a decrease in electrooptic coefficient r33. Of great importance is the synthesis of new EO polymers, which, along with a high coefficient of r33, have high optical transparency in C-range of spectrum. Increasing in transparency can be achieved by using fluorine-containing chromophores and polymers, since the replacement of light hydrogen atoms by heavier fluorine atoms leads to a shift of vibrational absorption bands towards longer wavelengths [10]. As a result, the windows of transparency for EO polymer material are opened in near-IR spectral region. In addition, the replacement of hydrogen atoms by fluorine atoms can increase the electronegativity of acceptor part of chromophore molecule, increasing its dipole moment.
In literature previously reported about creation of a number of fluorine-containing EO polymers in which either the chromophore or the polymer contained fluorine atoms [8, 9, 11]. This paper reports on the synthesis of a new fluorine-containing side-chain EO polymer based on DR1 dispersed red chromophore. The acrylic EO polymer having a high degree of fluorination has been obtained. Light-guiding films with a thickness of 0.5-4 µm were made from EO polymer. It has been shown that under the action of visible radiation, this polymer becomes lighter due to photodestruction of chromophore in the side chain. It has been found that refractive index n of polymeric material decreases in this process. This makes it possible to fabricate the channel waveguides, waveguide splitters and other elements of integrated optical devices in light-guiding polymer films by selective laser photobleaching.
1. Synthesis of fluorine-containing electro-optical polymer
Synthesis of polymers with EO chromophores in the side chain is usually carried out in three stages [1-3]. At the first stage, the chromophore itself is synthesized, that is then acylated with methacryloyl chloride (or with its equivalent) to form the ester. The third stage is radical copolymerization of resulting ester with acrylates or their fluorine-containing analogues. In this way, for example, a polymer based on alpha-fluoroacrylate with covalently attached DR1 chromophore was synthesized [11].
In this paper, for the first time, we performed the synthesis of methacrylic ester MADR1 copolymer with covalently attached chromophore DR1 and 2,2,3,3,4,4,5,5,6,6,7,7,7-tridecafluoroheptyl acrylate (FA1) by radical polymerization at ultrahigh pressure (without using of initiators). The synthesis scheme of EO polymer MADR1/FA1 is shown in Fig. 1.
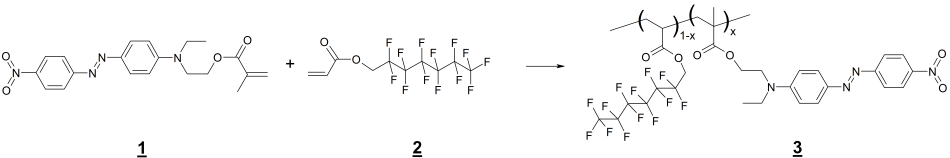
Figure 1. Synthesis scheme of EO polymer MADR1/FA1 3 by ultrahigh pressure method without using of initiators. 1 – methacrylic ester MADR1 with covalently attached chromophore DR1, 2 – fluorine-containing acrylic monomer FA1. Х is the mole fraction of MADR1 units in polymer macromolecule.
Synthesis of 2-(ethyl(4-((4-nitrophenyl)diazenyl)phenyl)amino)ethanol (DR1)
To synthesize DR1 chromophore, we modified the procedure [12] in terms of selecting of optimal conditions for dissolving the starting compound - 4-nitroaniline. 4-nitroaniline (7.0 g, 50 mmol) is dissolved in a mixture of water (150 ml), DMF (150 ml) and concentrated hydrochloric acid (25 ml) with stirring and heating to 30°C. A solution of sodium nitrite (3.60 g, 52 mmol) in 10 ml of water was added dropwise to resulting solution at 0-5°C and stirred for 30 min. A solution of N-ethyl-N-hydroxyethylaniline (10.00 g, 60 mmol) in 50 ml of 10% aqueous hydrochloric acid was added dropwise to reaction mixture at 0-5°C. The reaction mass is stirred for 30 min at 0-5°C and 1 h - at room temperature, poured onto ice, added the chilled water (600 ml) and neutralized with 10% NaOH aqueous solution to pH 7.0. The resulting precipitate is filtered off, washed with water (3×200 ml), dried and recrystallized twice from isopropanol. 9.05 g (58%) of DR1 are obtained as a red-brown crystals.
