Received: April 2022
DOI 10.17677/fn20714807.2022.03.03
Fluorine Notes, 2022, 142, 5-6
DECAY SEQUENCES - ION SERIES OF MASS SPECTRA OF BENZENE, 1,3,5,7-CYCLOOCTATETRAENE, [18]-ANNULENE, HEXAFLUOROBENZENE AND ITS ISOMERS
N. D. Kagramanov
A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 119991, GSP-1, Moscow, B-334, Vavilov St. 28
e-mail: ndkagram@gmail.com
Abstract: This research is devoted to the results of the analysis of decay sequences - ion mass spectra of benzene, 1,3,5,7-cyclooctatetraene, [18]-annulene, as well as hexafluorobenzene and its homologues. The difference between the primary abstraction groups in the spectra of benzene: .H, CH, .C2H,C3H3, C4H2, .C5H3 and hexafluorobenzene: .F and CF contradicts the analogy of their fragmentation pathways. Upon ionization and removal of one of the six π-electrons of benzene, its +.M cation-radical arises with the corresponding excitation energy and one of five variants of rearrangement of conjugations of π-electrons of its carbon core. The mass spectrum of benzene consists of six series of fragment ions. The first series of ions, including the successive detachment of six hydrogen atoms with the formation of the +C6 ion, is the result of the fragmentation of the excited +.M1, in which the existing π-conjugations of the carbon cycle are preserved and their rearrangement does not occur. The primary detachments of the CH, .C2H, C3H2, C4H2 , and .C5H3 groups of five other ionic series confirm that in excited +.M benzene, five variants of rearrangement of π-conjugations are realized, leading to the formation of ions +C5H5, +C4H5, +C3H4, +.C2H4 and +CH3. In contrast to the relatively intense peaks of series 1 (+C6H5 16%), series 3 (+.C4H4 20%; +C4H3 21%; +.C4H2 19%), and series 4 (+C3H3 14%), the peaks of series 2, 5 and 6 have minimum intensities. The mass spectrum of 1,3,5,7-cyclooctatetraene C8H8, in comparison with the spectrum of benzene, as a result of an increase in the number of possible π-electron conjugations of carbon atoms, includes eight series of ions. In the mass spectrum of [18]-annulene C18H18, the number of runs increases to 18. The mass spectra of benzene, [18]-annulene, and non-aromatic annulene-8 consist of 6, 18, and 8 series of ions, respectively, the number of which corresponds to the number of carbon atoms of these cycles. This coincidence is the result of the fact that, during the fragmentation of excited molecular radical cations, all possible variants of the rearrangement of their π-conjugations are realized, as well as the fragmentation variant-with the preservation of existing conjugations, when only the abstraction of hydrogen atoms occurs. The ionic series of benzene, 1,3,5,7-cyclooctatetraene C8H8, and [18]-annulene C18H18 fragment without branching or intersecting. In contrast to the six ionic series of benzene, hexafluorobenzene fragments with the formation of two series, that differ in the sequence of detachments. One of the series of ions includes successive abstractions of four fluorine atoms, CF and .F to form the +C5 ion. The other, after the primary detachment of the CF, branches, fragmenting both with the detachment of the CF and with the detachment of the .F. In contrast to the ionic series of the mass spectrum of benzene, in the spectra of hexafluorobenzene and its isomers, the rearrangement of π-conjugations of the ring does not occur, since none of the two ionic series formed is the result of the appearance of any new π-conjugation.
Keywords: ionic series of mass spectra, π-conjugations, CH-annulenes, regular fragment groups (CH)n, (CF)n, benzene, hexafluorobenzene.
Introduction
Decay sequences - ionic series of mass spectra of compounds containing regular fragment groups (C2H4)n and (CF2)n are considered in [1]. This report presents ion series of mass spectra of anulenes (CH)n n=6, 8 and 18, series of ions of hexafluorobenzene, hexafluorobenzene Dewar and also 1,1,1,6,6,6-hexafluoro-2,4-hexadiine from the NIST libraries.
The part of hydrocarbon chemistry that deals with isomers consisting only of methine units has attracted and continues to attract researchers [2]. The theory of conjugation, which arose from the theory of resonance L. Pauling and K. Ingold's mesomerism theory is constantly supplemented and expanded [3-5].
