Received: March 2022
DOI 10.17677/fn20714807.2022.03.01
Fluorine Notes, 2022, 142, 1-2
PREPARATION OF ORGANOCLAY BASED ON 1Н,1Н,13Н‑TRIHYDROPERFLUOROTRIDECAN-1-OL AND MONTMORILLONITE
S. V. Kudashev1, Z. M. Daniel1, F. Dauda1, V. N. Arisova1, A. I. Bogdanov1, V. F. Zheltobryukhov1, A. A. Fofonova2, E. A. Matushkin3
1 Volgograd State Technical University 400005 Russia, Volgograd, Lenin ave. 28
e-mail: kudashev-sv@yandex.ru
2 Volgograd State Medical University, 400131 Russia, Volgograd, Fallen Fighters sq. 1
3 Volgograd State Socio-Pedagogical University, 400005 Russia, Volgograd, Lenin ave. 27
Annotation. Organoclay was obtained by modification of layered montmorillonite aluminosilicate with 1Н,1Н,13Н-trihydroperfluorotridecan-1-ol. X-ray diffraction at small scattering angles was used to evaluate the possibilities for intercalation of polyfluorinated alcohol molecules into intralayer spaces of clay, and the elemental composition of samples was studied by energy-dispersive X-ray microanalysis.
Keywords: polyfluorinated alcohol, fluoropolymers, montmorillonite, composites, immobilization, organoclay, structure, elemental analysis.
Introduction
The bibliography describes in sufficient detail the features of creating compositions based on organoelement compounds (nitrogen-, phosphorus-, sulfur-, silicon- and organoboron substances) and layered fillers (montmorillonite, saponite, kaolin, vermiculite, graphite, etc.) for their subsequent use as modifying additives in polymers [1]. Films, filaments and monolithic products, including compositions of these compounds, show improved properties than the initial polymer.
Organophilization of montmorillonite clay (MMТ) by immobilization of various compounds provides the increase in affinity to organic polymer matrix [2–4]. When choosing methods for modifying MMТ, the nature of distribution of organoelement component at the mineral surface, in its intralayer spaces, as well as the stability of formed structures plays a significant role. There is only odd bits of information about using of organofluorine (including poly- and perfluorinated) compounds as clay modifiers (fluoroalkanes, fluoroalkylammonium salts, sodium fluoroalkylsulfonate) [1].
Modification of layered aluminosilicate (MMТ) by polyfluorinated telomer alcohols H(CF2CF2)nCH2OH (n = 1–5), which can delaminate in polymer matrix to single monolayers with thickness of about 1 nm, opens up the new prospects for creation of fluorine-containing composite materials with improved set of properties [4, 5]. In [6–14], the features of macromolecules intercalation for a number of heterochain polymers into intralayer spaces of MMТ with immobilized polyfluorinated alcohol, as well as the subsequent disintegration of clay particles to single monolayers, leading to a partial release of polyfluorinated alcohol molecules into polymer matrix volume and a resulting chemical and physical-chemical processes with its participation in the macromolecular system. It is of practical interest to further obtain new modifying MMT based additives and polyfluorinated alcohols with a degree of telomerization n > 5.
The purpose of this paper is to obtain a new MMT based organoclay and 1Н,1Н,13Н‑trihydroperfluorotridecan-1-ol (n = 6), to study its structure and elemental composition.
Experimental part
We used sodium MMT with specific surface area of 595 m2/g (by water) and cation exchange capacity of 100 meq/100 g (LLP “B-Clay”, Kazakhstan). Polyfluorinated alcohol with a degree of telomerization n = 6 (m.p. 129–130°C) was provided by CJSC “PiM Invest” (Dolgoprudny, Moscow reg.). The mass content of main substance in the sample was 90%.
The clay was modified with polyfluorinated alcohol by ultrasonic dispersion (frequency 40 kHz) of weighed equal portions in isopropanol (with analytical grade) at 70°C for 1 h. Then the solvent residues were evaporated from the clay and the product was dried at 50°C under vacuum. The amount of modifier absorbed by this clay was judged by gravimetric method. The content of 1Н,1Н,13Н-trihydroperfluorotridecan-1-ol in the modified MMT was 61.5 wt. %
Diffractograms within the scattering angles range 2θ = 0–8° were recorded at room temperature via Bruker D8 Advance automated diffractometer (reflection in Bragg-Brentano mode, CuКα radiation, λ = 1.5418 Å, Ni-filter). The experimental diffraction patterns were processed using Diffrac.Eva and Topas software packages. Qualitative and quantitative elemental analysis of clays was carried out via Versa 3D DualBeam (FEI) scanning electron microscope equipped with attachment for energy dispersive X-ray microanalysis.