LC/MS (M+, C16H18N4O3). Calculated: 314.34; Obtained: 315.1 [M+H]+, 337.1 [M+Na]+.
Synthesis of 2-(ethyl(4-((4-nitrophenyl)diazenyl)phenyl)amino)ethyl methacrylate (MADR1)
The MADR1 ester was synthesized according to procedure described in [13]. Triethylamine (3.35 g, 4.17 ml, 30.0 mmol) is added to a solution of DR1 (4.72 g, 15.0 mmol) in 40 ml of anhydrous methylene chloride, cooled to 0°C; a solution of freshly distilled methacrylic acid chloride (2.09 g, 1.905 ml, 20.0 mmol) is added dropwise in 10 ml anhydrous methylene chloride. The reaction mixture is stirred for 30 min at 0°C, and 1 h - at room temperature, washed with saturated NaCl solution (100 ml), 10% potassium carbonate solution (100 ml), evaporated in vacuum. Purification of technical product is carried out by column chromatography on silica gel 60‑100 nm in the system ethyl acetate/hexane, 1:4. 4.38 g (76%) MADR1 is obtained as a dark red powder.
LC/MS (M+, C20H22N4O4) calculation, 382.41; obtained, 383.1 [M+H]+, 405.1 [M+Na]+.
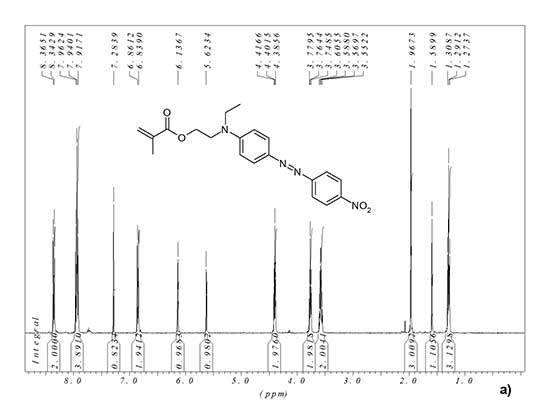
1H NMR (CDCl3), δ, ppm: 1.29 (t, 3H, CH3), 1.97 (s, 3H, CH3), 3.58 (q, 2H, CH2), 3.76 (t, 2H, CH2), 4.40 (t, 2H, CH2), 5.62 (s, 1H, CH=), 6.14 (s, 1H, CH=), 6.85 (d, 2H, CH arom.), 7.94 (m, 4H, CH arom.), 8.35 (d, 2H, CH arom.).
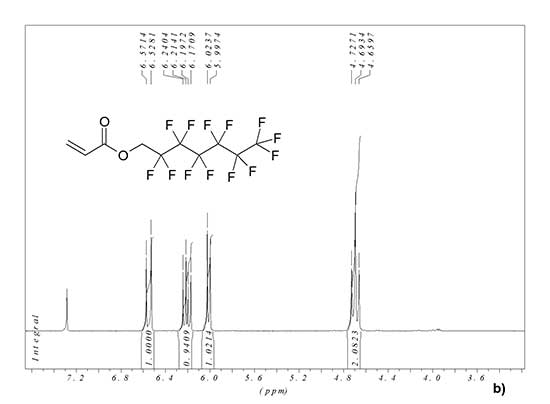
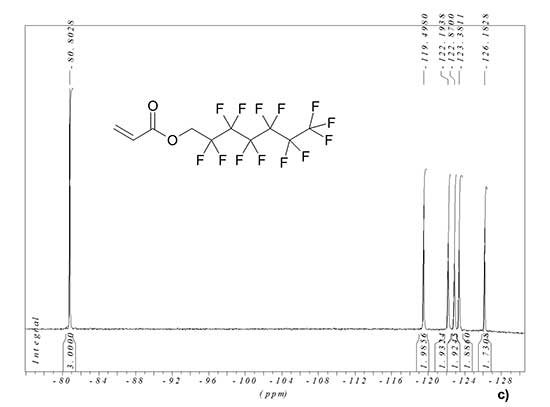
1H and 19F NMR spectra of synthesized monomer MADR1 and fluorine-containing acrylate FA1 manufactured by PIM Invest are shown in Fig. 2. In 1H NMR spectrum of FA1 monomer, the signal is 4.69 ppm corresponds to two hydrogen atoms of methylene group, and signals 6.02, 6.21 and 6.55 ppm – to protons in vinyl group (see Fig. 2b). In 19F NMR spectrum of FA1 monomer, the signal is -80.80 ppm corresponds to three fluorine atoms in terminal trifluoromethyl group, and the signals -119.50, -122.19, -122.87, -123.38 and -126.18 ppm – to five CF2 groups in polyfluorinated aliphatic chain of monomer (see Fig. 2c).