Due to the high lability of the structural isomers of benzene (1): benzvalene (2), Dewar benzene (3), prisman (4) and bicyclopropenyl (5), the mass spectrum was taken only for benzene. Unstable structural isomers of benzene were first obtained by photolysis of benzene [6]. Isomers of cyclooctatetraene (CH)8 arose during its flash pyrolysis [7] and photolysis [6].
Under the conditions of thermal or photochemical reactions, depending on the amount of acquired energy, new π-conjugations are realized in annulenes molecules, leading to the formation of the corresponding isomers.
During ionization by electrons, excitation of molecular radical cations occurs, the excess energy of which activates the rearrangement of π-conjugations.
As a result, the mass spectrum of annulene is the sum of ionic series of fragmentation of radical cations, with all possible π-conjugations.
Comparison of the ion series of the mass spectra of anulenes (CH)n n=6, 8 and 18, presented in this research, as well as the ion series of perfluorobenzene, perfluorobenzene Dewar and also 1,1,1,6,6,6-hexafluoro-2,4-hexadiine allows us to establish the presence or absence of π‑conjugations of their carbon atoms.
Ionic series of benzene, 1,3,5,7-cyclooctatetraene, [18]-annulene
While studying the mass spectrum of benzene, five primary processes of its fragmentation were identified for the first time: the loss of .H, H2, C2H2, .C3H3, and .CH3 with the formation of +C6H5, +.C6H4, +.C4H4, +C3H3, and +C5H3, respectively [8]. The decay of deutero-substituted benzenes, as well as deuterobenzene with one C13 isotope, was studied under electron impact conditions [9–10].
The mass spectrum of benzene (Fig. 1) consists of five ionic series resulting from the rearrangement of the carbon backbone in accordance with five possible variants of π-conjugations (series 2-6) [5]. Another series 1, corresponds to the preservation of the +C6 carbon backbone.
When the electron π1 is removed, an excited molecular radical cation +.M
arise without conjugation rearrangement. It fragments by successive detachments of 6 hydrogen atoms
with the formation of the +.C6 ion (m/z 72 0.2%).
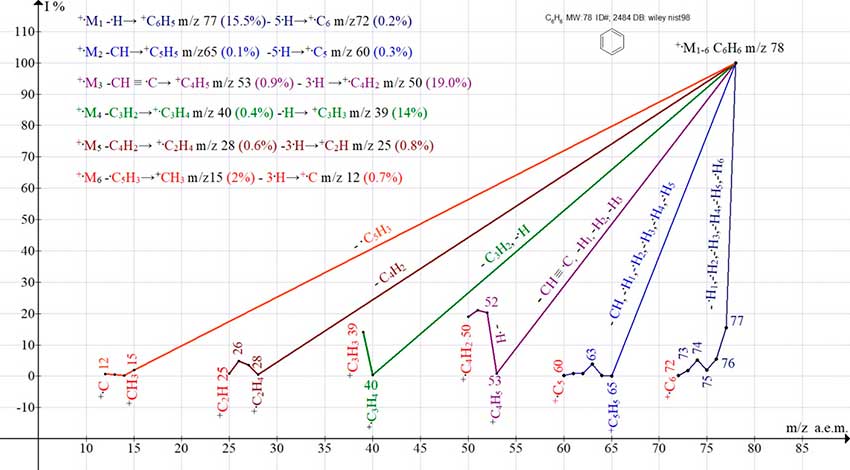
Figure 1. Mass spectrum of benzene C6H6 ID#: 2484 DB: wiley_nist98 and six series of its ions.
As a result of the removal of the π2 electron (C1-C5 conjugation), the carbine CH is ejected, the +C5H5 ion is formed, which fragments by successive detachments of five hydrogen atoms, turning into the +C5 ion (m/z 60 0.3%).
As a result of the removal of the π3 electron (C1-C2 conjugation), .C2H is ejected, the +C4H5 ion appears, the detachment of three hydrogen atoms from which leads to the +.C4H2 ion (m/z 50 19%).
The removal of the π4 electron (C1-C3 conjugation) leads to the emission of C3H2, and the +C3H4 ion (m/z 40 0.4%) is formed. The subsequent detachment of the .H atom leads to the +C3H3 ion (m/z 39 14%).
