Received: January 2022
DOI 10.17677/fn20714807.2022.01.03
Fluorine Notes, 2022, 140, 5-6
FRAGMENTATION SEQUENCES - ION MASS SPECTRA SERIES n-ALKYLHALGENIDES,
α, ω-DIHALOALKANES, α, ω-DIHALOPERFLUOROALKANES.
EFFECT
OF TERMINAL HALIDE MASS AND CHAIN MOLECULAR WEIGHT
N. D. Kagramanov
A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 119991, GSP-1, Moscow,
B-334, Vavilov St. 28
e-mail: ndkagram@gmail.com
Abstract: In the mass spectra of eicosane C20H42, 1-bromo eicosane and 1,20-dibrom eicosane (from the NIST libraries), the decay sequences were established - six and, accordingly, eight ion series. The mass spectra of α,ω-dichloro, -dibromo and -diiodo perfluoroalkanes consist of four ionic series. With an increase in the molecular mass of homologues of n-alkyl halides and α,ω‑dihaloalkanes, a decrease in the intensities of the +C4H8Hal and +C5H10Hal peaks is the result of a decrease in the stabilizing ability of the halide atom mass, which is distributed over the entire chain from +.M to +.C4H8Hal. Terminal halide atoms in the alkyl and perfluoroalkyl chains stabilize the series of ions from +.M to fragment ions, the peaks of which have maximum intensities and minimum masses. The minimum masses of fragments stabilized by halides have similar values: 56 and 70 Da (C4H8, C5H10) in alkyl and 50 and 100 Da (CF2, C2F4) in perfluoroalkyl chains. Using the examples of the mass spectra of α,ω-dihalobutanes and α,ω-dihaloperfluorobutanes, it was confirmed that the peak intensity of the ion formed during the detachmnent of the terminal halide is directly proportional to the mass of the detached halide and inversely proportional to the mass of the alkyl or perfluoroalkyl chain. In a series of gradually increasing fragment ion peaks of the mass spectra of n-alkanes and cycloalkanes, all the penultimate peaks have maximum intensities, both because of the optimal mass-energy ratios, and the increasing possibility of cyclization or rearrangement. With decreasing chain length, the probability of cyclization or rearrangement of a linear ion increases. On the other hand, in the spectra of α,ω-dihaloperfluoroethanes, due to the close arrangement of terminal halide atoms and mutual stabilization of masses, their fragmentation differs from the fragmentation of higher homologues. For a graphical assessment of the energy redistributions of ions and neutral ethylene molecules occurring during fragmentation, a method of fictitious ethylene intensities is proposed. In a significant range of masses, the graph of ethylene intensities, its symmetric and consistent growth, mirrors the graph of the intensities of alkyl ions. With an increase in the peak intensities of alkyl ions, the corresponding intensities of ethylene, as well as their polarity, change nonsymbatically, which gives information about the formation and final decays of rearrangement ions. The plots of fictitious ethylene detachment rates in the mass spectra of eicosane and 1-bromo pentacosane allow us to conclude that the base alkyl ion +C4H9 m/z 57 - is a rearrangement tertiary ion +C(CH3)3, which, upon the final detachment of ethylene, again rearranges into a linear ion.
Keywords: ionic series of spectra, n-alkanes and n-alkyl halides, regular fragment groups (C2H4)n, (CF2)n, effect of the mass of the detached radical and the chain mass, successive increase in peak intensities, intense peaks completing fragmentation, method of fictitious ethylene intensities.
Introduction
Primary detachments, fragmentation algorithms and subsequent processes occurring in the mass spectra of n-alkanes, n-perfluoroalkanes, n-carboxylic acids, cycloalkanes, perfluoropolycycloalkanes were previously reported in [2-4].
In the article [1] devoted to the effect on the fragmentation of alkyl halides and α,ω‑dihaloalkanes of the mass of the detached radical and the molecular mass of the homologues, the analysis of the spectra was carried out without considering their ionic series. This did not allow us to draw more substantiated conclusions regarding the reasons for the decrease in the peak intensities of halogen-containing ions with an increase in the molecular mass of homologues, and to explain the effect of the mass of the detached halide and the mass of the chain on the peak intensity of the resulting ion. In the present research, by examples of C20 homologues, all ionic series of fragmentation of eicosane, 1-bromo eicosane, 1,20-dibromo eicosane are considered in detail.