|
|
|
Figure 2. 1H NMR spectrum of MADR1 monomer (a) and fluorine-containing acrylic monomer FA1 (b). 19F NMR spectrum of FA1 monomer (c). The spectra were recorded via Bruker AM-300 NMR spectrometer (300 MHz) in CDCl3. The insets show the structures of monomers.
Synthesis of EO polymer at ultrahigh pressure
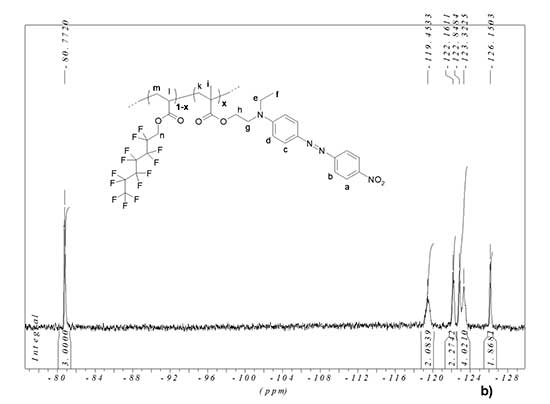
Synthesis of EO polymer MADR1/FA1 was carried out in Teflon ampoules with volume of 10 ml in molds of cylinder-piston type at pressure of 7000 atm and temperature of 70°C. No radical polymerization initiators were used. The reaction time varied from 96 to 192 h. Before synthesis, FA1 monomer was distilled in argon atmosphere. Then MADR1 and FA1 monomers were dissolved in DMF at a concentration of no more than 8 wt % (weakly concentrated solutions were used in order to prevent cross-linking of polymers). This solution was placed in Teflon ampoule and degassed to remove dissolved oxygen, which is known to be an inhibitor of radical polymerization reaction. After synthesis, this ampoule was removed from the mold, and methanol was added to polymer solution in DMF. This led to cloudiness of mixture and precipitation of polymer. The EO polymer was removed from solution by centrifugation. 1H and 19F NMR spectra of resulting EO polymer MADR1/FA1 were recorded via Bruker AM-300 spectrometer and are shown in Fig. 3.
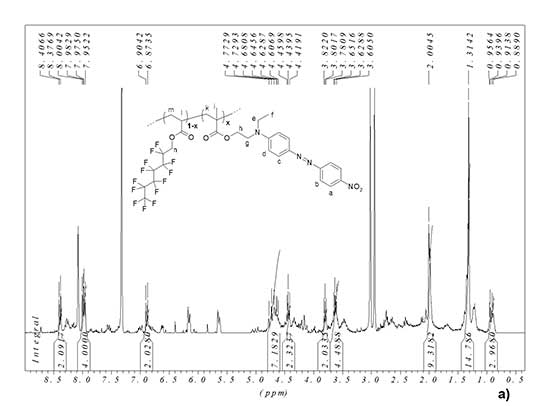
1H NMR (CDCl3) δ, ppm: 8.39 (d, 2H, CHa), 7.97 (m, 4H, 2CHb+2CHc), 6.88 (d, 2H, CHd), 4.61‑4.77 (m, 2H, CH2n), 4.44 (t, 2H, CH2h), 3.80 (t, 2H, CH2g), 3.63 (q, 2H, CH2e), 2.00, 1.31 (m, copolymer chain protons + 3H, CH3f), 0.96-0.90 (m, 3H, CH3i) (see Fig. 3a).
