Received: March 2022
DOI 10.17677/fn20714807.2022.02.01
Fluorine Notes, 2022, 141, 1-2
X-RAY DIFFRACTION STUDY OF MONTMORILLONITE MODIFIED WITH TETRAFLUOROPHTHALIC ACID AND ITS ANHYDRIDE
S. V. Kudashev1, Z. M. Daniel1 F. Dauda1, V. N. Arisova1, A. I. Bogdanov1, V. F. Zheltobryukhov1, A. A. Fofonova2E. A. Matushkin3
1Volgograd State Technical University,400005 Volgograd, Lenin av., 28.
e-mail: kudashev-sv@yandex.ru
2Volgograd State Medical University, 400131 Pavshikh Bortsov sq., 1.
3Volgograd State Socio-Pedagogical University, 27 Lenin Avenue, Volgograd, Russia, 400005
Abstract: Structure of montmorillonite modified with tetrafluorophthalic acid and its anhydride was studied by X-ray diffraction analysis at small- and wide diffraction angles. X-ray phase analysis established the composition of initial clay, including 98 % by weight of montmorillonite. It was shown that immobilization of tetrafluorophthalic acid and tetrafluorophthalic anhydride is accompanied by formation of organomineral structures, both at the surface and in the interlayer spaces of aluminosilicate.
Keywords: tetrafluorophthalic anhydride, tetrafluorophthalic acid, organofluorine compounds, fluoropolymers, montmorillonite, immobilization, structure.
Introduction
One of the ways to develop polymeric materials with improved set of properties is the creation of fluorine-containing composites containing organomineral components [1–4]. Using of layered montmorillonite clays (MMT), which are characterized by particle shape anisotropy (as carriers of organofluorine compounds of various chemical structures [5]), opens up the fundamentally new possibilities for obtaining fluorinated materials. As a rule, the appending of poly-, perfluorinated compounds and MMT into macromolecular systems is accompanied by increase in thermal stability, wear resistance and level of physicomechanical characteristics of resulting composites [1–5], and also by decrease in its flammability and gas permeability.
Formation of organophilic layers, i.e. organophilization of clay, will facilitate the penetration of polymer macromolecules into its interlayer galleries by increasing the distance between layers [6]. Herewith, the features of immobilization at MMT tetrafluorophthalic acid and its anhydride, as well as the structural studies of these organoclays, are not described in the literature. The presence of reactive groups in the composition of these compounds determines the possibility of their further chemical transformations with appending of immobilized forms of these substances at the stage of synthesis or processing of polyamides, polyesters and polyurethanes.
The purpose of this paper is to study MMТ, modified with tetrafluorophthalic acid and tetrafluorophthalic anhydride using small- and wide angle X-ray diffractometry.
Experimental part
We used sodium MMT with specific surface area of 595m2 g-1 (by water) and cation exchange capacity of 100 mg-eq/100 g (LLP «B-Clay», Kazakhstan). Tetrafluorophthalic acid (m.p. 150–155°С) and tetrafluorophthalic anhydride (m.p. 94–96 °С) were provided by P&M-Invest Company (Dolgoprudny, Moscow reg,). The mass content of main substance in the samples was 97%.
Clay modification by fluorinated compounds was carried out by means of ultrasonic dispersion (at frequency40 kHz) in analytical grade cyclohexanone medium at 50 °C for 2 h. Structural studies of vacuum-dried samples were carried out at room temperature via Bruker D8 Advance automated diffractometer (by Bragg-Brentano reflection method, CuКα, radiation, λ = 1.5418 Å, Ni-filter). The experimental diffraction patterns were processed using «Diffrac.Eva» and «Topas» software packages. Diffraction patterns were recorded for air-dried samples of initial MMT and two organoclays containing respectively, 5% wt. acid and its anhydride.
Registration of diffraction patterns of initial MMT at wide angle range (in Debye-Scherrer geometry, transmission method) was carried out at a wavelength of X-ray radiation 1 Å (RDE «Kurchatov Institute», station «Structural Materials Science» of Kurchatov Synchrotron Radiation Source). The crystalline standard Na2Al2Ca3F14 (a = 10.24 Å) was used as a calibrant of angular scale.
Discussion of results
Using X-ray phase analysis (in transmission mode), it was revealed that X-ray pattern of initial clay is represented by a significant amount of crystalline phases (in wt. %): 98 - ММT, 1.2 - gypsum, 0.3 - quartz and silica modifications, 0.2 - calcite, 0.2 - phosphates, 0.1 - feldspars. Characteristic line with a pronounced asymmetry 23.3 (d = 4.46 Å), observed earlier by the authors in [7], refers to MMT phase and corresponds to intralayer ordering of layered aluminosilicate structure. Its shape indicates a two-dimensional nature (there is no orientational ordering between adjacent layers of aluminosilicate structure).
In passing from initial MMT to clay samples containing immobilized tetrafluorophthalic acid and its anhydride, two types of changes appear at the diffraction patterns (in reflection mode), see Table 1 below.