Discussion of results
Increased acidity of 1H,1H,13H-trihydroperfluorotridecan-1-ol, associated with negative I‑effect of fluorine atoms, leads to partial displacement of polysilicic and carbonic acids from salts, as well as the reaction of metal oxides, that make up the mineral basis of clay, with this alcohol, contributing to formation of polyfluorinated alcoholates [H(CF2CF2)6CH2O]yMx (where M = Na, K, Ca, Mg, etc.) and “fixation” of modifier both at the surface of mineral and in its interlayer spaces (irreversibility of alcohol sorption at clay). Another possible “retaining” factor is formation of H‑bonds network between fluorine and hydrogen atoms of ~Si(Al)–OH groups, which abound at the surface of layered aluminosilicate:
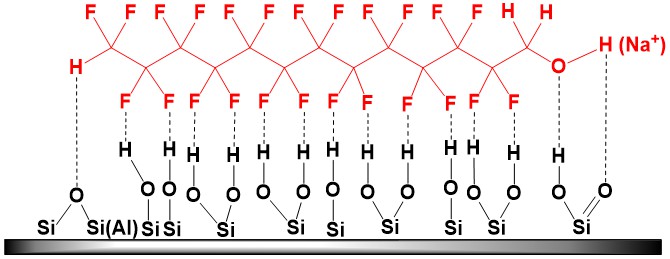
Accumulation of polar polyfluorinated alcohol in the layered aluminosilicate causes a shift in basal reflection to the region of small angles with characteristic increase in the interplanar spacing (see Table below). This shift is associated with processes of intercalation of alcohol-telomere molecules in MMT clay galleries and formation of hydrophobic organomineral complexes.
Table 1. Change in values of interplanar distance (d001) and intensity of reflection (I) in clay samples according to X-ray diffraction at small scattering angles
|
Parameter |
Sample |
|
|
initial clay |
organoclay |
|
|
d001, nm |
1,25 |
2,90 |
|
I, cps |
73,70 |
15,80 |
Using of energy dispersive X-ray microanalysis made it possible to establish the polyelement composition of organoclay (see Fig. 1 below). Sulfur in the original aluminosilicate is present in the form of microscopic scattered plates of gypsum (0.03 at.%), sulfide sulfur is absent. The content of some elements (calculated as oxides) in MMT is as follows (in wt. %): 3.80 (Na2O), 2.25 (MgO), 16.57 (Al2O3), 53.72 (SiO2), 1.08 (K2O) and 3.03 (Fe2O3).
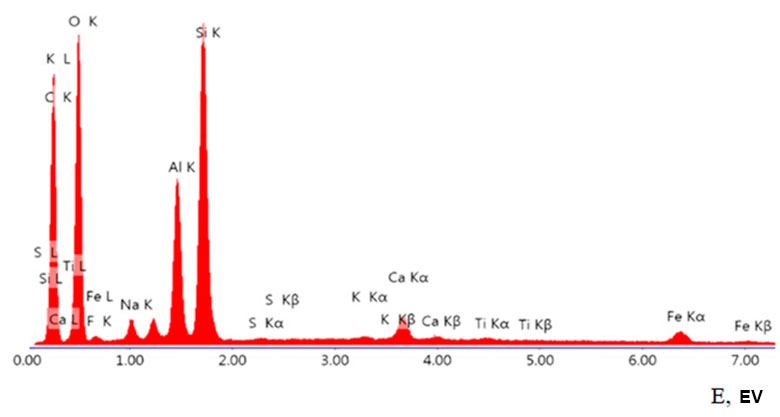
Figure 1. Elemental analysis of organoclay (where E is binding energy).
Conclusion
Modification of MMТ by polyfluorinated alcohol (n = 6) leads to its intercalation into intralayer spaces of this layered aluminosilicate and formation of organomineral structures, which will increase the compatibility of this fluorine-containing filler with polymer matrix, and also facilitate the formation of composite intercalation and exfoliation morphologies.