In 19F NMR spectrum of MADR1/FA1 polymer, the signal is -80.77 ppm corresponds to three fluorine atoms in terminal trifluoromethyl group of FA1 monomer units in EO polymer, and the signals are -119.45 ppm, -122.16 ppm, -122.85 ppm, -123.32 ppm, -126.15 ppm – corresponds to five CF2 groups of polyfluorinated aliphatic chain in EO polymer (see Fig. 3b). From a comparison of integral intensities of signals for chromophore fragment and signals for methylene protons in polyfluorinated fragment (4.60-4.77 ppm), it follows that the ratio of chromophore and polyfluorinated fragments in EO polymer is ~1:3.
|
|
Figure 3. 1H (a) and 19F (b) NMR spectra of fluorine-containing EO polymer MADR1/FA1. The spectra were recorded via Bruker AM-300 NMR spectrometer (300 MHz) in CDCl3. The insets show a fragment of polymer structure. x is the mole fraction of MADR1 units in the polymer macromolecule.
To estimate the molecular weight Mw of resulting MADR1/FA1 polymer, the hydrodynamic diameter D of molecular globules of this polymer in chloroform was measured using a 90Plus_Zeta nanoparticle/protein size analyzer (Brookhaven Instruments Corp., USA), see Fig. 4. As can be seen from Fig. 4, the average hydrodynamic diameter is <D> = 0.4 nm. This indicates that molecular weight of polymer does not exceed Mw 1 × 104 g/mol. To increase Mw, one should increase the synthesis time.
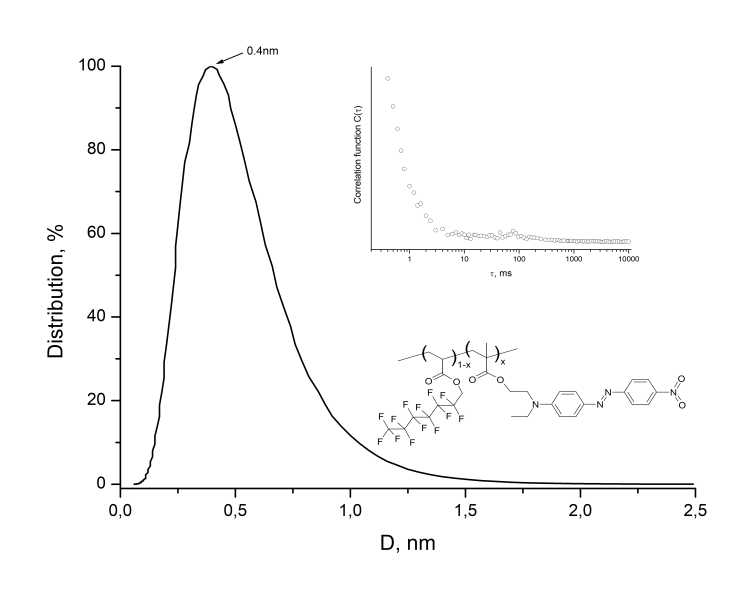
Figure 4. Distribution histogram of macromolecular globules of fluorine-containing EO polymer MADR1/FA1, measured in chloroform. D is hydrodynamic diameter of globules. The insets show the form of autocorrelation function and a fragment of polymer structure.
2. Study of properties of electro-optical polymer
Study of optical properties of resulting polymer was carried out by methods of UV/VIS spectroscopy, IR Fourier spectroscopy and prism excitation of waveguide modes. In Fig. 5 shows the absorption spectrum of MADR1/FA1 film at the quartz substrate in UV- and visible wavelength range, measured via Cary50 spectrometer. As follows from Fig. 5, the EO spectrum of polymer contains absorption bands centered near = 462, 352 and 265 nm.