Experimental part
Samples of C4-C10 α,ω-dibromoperfluoroalkanes, the spectra of which are not available in the NIST libraries, were provided by SIA P&M-Invest. Their mass spectra of ionization by electrons were recorded on a Finnigan Polaris Q chromatomass spectrometer in the mass range 30-700 Da, at an energy of 70 eV (capillary column Rtx-5MS 5% diphenyl / 95% dimethylpolysiloxane 30m, 0.25 mm inner diameter, max. Temp. 350C).
Ionic series of eicosane, 1-bromo nonane, 1-bromo eicosane, 1-bromo pentacosane, 1,20-dibromo eicosane
Eicosane spectrum, C20H42 MW 2820 NIST #: 341441 ID #: 25337 DB: mainlib includes six ion series. The detachment order, in the form of 6 fragmentation sequences, is shown in Fig. 1. Primary and subsequent detachments, occurring in six series, culminate in the formation of the 6 most intense ion peaks (m/z 57, 71, 41, 55, 56 and 70) (Fig. 1).
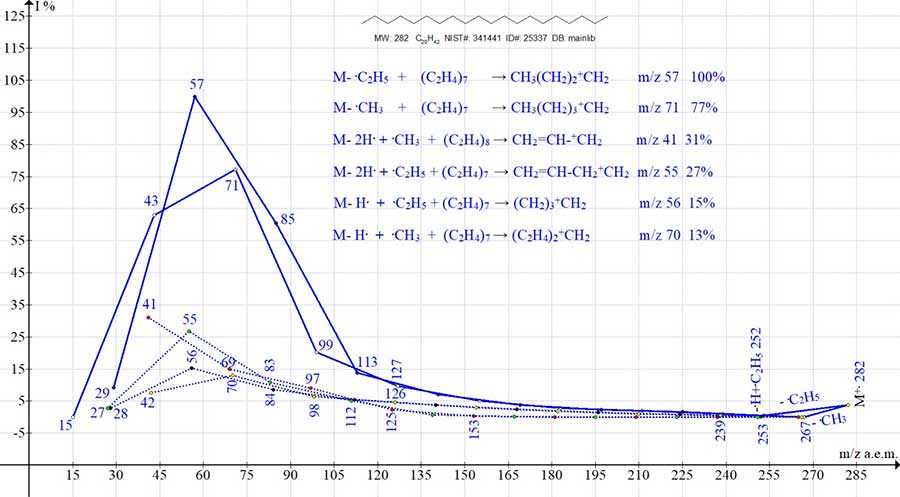
Figure 1. Six series of eicosane mass spectrum ions C20H42 MW: 282 NIST #: 341441 ID #: 25337 DB: mainlib. The two main alkyl series are marked with solid lines. Four less intense series are marked with dashed lines.
Of the six ion series of eicosane C20H42, the most intense are the two main alkyl series of peaks arising from the elimination of methyl and ethyl radicals and subsequent emissions of ethylene. Their most intense peaks are: the base peak +C4H9 with m/z 57 (multiple of the ethylene and ethyl group), as well as the peak +C5H11 with m/z 71 (77%) (multiple of two ethylene groups and a methyl group). Two less intense series of alkenyl peaks result from the abstraction of two hydrogen atoms from one of the terminal groups C2H5 M+. with the formation of a stabilizing vinyl group. The detachment of methyl and ethyl radicals from the opposite terminal group C2H5 and subsequent emissions of ethylene lead to two alkenyl series, ending with peaks of medium intensity +C3H5 with m/z 41 (31%) and +C4H7 with m/z 55 (27%). The two least intense series of olefin peaks are formed upon the detachment of a hydrogen atom from one of the terminal groups CH3 +.M, the detachment of a methyl (ethyl) radical from the opposite terminal group C2H5, and subsequent emissions of ethylene. The most intense peaks of the olefin series are: peak +C4H8 with m/z 56 (15%) (multiple of two ethylene groups) and peak +C5H10 with m/z 70 (13%) (multiple of ethylene and propylene groups). The spectrum of 1-bromo eicosane C20H41Br MW: 360 NIST #: 232983 ID #: 24229 DB: mainlib (Fig. 2) includes eight ion series. Six alkyl series leading to intense peaks with m/z 57, 43, 55, 41, 56 and 70 a.m.u. (blue graphs), and two bromoalkyl series with intense peaks m/z 135 and 149 (red graphs).