Table 1. Indices (hkl), interplanar distances (d) and diffraction intensity (I, %) of reflections for clay samples (at wide diffraction angle range)
|
Indices hkl |
Clay modifier |
|||||
|
None |
Tetrafluorophthalic acid |
Tetrafluorophthalic anhydride |
||||
|
d, Å |
I, % |
d, Å |
I, % |
d, Å |
I, % |
|
|
(003) |
5,00 |
61 |
4,48 |
55 |
4,45 |
50 |
|
(110) |
4,47 |
32 |
4,42 |
30 |
4,37 |
27 |
|
(004) |
3,75 |
31 |
3,61 |
30 |
3,60 |
24 |
|
(005) |
3,01 |
60 |
3,00 |
54 |
2,57 |
50 |
|
(200) |
2,59 |
40 |
2,56 |
38 |
2,50 |
34 |
|
(006) |
2,49 |
75 |
2,45 |
71 |
2,40 |
70 |
|
(007) |
2,15 |
15 |
2,10 |
10 |
2,00 |
9 |
|
(008) |
1,85 |
20 |
1,80 |
13 |
1,76 |
10 |
|
(009) |
1,67 |
30 |
1,61 |
27 |
1,60 |
20 |
|
(060) |
1,50 |
28 |
1,47 |
21 |
1,42 |
20 |
First of all, the lines of MMT structure, initially observed at diffraction angle range 2θ = 15–75, are shifted for organoclays towards smaller angles (Δ2θ = 1–3). This shift is associated with processes of intercalation of tetraphthalic acid molecules and its anhydride into interlayer spaces of this clay. Another strong change in diffraction patterns is associated with appearance of a peak near 2θ = 21 (d = 4.94 Å), due to formation of separate phase by tetrafluorophthalic acid and its anhydride (including multicenter proton-donor and proton-acceptor interactions with functional groups of aluminosilicate plates [6, 8]), both at MMT surface and its interlayer spaces. The intensity of this component is maximum (I = 45%) in the case of organoclay containing immobilized tetrafluorophthalic anhydride (I = 18% for MMT modified with tetrafluorophthalic acid).
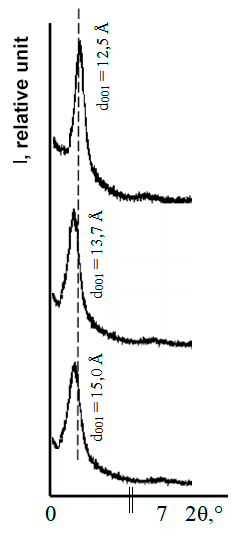
According to small-angle diffractometry data (see Fig. 1 below), at a fixed content of organofluorine compound (5% wt.) in clay, increasing in interplanar distance between aluminosilicate layers (according to position of small-angle basal reflection d001) is maximum for MMT containing tetrafluorophthalic anhydride. These changes (including some increasing in asymmetry of small-angle reflection line) are associated with partial chemical interaction of carboxyl groups of tetrafluorophthalic acid with mineral base of clay (metal oxides, displacement of polysilicic acids from salts) [6], which hinders the further penetration of molecules of this acid into interlayer spaces of MMT.
|
|
Figure 1. Small angle diffraction patterns for clay samples: a - initial MMC; b - contains the tetrafluorophthalic acid; c - contains the tetrafluorophthalic anhydride. |
Conclusion
Aggregative data analysis of small- and wide angle diffractometry (d001 values, diffraction intensity, position of reflections and line shapes) indicates that chemical structure of organofluorine modifier determines its ability to intercalate into MMT interlayer space. The immobilization of tetrafluorophthalic acid at the clay is accompanied by its predominant chemical interaction with MMT mineral basic to formation of corresponding salts, which leads to a lower d001 value (in comparison with d001 value for organoclay containing immobilized tetrafluorophthalic anhydride).
References
- Features of Structural Transformations and Properties of Polycaproamide Modified with Polyfluorated Alcohol Immobilized on Montmorillonite, S. V. Kudashev, I. А. Zvereva, М. V. Chislov, V. M. Shapovalov, A. M. Valenkov, N. V. Kuznetsova, Russian Journal of Applied Chemistry, 2020, 93(6), 854-860.
- Reducing the Combustibility of Polycaproamide Using a Mixture of Polyelemental Flame Retardants, S. V. Kudashev, V. M. Shapovalov, A. M. Valenkov, V. N. Arisova, A. I. Bogdanov, V. F. Zheltobryukhov, Fibre Chemistry, 2020, 51(5), 346-349.
- Study of Ozone Aging of Fluorine-Containing Polydienurethane Elastomers, S. V. Kudashev, V. P. Medvedev, O. O. Tuzhikov, Protection of Metals and Physical Chemistry of Surfaces, 2019, 55(2), 359-362.
- Investigation of Supermolecular Structure of Fluorine-Containing Polyethyleneterephthalate Monofibers, S. V. Kudashev, T. E. Sukhanova, P. N. Yakushev, V. V. Rоdaev, V. M.Vasyukov, V. N. Arisova, A. I. Bogdanov, Fibre Chemistry, 2018, 50(1), 19-23.
- Methods of introducing poly- and perfluorinated fragments in to a macromolecular system (Review), S. V. Kudashev, Fluorine notes, 2020, 3(130), 3-4. DOI: 10.17677/fn20714807.2020.03.02. – URL: http://en.notes.fluorine1.ru/journals/by_issue/130.
- Modification of Na+-montmorillonite with mono- and bis(polyfluoroalkyl) phthalates, S. V. Kudashev, V. F. Zheltobryukhov, O. A. Barkovskaya, V. M. Dronova, K. R. Shevchenko, Russian Journal of Applied Chemistry, 2013, 86(70), 1010-1015.
- Structure of a Composite Material Based on Polyfluorinated Alcohol and Montmorillonite, S. V. Kudashev, Yu. M. Shulga, Russian Journal of Physical Chemistry A, 2018, 92(10), 1953‑1958.
- Rakhimova, N. A. Hydrophobic and organophilic properties of polyfluoroalkyl-oligo-ε-caproamide as a Na+-montmorillonite modifier, N. A. Rakhimova, S. V. Kudashev, Russian Journal of General Chemistry, 2011, 81(2), 369-373.
ARTICLE INFO
Received 20 March 2022
Accepted 31 March 2022
Available online April 2022
Recommended for publication by PhD A. Tyutyunov
eLIBRARY Document Number (EDN) EDOPGR

Fluorine Notes, 2022, 141, 1-2