When the π5 electron is removed (C1-C4 conjugation), С4H2 is detached, and the +.C2H4 ion (m/z 39 0.6%) is formed. The subsequent detachment of 3 hydrogen atoms from it leads to the +.C2H ion (m/z 25 0.8%).
As a result of the removal of the π6 electron (C5-C1 conjugation), breaking is happening C5H3 end the +CH3 ion (m/z 15 2%) is detached. The +CH3 ion (m/z15 2%), which loses three hydrogen atoms, forming the +C1 ion (m/z 12 0.7%).
The mass spectra of the linear isomers of benzene: 1,3-hexadiene-5-yne, 1,5-hexadiene-3-yne and 2,4-hexadiine, as well as the mass spectrum of benzene, consist of 6 series of ions. The series of their spectra differ from those of benzene mainly by changes in the intensities of some peaks, as well as by the maximum possible number of emissions of hydrogen atoms by ions with m/z 52 and 39.
Although the mass spectrum of 1,5-hexadiine contains the same set of peaks as spectrum of benzene, however, since π-conjugation of its terminal CH2 groups is impossible, the peak intensities in the spectrum of 1,5-hexadiine differ sharply from the peak intensities in the spectrum of benzene. The base peak of the mass spectrum of 1,5-hexadiine +C3H3 appears when the molecule splits in half.
In [11], the processes of ionization and fragmentation of seven C8H8 isomers (styrene, cyclooctatetraene, cubane, benzocyclobutene, syn- and anti-tricyclo[4.2.0.0.2.5]-3,7-octadiene) were studied.
The structures of the fragment ions were postulated by comparing their heat of formation with the heat of formation of other ions with the corresponding mass-to-charge ratio.
In contrast to aromatic anulenes with five, six, and seven carbon rings, the four-membered cyclobutadiene ring has no aromatic hardening, and both of its double bonds are localized in certain places of the molecule [12]. Similarly to cyclobutadiene, the eight-membered ring of carbon atoms with four double bonds of cyclooctatetraene is also not aromatic: π-electrons in it are localized and not conjugated, i.e., they do not form a common closed cloud [12]. In contrast to the planar benzene molecule, the cyclooctatetraene molecule has a bath conformation [3].
However, under conditions of electron ionization, in the excited molecular radical cation +.C8H8, the π-orbitals are rearranged, leading to the appearance of new π-conjugations.
Figure 2 shows eight ion series of the mass spectrum of 1,3,5,7-cyclooctatetraene. The series do not intersect and do not branch out.
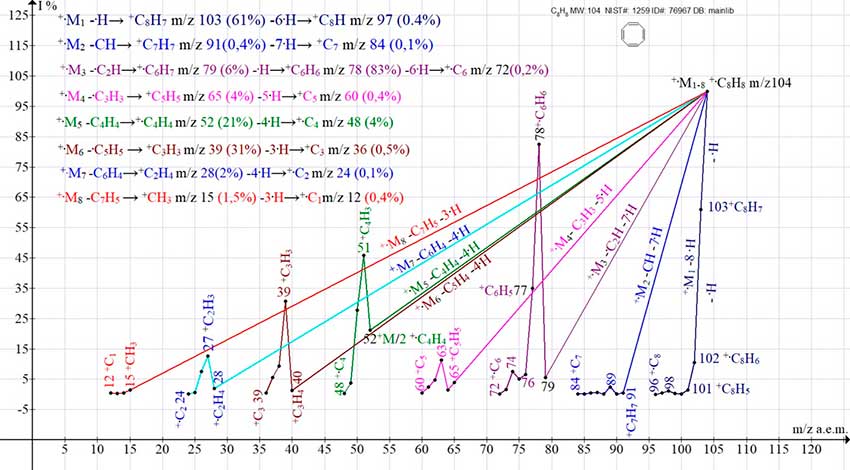
Figure 2. Eight series of mass spectrum ions of 1,3,5,7-cyclooctatetraene C8H8 MW: 104 NIST#: 1259 ID#: 76967 DB: mainlib.