References
- Methods of introducing poly– and perfluorinated fragments in to a macromolecular system (Review), S. V. Kudashev, Fluorine notes, 2020, 3(130), 3-4. DOI: 10.17677/fn20714807.2020.03.02. – URL: http://ru.notes.fluorine1.ru/journals/by_issue/130.
- Modification of Na+-montmorillonite with mono- and bis(polyfluoroalkyl) phthalates, S. V. Kudashev, V. F. Zheltobryukhov, O. A. Barkovskaya, V. M. Dronova, K. R. Shevchenko, Russian Journal of Applied Chemistry, 2013, 86(7), 1010-1015.
- Hydrophobic and organophilic properties of polyfluoroalkyl-oligo-ε-caproamide as a Na+‑montmorillonite modifier, N. A. Rakhimova, S. V. Kudashev, Russian Journal of General Chemistry, 2011, 81(2), 369-373.
- Organophilization of Na+-montmorillonite with polyfluorinated alcohols, N. A. Rakhimova, S. V. Kudashev, Russian Journal of Applied Chemistry, 2010, 83(11), 2035-2040.
- Structure of a Composite Material Based on Polyfluorinated Alcohol and Montmorillonite, S. V. Kudashev, Yu. M. Shulga, Russian Journal of Physical Chemistry A, 2018, 92(10), 1953‑1958.
- Features of Structural Transformations and Properties of Polycaproamide Modified with Polyfluorated Alcohol Immobilized on Montmorillonite, S. V. Kudashev, I. А. Zvereva, М. V. Chislov, V. M. Shapovalov, A. M. Valenkov, N. V. Kuznetsova, Russian Journal of Applied Chemistry, 2020, 93(6), 854-860.
- Reducing the Combustibility of Polycaproamide Using a Mixture of Polyelemental Flame Retardants, S. V. Kudashev, V. M. Shapovalov, A. M. Valenkov, V. N. Arisova, A. I. Bogdanov, V. F. Zheltobryukhov, Fibre Chemistry, 2020, 51(5), 346–349.
- X-Ray Structure and PMR Spectra of Polycaproamide Modified by a Polyfluorinated Alcohol, S. V. Kudashev, N. А. Zhuravlev, V. M. Shapovalov, T. A. Denisova, A. M. Valenkov, V. F. Zheltobryukhov, Fibre Chemistry, 2019, 51(1), 9-13.
- Study of Ozone Aging of Fluorine-Containing Polydienurethane Elastomers, S. V. Kudashev, V. P. Medvedev, O. O. Tuzhikov, Protection of Metals and Physical Chemistry of Surfaces, 2019, 55(2), 359-362.
- Composites of Reduced Flammability Based on Amorphous Elastic Polyurethane and Halogen-containing Flame Retardant, S. V. Kudashev, V. P. Medvedev, Russian Journal of Applied Chemistry, 2018, 91(3), 483-486.
- Fluorine-Containing Polyamide Composites with Reduced Combustibility, S. V. Kudashev, V. N. Arisova, Protection of Metals and Physical Chemistry of Surfaces, 2018, 54(6), 1165-1168.
- Investigation of Supermolecular Structure of Fluorine-Containing Polyethyleneterephthalate Monofibers, S. V. Kudashev, T. E. Sukhanova, P. N. Yakushev, V. V. Rоdaev, V. M.Vasyukov, V. N. Arisova, A. I. Bogdanov, Fibre Chemistry, 2018, 50(1), 19-23.
- Production and Properties of Fluorine-Containingpolyester Composite Monofilaments, S. V. Kudashev, V. V. Rоdaev, V. M. Vasyukov, V. N. Arisova, A. I. Bogdanov, T. I. Danilenko, V. F. Zheltobryukhov, Fibre Chemistry, 2018, 4(5), 327-329.
- Production of fluorine-containing composite materials based on polydieneurethane and montmorillonite, S. V. Kudashev, A. V. Nistratov, V. N. Arisova, T. I. Danilenko, V. F. Zheltobryukhov, International Polymer Science and Technology, 2018, 45(1), 11-14.
ARTICLE INFO
Received 20 March 2022
Accepted 31 March 2022
Available online June 2022
Recommended for publication by PhD V. Don
eLIBRARY Document Number (EDN) QNBVYD

Fluorine Notes, 2022, 142, 1-2