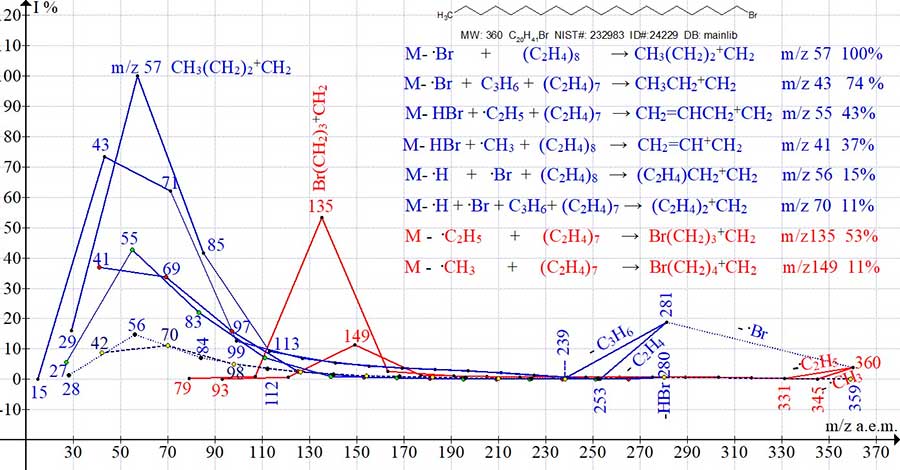
Figure 2. Eight series of 1-bromo eicosane mass spectrum ions C20H 41Br MW: 360 NIST #: 232983 ID #: 24229 DB: mainlib.
The two most intense series of alkyl ions ending in peaks with (m/z 57 and 43) and two series of bromoalkyl ions ending in peaks with (m/z 135 and 149), are the result of two parallel fragmentations, alkyl and bromoalkyl, starting from opposite terminal groups of the molecular radical cation. The remaining four series of peaks: two alkenyl (m/z 55 and 41), and two olefin (m/z 56 and 70) are formed in two accompanying processes of alkyl fragmentation because of fragmentation of both terminal groups of the molecular cation radical. When the number of methylene groups is odd up to the most intense peak of the series, as in the case of the alkyl series with a peak of m/z 43, or in the olefin series with a peak of m/z 70, then in these series first there is one detachment of propylene – C3H6, and then successive emissions of ethylene.
The spectrum of 1-bromo nonane C9H19Br MW: 206 NIST #: 9449 ID #: 120721 DB: mainlib (Fig.3) includes eight ion series. Each of the eight series ends with the peak having the maximum intensity in this series (m/z 43, 57, 41, 55, 56, 42, 135, and 149). The two olefin ion series culminate in peaks with m/z 42 and 56.
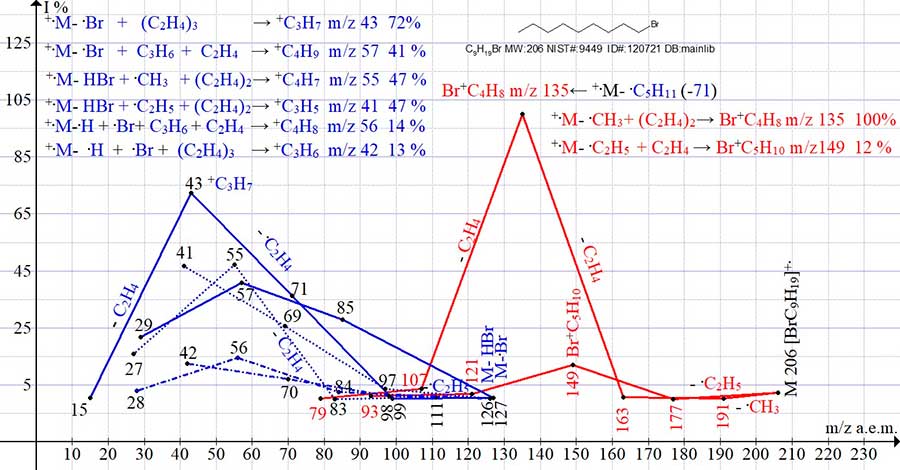
Figure 3. Eight series of 1-bromo nonane mass spectrum ions C9H19Br MW: 206
NIST #: 9449 ID #: 120721 DB: mainlib.