After primary detachments leading to disruption of π-conjugation, the subsequent ejection of a hydrogen atom in series 3, 4, 5, 6, and 7 leads to a sharp increase in the peak intensities of the corresponding ions because of the appearance of new π-conjugations and stabilization. The most intense series of the spectrum is the three +.M3 series. It arises upon detachment of .C2H and ejection of .H, with the formation of the benzene radical cation +.C6H6 m/z 78 (83%), which fragments with subsequent abstractions of six hydrogen atoms. The mass spectra of C8H8 isomers: bicyclo[4.2.0]octa-1,3,5-triene and styrene, also consisting of eight series of ions, differ from the spectrum of cyclooctatetraene by the lower intensities of their fragment peaks.
In the [18]-annulene molecule, at a large ring size, the internal protons do not shield each other. The arrangement of hydrogen atoms occurs without significant distortion of bond angles. [18]-annulene has properties that allow it to be classified as aromatic [3].
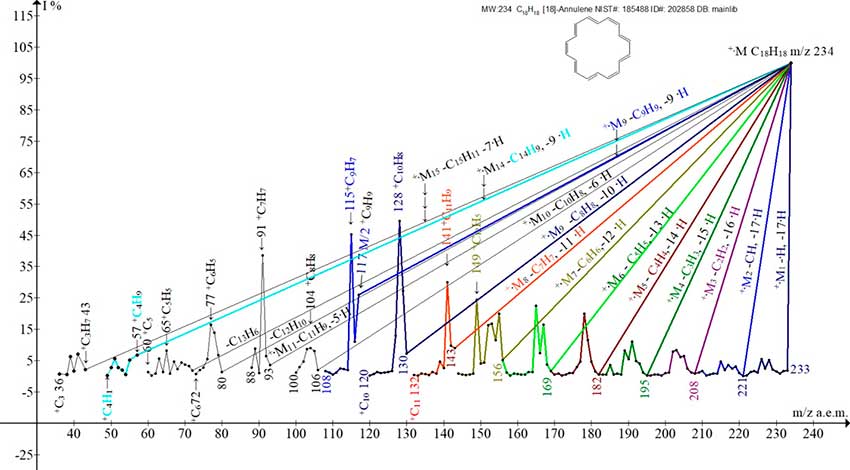
Figure 3. Fifteen ion series of the mass spectrum of [18]-anullene C18H18 MW: 234 NIST#: 185488 ID#: 202858 DB: mainlib.
The mass spectrum of [18]-annulene should consist of 18 ion series. The spectrum presented in (Fig. 3), consisting of 15 series, is not a complete spectrum of [18]-annulene, since it lacks at least 2 low-intensity series, culminating in the formation of +C2 and +C1 ions.
Ionic series of fluorobenzene, 1,2-difluorobenzene, 1,2,4-trifluorobenzene, hexafluorobenzene, Dewar's hexafluorobenzene, 1,1,1,6,6,6-hexafluoro-2,4-hexadiine
Hexafluorobenzene obtained by pyrolysis of CFBr3 [13] was first studied by mass spectrometry and its mass spectrum was compared with that of benzene [14].The mass spectra of fluorinated benzenes from fluorobenzene to hexafluorobenzene, the values of their ionization potentials and the potentials for the appearance of ions under electron impact and photoionization conditions, as well as the values of the C-H and C-F bond energies are presented in the review [15].
The electronic delocalization of benzene and hexafluorobenzene molecules was analyzed under conditions of an induced magnetic field. A detailed analysis of magnetic descriptors (RCS, NICS or Bindz) led the authors to the conclusion that benzene is more aromatic than hexafluorobenzene, and benzene fluorination reduces aromaticity due to a decrease in the number of π ring conjugations [16].
Analysis of the ion series of the mass spectra of fluorobenzene, 1,2-difluorobenzene, 1,2,4-trifluorobenzene, hexafluorobenzene and its isomers, makes it possible to trace the corresponding changes in the number of ion series and the nature of their fragmentation. Replacing one hydrogen atom of benzene with a fluorine atom (Fig. 4) leads to an increase in the number of series of its mass spectrum from seven series to thirteen.