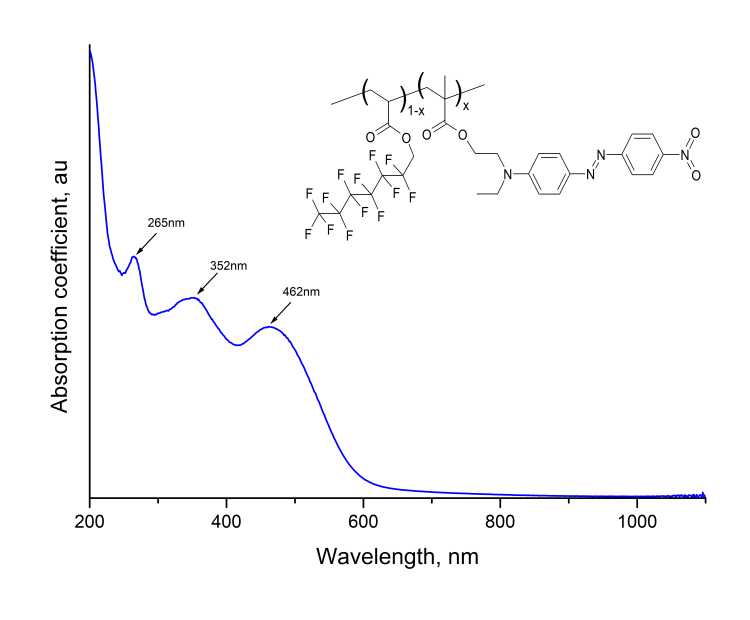
Figure 5. Transmission spectrum of MADR1/FA1 copolymer film measured via Cary50 spectrometer. The inset shows a fragment of polymer structure.
Fig. 6 shows the transmission spectrum of MADR1/FA1 polymer film at KBr substrate measured via Shimadzu 8400S Fourier spectrometer. Fig. 6 also shows that the main absorption bands of polymer lie in the region of wavelengths near 6 μm (fundamental vibrational wavelength for C-H bonds) and 8 μm (fundamental vibrational wavelength for C-F bonds). In telecommunications C-wavelength range of 1530-1565 nm, the light absorption in this polymer is determined by overtone wavelengths for these bonds and, as follows from Fig. 6, is little.
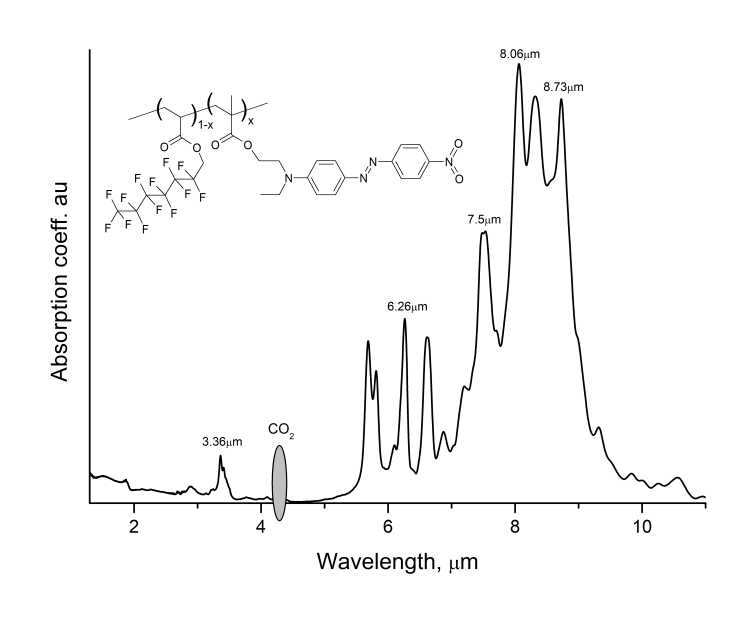
Figure 6. Absorption spectrum of MADR1/FA1 polymer film in IR range measured via Shimadzu8400 FTIR spectrometer.
3. Photobleaching of electro-optical polymer
The films from synthesized EO polymer were formed by centrifugation from MADR1/FA1 solutions in DMF at the quartz substrates. The absorption spectrum of the freshly applied film corresponds to curve 1 in Fig. 7. Curves 2 and 3 in this figure correspond to absorption spectra of film after exposure to light radiation with wavelength of 200-600 nm, i. e. in the absorption bands of chromophore.