The spectrum of 1-bromo-pentacosane (Fig. 4) includes eight ion series but compared to the spectrum of 1-bromo-nonane (Fig. 3), with an increase in the weight of the alkyl chain by 224 a.m.u., a threefold decrease in intensity occurs in the peak + C4H8Br with m/z 135 and the change of the maximum peak of the alkyl ion +C3H7 (72%) to the base peak +C4H9 (100%).
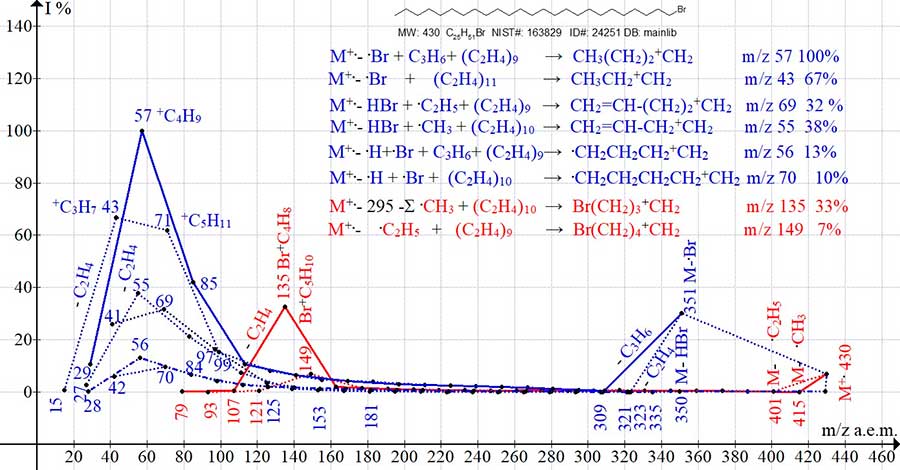
Figure 4. Eight series of 1-bromo pentacosane mass spectrum ions C25H51Br MW: 430
NIST #: 163829 ID #: 24251 DB: mainlib.
The dependences of the intensities (I rel.%) of 4-alkyl and 2-bromoalkyl peaks of the spectra of C1-C34 alkyl bromides homologues to the number of carbon atoms of the alkyl chain (the mass of the alkyl chain) are shown in (Fig. 5.).
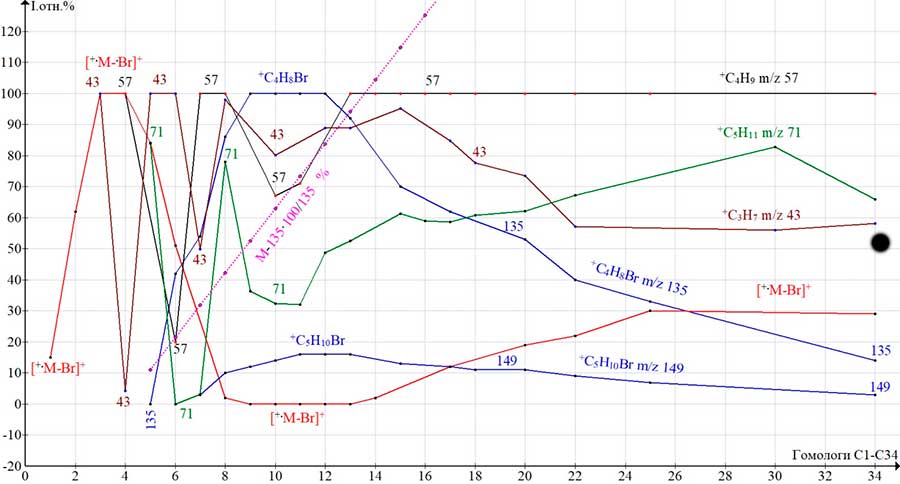
Figure 5. Dependences of the intensities (I rel.%) of the peaks, in the spectra of alkyl bromides on the molecular weight of homologues (C1 –C34).