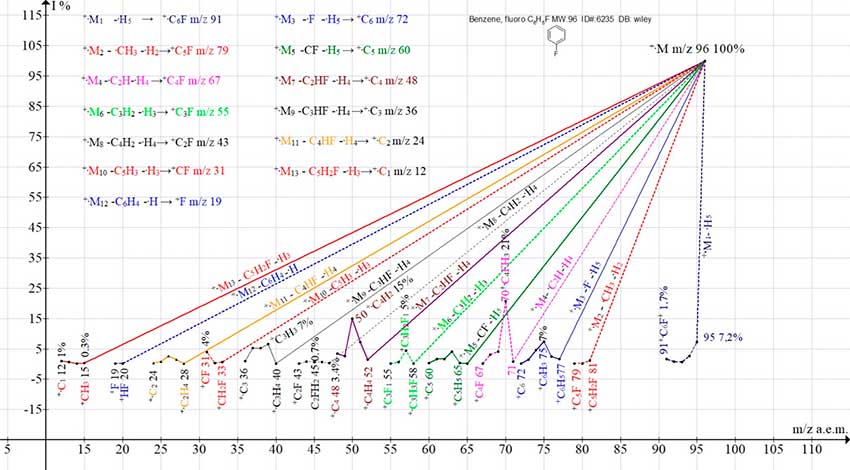
Figure 4. Thirteen series of ions of the mass spectrum of fluorobenzene C6H5F ID#: 6235 DB: wiley_nist98.
Six ionic series of benzene (series 3, 5, 7, 9,11,13), ending with six carbon ions (+C6,+C5,+C4, +C3, +C2,+C1) are preserved in the spectrum of fluorobenzene (Fig. 4).Seven more new fluorine-containing ion series appear, ending with six fluorocarbon ions: +C6F m/z 91 (1.7%), +C5F m/z 79 (0.1%), +C4F m/z 67 (0.1%), +C3F m /z 55 (0.2%), +C2F m/z 43 (0.1%), +CF m/z 31 (3.9%) (series 1, 2, 4, 6, 8,10) (Fig. 4). The seventh series of the spectrum is series (12) - the detachment of the C6H4 molecule with the formation of the +HF ion (0.1%).
Replacement of one hydrogen atom of benzene by fluorine does not disrupt the π‑conjugations of the +.C6H5F radical-cation. However, compared to the mass spectrum of benzene, the peak intensities of +CH3, +.C2H4, +C3H3, +C4H4 ions in the spectrum of fluorobenzene decrease by 6, 3, 2, and 10 times. The only exception is the +C5H3 ion peak, whose intensities are the same (4%) in both spectra.
Replacing two hydrogen atoms of benzene with fluorine (Fig. 5) leads to an increase in the number of series of fluorine-containing ions to nine and a decrease in the number of series of hydrocarbon series of ions to four. Total number of ion series in the spectrum 1,2-difluorobenzene, equal to thirteen, is retained, as in the spectrum of fluorobenzene.
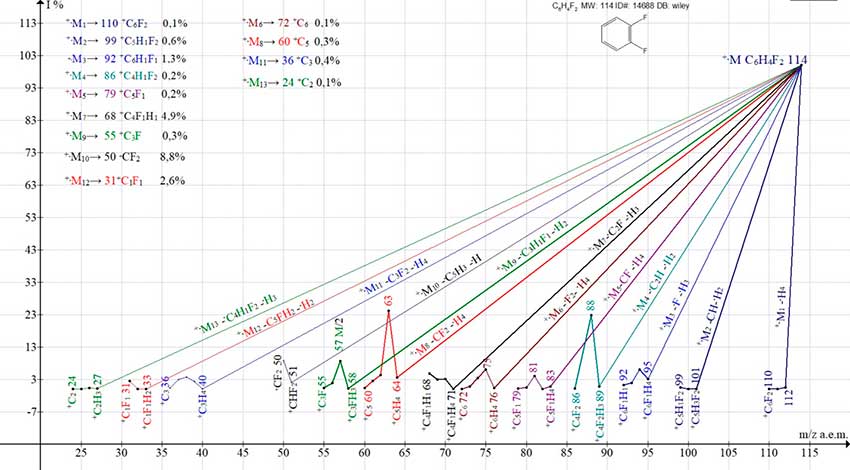
Figure 5. Thirteen series of ions in the mass spectrum of 1,2-difluorobenzene C6H4F2 ID#: 14688 DB: wiley_nist98.
The possibility of π-conjugations between the four CH bonds of the six-membered ring of difluorobenzene is retained. However, π-conjugation of hydrogen- and fluorine-substituted carbon atoms of 1,2-difluorobenzene probably also takes place.
The substitution of three hydrogen atoms of benzene for fluorine leads to a decrease in the number of the formed series to 8, while series 3, 4 and 5 branch (Fig. 6).