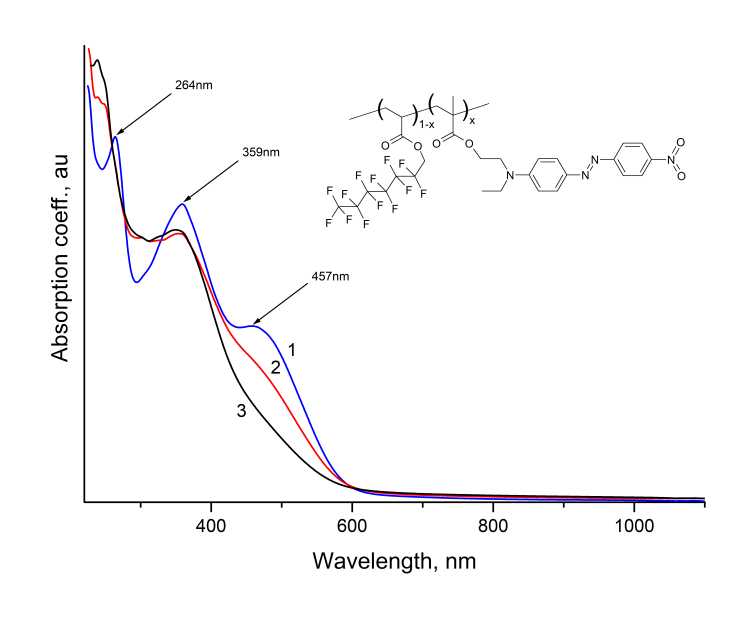
Figure 7. Absorption spectra of a film made of MADR1/FA1 EO polymer in visible- and near-IR wavelength ranges. 1 – before exposure, 2 - in process of exposure, 3 - after exposure by radiation in 200 - 600 nm range. The inset shows the molecular structure of EO polymer.
It follows from comparison of curves 1, 2 and 3 that during exposure the intensity of absorption bands with centers near 460, 360 and 265 nm decreases. This change is due to irreversible photodestruction of chromophore with loss of color (due to effect of photobleaching [6, 14]). It is known that photodestruction of DR1 chromophore molecules is accompanied by a change in their polarizability and, as a consequence, a change in refractive index n of polymer material [8, 15]. To determine the change in refractive index n in MADR1/FA1 EO polymer, the light-guiding films 1-3 µm thick were fabricated at silicon substrates. n of polymer before and after photobleaching was measured by resonant excitation of waveguide modes in the film at a wavelength of 632.8 nm using Metricon/2010M prism coupler (Metricon corp., USA). It was found that refractive index of polymer decreases during photobleaching, and decrease reaches n 0.02. Such change in n is sufficient for formation of channel optical waveguides with numerical aperture NA = 0.17. The synthesized fluorine-containing EO polymer can be used to manufacture various elements of integrated optical devices, in particular, integrated optical modulators based on Mach-Zehnder waveguide interferometers [16].
Conclusion
A new fluorine-containing EO polymer with covalently attached DR1 chromophore in the side chain was synthesized by ultrahigh pressure method without using of initiators. This polymer has high optical transparency in telecommunication C - wavelength range 1530-1565 nm. It has been shown that under the action of light radiation in range of 200-600 nm, photobleaching of polymer occurs, accompanied by a decrease in its refractive index n. The measured decrease in n at a wavelength of 632.8 nm was n = 0.02, which makes it possible to form various elements of integrated optical devices. The resulting polymer is promising for creation of waveguide optical modulators operating in near-IR spectral region.
Acknowledgments
This work was supported by the Ministry of Science and Higher Education of RF within the State assignment FSRC «Crystallography and Photonics» RAS (synthesis of EO polymers and investigation of their optical properties), within the State assignment INEOS RAS No.075‑00697‑22‑00 (synthesis and analysis of monomers with attached chromophores).
References
- Dalton. L., Benight S., Theory-Guided Design of Organic Electro-Optic Materials and Devices, Polymers, 2011, 3, 1325-1351.
- Liu J., Xu G., Liu F., Kityk I., Liu X., Zhen Z., Recent advances in polymer electro-optic modulators, RSC Advances, 2015, 5, 15784-15794.