In the spectra of C3-C8 homologues, the base alkyl ions change. In C3 and C4 (43 - in C3, 57 ‑ in C4) it is the result of the detachment of the .Br atom. In the spectrum of C5, with the abstraction of the methyl radical, a weak peak +C4H8Br m/z 135 (0.2%) is formed. In the C6-C8 spectra, its intensity increases to 86%, while the intensity of the M-Br peaks decreases from 51 to 2.3%. Nevertheless, in the spectra of C7-C8, the peak of the alkyl ion with m/z 57 remains basic. The main fragmentation processes occurring in the C3-C8 spectra are:
+C3H7Br - .Br → +C3H7 m/z 43 (100%)
+C4H9Br. - .Br → +C4H9 m/z 57 (100%)
+C5H11Br - .Br → +C5H11 m/z 71 (84%) - C2H4 → 43 (100%)
+C5H11Br - .CH3 → +C4H8Br m/z 135 (0.2%)
+C6H13Br - .Br → +C6H13 m/z 85 (51%) - (C3H6) → +C3H7 m/z 43 (100%)
+C6H13Br - .C2H5 → +C4H8Br m/z 135 (42%)
+C7H15Br - .Br → +C7H15 m/z 99 (0.2%) - (C3H6) → +C4H9 m/z 57 (100%)
+C7H15Br - .CH3 + C2H4 → +C4H8Br m/z 135 (54%)
+C7H15Br - .C2H5 → +C5H10Br m/z 149 (3%)
+C8H17Br - .Br → +C8H17 m/z 113 (2.3%) - (C3H6) → +C5H11 m/z 71 (78%)
+C8H17 m/z 113 (2.3%) - (C2H4) → +C6H13 m/z 85 (2.3%)
+C6H13 m/z 85 (2.3%) - (C2H4) → +C4H9 m/z 57 (100%)
+C8H17Br - .C2H5 + C2H4 → +C4H8Br m/z 135 (86%)
+C8H17Br - .CH3 + C2H4 → +C5H10Br m/z 149 (10%)
In the spectra of homologues C8-C12 (Fig. 5), the base peak becomes +C4H8Br m/z 135. The intensity of the M-Br peak decreases to 2.3% in C8 and 0.7% in C12. The almost complete cessation of bromine detachment causes a decrease in the peak intensities of the base alkyl ions +C3H7, +C4H9, and +C5H11, since the detachment of .Br is necessary for their occurrence. Starting from the C13 homologue, there is a rather sharp decrease in the intensity of the +C4H8Br peak, which is inversely proportional to the increase in the ratio of the mass of the bromoalkyl chain (M-135 100%) to the mass of its most intense ion with m/z135 (purple graph). The decrease in the intensity of the +C5H10Br peak, which has a large mass, occurs less abruptly. In the spectra of homologues C14-C25, the intensity of the M-.Br peak begins to increase, and +C4H9 again becomes the base peak of the spectrum. When the mass of the alkyl chain (M-135) becomes greater than the mass of the maximum bromoalkyl ion +C4H8Br m/z 135, the intensity of its peak decreases, since the stabilization of the bromine atom extends to the entire alkyl chain from +.M to ion with m/z 135.