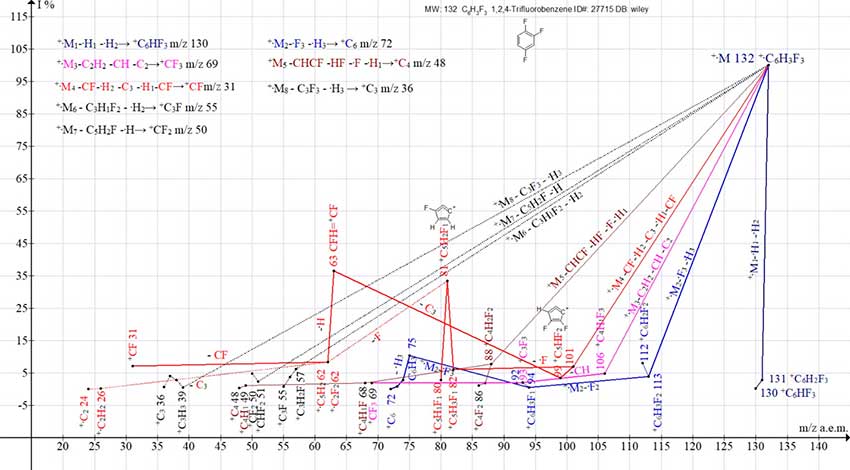
Figure 6. Eight series of mass spectrum ions of 1,2,4-trifluorobenzene C6H3F3 ID#: 27715 DB: wiley_nist98.
In contrast to the mass spectrum of benzene, which consists of six independent, non‑intersecting and non-branching ion series, in the spectrum of hexafluorobenzene their number is reduced to two series (Fig. 7).
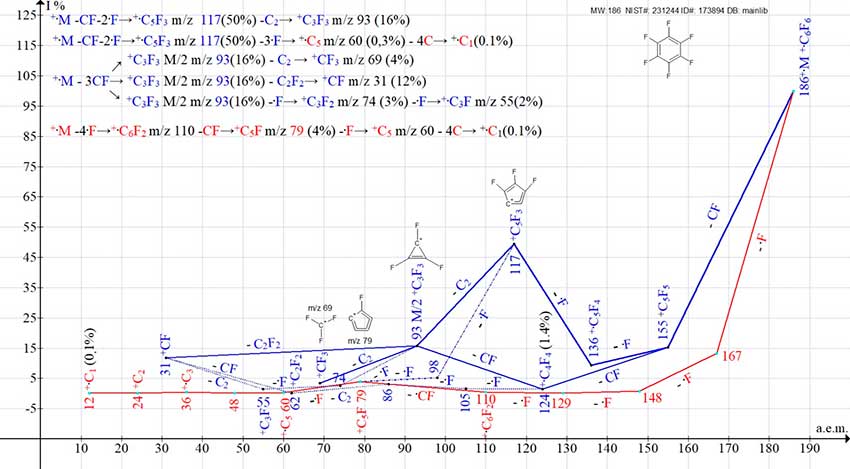
Figure 7. Two series of mass spectrum ions of hexafluorobenzene C6F6 MW: 186 NIST#: 231244 ID#: 173894 DB: mainlib.
One of the two series, starting with the detachment of four .F atoms (marked, in red in Fig. 7), ends with the +.C5 ion, which fragments by successive detachments of carbon atoms. It resembles the first series of benzene ions, formed as a result of the successive detachment of six hydrogen atoms.
Another, more intense and branched series (marked in blue) begins with the detachment of fluorocarbine CF. Subsequent alternating detachments of fluorine and CF atoms lead to the formation of rearrangement ions +C5F3 m/z 117, +C3F3 m/z 93, and +CF3 m/z 69.
The fragmentation of this series is irregular, since the same ion, such as +C5F5, fragments both with the .F detachment and with the CF detachment. During the fragmentation, the detached particles and radicals are replaced. This series, marked in blue, branches into two series, which then merge and then branch again.
Unlike the six ionic series of benzene (Fig.1), the two ionic series of hexafluorobenzene (Fig. 7) do not agree with any of the possible rearrangements of the π-conjugations of the six-membered ring.
The series, fragmenting by four successive detachments of fluorine atoms (marked in red in Fig. 7) is consistent with the presence of π-conjugations of the six-membered cycle +.С6F6, but is not the result of their rearrangement.