- Zhang H., Oh M.C., Szep A., Steier W.H., Zhang C., Dalton L.R., Erlig H., Chang Y., Chang D.H., Fetterman H.R., Push-pull electro-optic polymer modulators with low half-wave voltage and low loss at both 1310 and 1550 nm, Applied Physics Letters, 2001, 78(20), 3136-3138.
- Zheng C.T., Zhang L.J., Qv L.C., Liang L., Ma C.S., Zhang D.M., Cui Z.C., Nanosecond polymer Mach-Zehnder interferometer electro-optic modulator using optimized micro-strip line electrode, Opt. Quant. Electron., 2013, 45(3), 279-293.
- Nazmieva G.N., Vakhonina T.A., Ivanova N.V., Mukhtarov A.Sh., Smirnov N.N., Yakimansky A.V., Balakina M.Yu., Sinyashin O.G., Testing of the ways for synthesis of new nonlinear optical epoxy-based polymers with azochromophores in the side chain, European Polymer Journal, 2015, 63, 207-216.
- Sokolov V.I., Akhmanov A.S., Asharchuk I.M., Goryachuk I.O., Khaydukov K.V., Nazarov M.M., Formation of channel optical waveguides in polymethylmethacrylate with embedded electro-optic chromophore DR13 by the photoinduced bleaching method, Optics and Spectroscopy, 2017, 122(3), 128-134.
- Michel S., Zyss J., Ledoux-Rak I., Nguyen C.T., High-performance electro-optic modulators realized with a commercial side-chain DR1-PMMA electro-optic copolymer, Proceedings of SPIE Organic Photonic Materials and Devices XII, 2010, 7599, 75990I-1-75990I-14.
- V.I. Sokolov, A.S. Ahmanov, Е.S. Vasilenko, I.O. Goriachuk, S.I. Molchanova, J. E. Pogodina, E.V. Polunin, Synthesis and investigation of optical properties of fluorine containing chromophore Disperse Orange DO1, Fluorine notes, 2018, 5(120), 1-2.
- Sokolov V.I., Ahmanov A.S., Asharchuk I.M., Goriachuk I.O., Zavarzin I.V., Pogodina J.E., Polunin E.V., Laser formation of light guides In electro optical polymers with fluorine containing chromophores in a side chain, FluorineNotes, 2018, 6(121), 5-6.
- W. Groh., Overtone absorption in macromolecules for polymer optical fibers, Makromol. Chem., 1988, 189, 2861-2874.
- I. McCulloch, H. Yoon, Fluorinated NLO polymers with improved optical transparency in the Near Infrared, Journal of Polymer Science, Part A: Polymer Chemistry, 1995, 33, 1177-1183.
- S.H. Hosseini, M. Mohammadi, Preparation and characterization of new poly-pyrrole having side chain liquid crystalline moieties, Materials Science and Engineering C, 2009, 29, 1503-1509.
- E. Perju, E. Cuervo-Reyes, S. Shova, D.M. Opris, Synthesis of novel cyclosiloxane monomers containing push-pull moieties and their anionic ring opening polymerization, RSC Adv., 2018, 8, 7569-757.
- D. Bosc, F. Foll, B. Boutevin, A. Rousseau., Synthesis of a novel difunctional NLO azo-dye chromophore and characterizations of crosslinkable copolymers with stable electrooptic properties, Journal of Applied Polymer Science, 1999, 74(4), 974-982.
- Nakanishi M., Sugihara O., Okamoto N., Hirota K., Ultraviolet photobleaching process of azo dyedoped polymer and silica films for fabrication of nonlinear optical waveguides, Applied Optics, 1998, 37(6), 1068-1073.
- . I.Y. Denisyuk, Y.E. Burunkova, S.A. Pozdnyakova, V.K. Balya, D.I. Zhuk, M.I. Fokina, An electro-optic polymer modulator for radio photonics, Optics and Spectroscopy, 2015, 119(4), 719‑723.
ARTICLE INFO
Received 03 December 2022
Accepted 15 December 2022
Available online December 2022
Recommended for publication by PhD V. Don
eLIBRARY Document Number (EDN) KJOIOB

Fluorine Notes, 2022, 145, 1-2