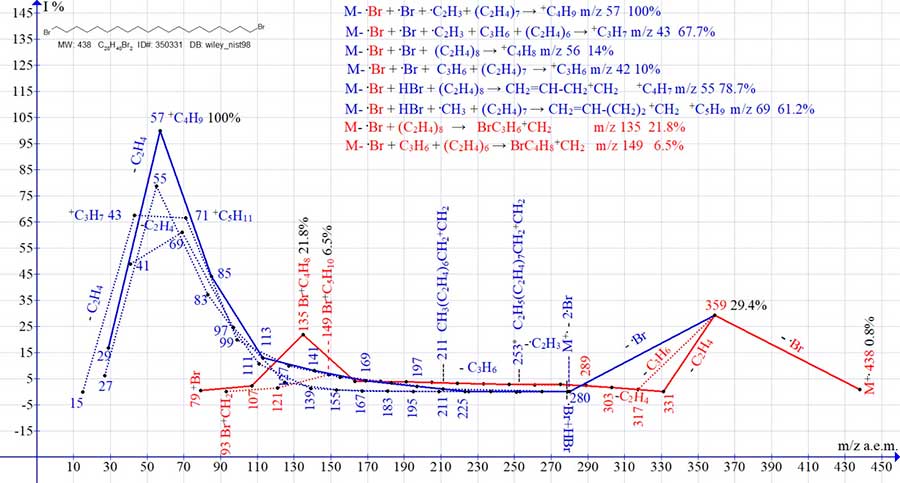
Figure 6. Eight series of mass spectrum ions of 1,20-dibromo eicosane C20H40Br2 MW: 438
NIST #: 163838 ID #: 24717 DB: mainlib.
The spectrum of 1,20-dibromo eicosane (Fig. 6) includes eight ion series, each of which ends with a peak having the maximum intensity in this series (m/z 57, 43, 56, 42, 55, 69 and 135, 149). In both alkyl series of the spectrum of 1,20-dibromo eicosane, after the detachment of two bromine atoms, as in the fragmentation of cycloalkanes [4], the release of the vinyl radical occurs with the formation of a terminal methyl group. In three series of 1,20-dibromo eicosane ions (Fig. 6): in one of the alkyl series (with the maximum peak at m/z 43), in one of the olefin series (with the maximum peak at m/z 42), as well as in one of the bromoalkyl series (with a maximum peak m/z 149) containing an odd number of methylene groups produces propylene emissions. Compared to the mass spectrum of 1-bromo eicosane (Fig. 2) in the spectrum of 1,20-dibromo eicosane (Fig. 6), the intensity of the +C4H8Br peak decreases more than twofold from 53% to 22%. The reason for such a decrease in the peak intensity with m/z 135 is apparently a twofold increase in the probability of detachment of bromine atoms.
Influence of the mass of the opened halide on the intensity of the [+.M -.Hal] peak in the mass spectra of 1,4-dihalobutanes
Modern studies on the effect of the homologues molecular weight on their fragmentation, and the intensity of the formed characteristic, and basic ions are practically absent. The exception is the MS/MS study of peptide fragmentation, in which the authors of [5] found the effect of the peptide mass on the growth and decrease in the number of ions, as well as the intensities of their peaks in series of two different types: -y and -b, formed as a result of peptide chain breaks. The sequence of the removal of alkyl substituents in N-(tert-alkyl)-amines and -acetamides in accordance with the increase in their masses, as well as the increase in the intensity of the ion peaks formed with an increase in the mass of the removed alkyl radicals are described as the "ionic mass effect" and are quantitatively calculated in the work of Zagorsky [6]. In Russian reviews on mass spectrometry, this effect is called the rule of emission of the maximum alkyl radical [7]. Examples of the application of the rule for the cases when the mass of the total detached radical is greater than the mass of the formed ion, apparently, are absent. Influence of the molecular weight of a detached halide atom on the intensity of the resulting +.M - .Hal = +C4H8Hal is confirmed by the spectra of α,ω-dihalobutanes: 1,4-diiodo-, 1,4-dibromo- and 1,4-dichloro- and 1,4-difluoro butanes (Table 1).