Unlike the unstable C6H6 Dewar benzene, C6F6 Dewar benzene is stable. Its mass spectrum (Fig. 8), as well as the mass spectrum of hexafluorobenzene, consists of two ion series that branch and intersect.
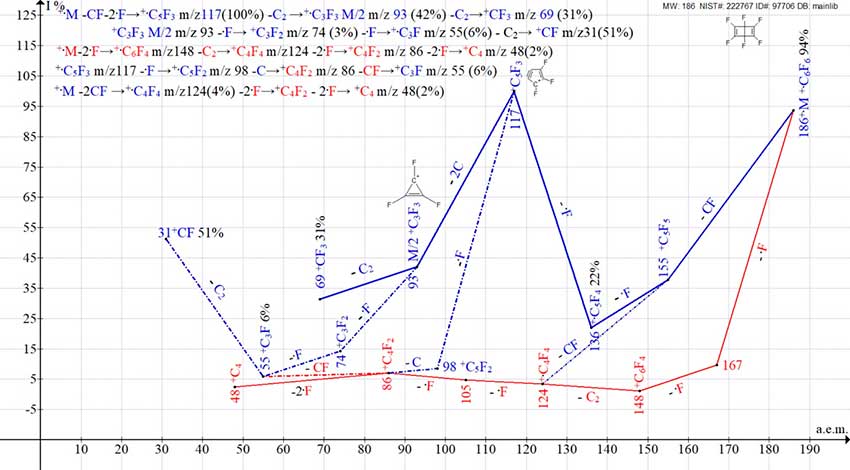
Figure 8. Two mass spectrum ion series of 1,2,3,4,5,6-hexafluoro-bicyclo[2.2.0]hexa-2,5-diene (Benzene Dewar) C6F6 MW: 186 NIST#: 222767 ID#: 97706 DB: mainlib.
The presence in the 1,1,1,6,6,6-hexafluoro-2,4-hexadiine molecule (Fig. 9) of two terminal trifluoromethyl groups adds successive fragmentation detachments .F and CF2, as well as CF2 and .F to the scheme of its decomposition.
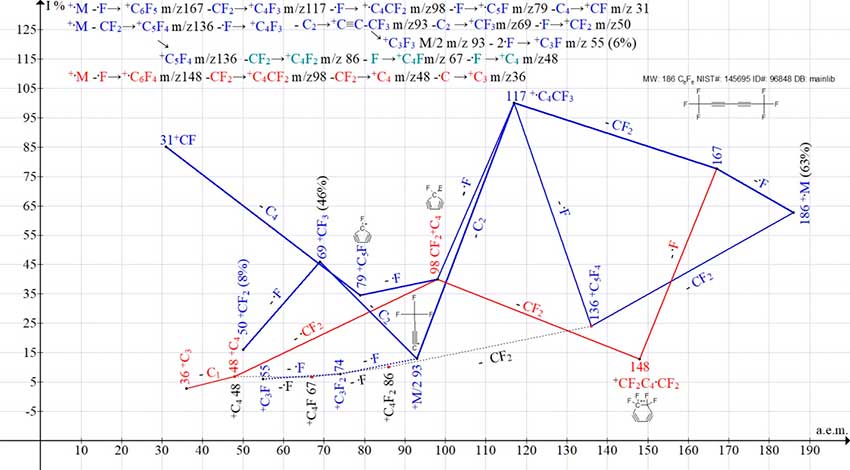
Figure 9. Two mass spectrum ion series of 1,1,1,6,6,6-hexafluoro-2,4-hexadiine C6F6 MW: 186 NIST#: 145695 ID#: 96848 DB: mainlib.
In the spectrum (Fig. 9), the base peak is the +C4CF3 m/z 117 +.M -.F –CF2 peak. Due to the π-conjugation of the diinyl groups, the peak intensity of the +C3F3 M/2 m/z 93 ion does not exceed 13%.
Conclusion
An analysis of the ionic series of benzene, annulenes (CH)n n= 8 and 18 allows us to conclude that, in the excited state,+.M aromatic benzene and [18]-annulene, as well as non-aromatic cyclooctatetraene (CH)8, fragment in the same way. All possible variants of the rearrangement of π‑conjugations of carbon atoms are realized in their spectra.
The replacement of one hydrogen atom by a fluorine atom in the benzene molecule, leads to the appearance of seven new fluorine-containing ionic series, which confirms the presence of π‑conjugations not only between CH groups, but also between CH and CF groups. In the mass spectrum of 1,2-difluorobenzene, the number of hydrocarbon ion series decreases from six to four series. Further substitution of hydrogen atoms for fluorine atoms leads not only to a decrease in the amount of series of hydrocarbon ions, but also to a decrease in the number of fluorine-containing series.
In the mass spectrum of hexafluorobenzene, one of the two formed ionic series arises as a result of successive abstractions of four fluorine atoms and CF, which is consistent with the π‑conjugation of at least 4 carbon atoms of its cycle.
Another branching series of hexafluorobenzene ions arises as a result of alternating detachments of fluorine and CF atoms, which do not confirm the rearrangement of π-conjugations of the carbon atoms of the cycle and the appearance of new π-conjugations.
The difference between the primary detachment groups in the spectra of benzene: .H, CH, .C2H, C3H3, C4H2, .C5H3 and hexafluorobenzene: .F and CF namely, the absence of detachments of .C2F, C3F3, C4F2, and .C5F3, contradicts the analogy of their fragmentation paths. indicates the impossibility of rearranging the existing π-conjugations +.M hexafluorobenzene.
Acknowledgments
This work was supported by the Ministry of Science and Higher Education of Russian Federation, using scientific equipment of INEOS RAS Molecule Structure Study Center.
References
- Kagramanov N.D., Fragmentation sequences - ion series of mass spectra of n-alkyl halides, α,ω-dihaloalkanes, α,ω-dihaloperfluoroalkanes, Fluorine notes, 2022, 1(140), 5-6.
- Scott L.T., Jones M. Jr., Rearrangements and interconversions of compounds of the formula (CH)n, Chemical Reviews, 1972, 72(2), 181-203.
- Gorelik M.V., The current state of the problem of aromaticity, Uspekhi khimii, 1990, 59(2), 197-228. (in Russian)
- Minyaev R. M., Minkin V.I., Gribanova T.N., Orbital stabilization of superstresses D3d of benzene conformation, Doclady AN, 2003, 393(3), 345-348. (in Russian)
- Rodionova E. V., Tomilin O. B., Fomina L. V., and Rodin E. A., Study of thermal transformations of benzene valence isomers, 2018, collection of scientific articles Ed. Ivanovo state. University. named after N.P. Ogaryov. (in Russian)
- Ward H. R. and Wishnok J. S., J. Amer. Chem. Soc., 1968, 90, 1085.
- Criegee R. and Schr.o.der G., Agew. Chem., 1959, 71, 70.
- Franklin J. L. and Field F. H., J. Chem.Phys., 1953, 21, 2082.
- Pryor William A., Henderson R.W., J. Amer. Chem. Soc., 1970, 2, 7236-7238.
- Meyerson Seymor, Perry W. O., Beynon J. H., Baitinger W. E.; at all J. Amer. Chem. Soc., 1970, 92(24), 7236-7238.
- Franklin J. L. and Carroll S.R., J. Amer. Chem. Soc., 1969, 22, 5940-5946.
- Shchukarev S. A., Lectures on the general course of chemistry, Volume II, Publishing house of the Leningrad University, 1964, 443 pages, p. 365 (in Russian)
- Desirant, Y., Bull. Acad. Belg. Cl. Sci., 1955, 41, 759.
- Dibeler, V. H., Reese, R. M. and Mohler, F. L., J. Chem. Phys., 1957, 26, 304.
- Advances in fluorine chemistry, Vol. 2, London, Butterworths, 1961, Majer J. R., Mass spectrometry of fluorine compounds, Fluorinaed Benzenes, 83-92.
- Torres-Vega J. J., Vasquez-Esinal A., Ruiz L., Fernandez-Herrera M. A., Alvarez-Thon L., Merino Gabriel, and Tiznado William, ChemistryOpen, 2015, 4, 302-307.
ARTICLE INFO
Received 28 April 2022
Accepted 16 May 2022
Available online June 2022
Recommended for publication by PhD M. Manaenkova
eLIBRARY Document Number (EDN) RSJOOJ

Fluorine Notes, 2022, 142, 5-6