Table 1. Intensities of +C4H8Hal peaks in the spectra of α,ω-dihalogenbutanes I % and their % Hal/ m/z values corresponding to the detached masses of halides.
|
Mass spectrum C4H8Hal2 |
Library |
Formula: |
MW and - Hal % |
I % peak +C4H8Hal |
% Hal / m/z I |
|
Butane, 1,4-diiodo- |
ID#:259815 DB:wiley nist98 |
C4H8I2 |
MW 310 - I 41% |
m/z 183 100% |
m/z I 127 100% |
|
Butane, 1,4-dibromo- |
ID#:134901 DB:wiley nist98 |
C4H8Br2 |
MW 214 - Br 37% |
m/z 135 63% |
Br 79 - 62,2% |
|
Butane, 1,4-dichloro- |
ID#:20972 DB#: mainlib |
C4H8Cl 2 |
MW 126 -Cl 28% |
m/z 90 30% |
Cl 35 - 27,6% |
|
Butane,1,4-difluoro- |
No spectrum |
C4H8F2 |
MW 96 - F 20% |
m/z 75 ? % |
F 19 - 15% |
In the spectrum of C4H8I2 (Table 1), with the detachment of radical I., a basic peak with m/z 183 (M-127) is formed. With the detachment of bromine and chlorine atoms, the intensities of the +.M - .Hal peaks (Table 1) decrease to 63% and 30%, respectively. The decrease in intensities occurs in proportion to the molecular masses of the detached .Hal, relative to the mass of the iodine atom taken as 100%.
The peak intensities of the +C4H8Hal ions formed (Table 1) Correspond to the values of the masses of the detached atoms .Hal. Since the detachment of a halogen atom from α,ω-dihaloalkane is the primary process, the energy is +.M sufficient to break any C4H8Hal2 bond. Compared to the effect of detachment of the halide mass, which leads to a decrease in vibrational excitation and an increase in the peak intensity of the formed ion, the difference in the bond energies of the detached atoms of halides, with the exception of fluorine, in the spectra of α,ω-dihalides, does not significantly affect the intensity of the resulting peak.
The choice of the C4 spectra (Table 1) was not made by chance. If in the mass spectrum of BrC4H8Br the detachment of any of the two terminal bromine atoms leads to the appearance of a stable ion +C4H8Br (63%), then in the spectra of C5-C6 dibromoalkanes a certain conflict of interest arises for terminal Br atoms for possessing a large number of methylene groups. The conflict remains unresolved. Instead, the process of detachment +.M -.Br + HBr is enhanced (Fig. 6), which almost completely blocks the formation of the +C4H8Br ion (0,4% - 1,8%). This conflict somewhat weakens in the C7 spectrum, in which the intensity of the +C4H8Br peak is only 16%. The conflict completely ends only in C8, when the peak of the +C4H8Br ion can be formed during the decay of +.M/2, as a result of the detachment of the radical Br(CH2)3CH2+.CH2(CH2)3Br → +C4H8Br + .C4H8Br. However, in this case, the intensity of the +C4H8Br peak in the spectrum of C8, in comparison with C4, decreases by almost a factor of 2 - to 29%. The likely reason for the decrease in the intensity of the +C4H8Br peak is a twofold increase in the possibility of fragmentation of a symmetric compound with the detachment of two bromine atoms.
Influence of the mass of the detached halide on the intensity of the peak [+.M -.Hal] in the mass spectra of 1,4-dihaloperfluorobutanes
A similar effect of the influence of the mass of the detached .Hal radical on the intensity of the resulting fragment ion +.M -.Hal is also observed in the spectra of α,ω-dihaloperfluorobutanes (Table 2).
In the mass spectrum of C4F8I2 (Table 2), with the detachment of the .I radical, a basic peak with m/z 327 (M-127) is formed. With the detachment of bromine and chlorine atoms, the intensity of the +.M - .Hal peak (Table 2) decreases to 62% and 8%, respectively. If the decrease in the peak intensities formed during the detachment of .I and .Br atoms occurs in proportion to their molecular masses, then during the detachment of .Cl and .F atoms, the decrease in the peak intensities occurs by three and, accordingly, five times greater than the expected intensities proportional to the detached masses of .Cl and .F atoms.
Table 2. Intensities of +C4F8Hal peaks in the spectra of dihaloperfluorobutanes I% and their calculated % Hal values corresponding to the detached masses of halides.
|
Mass spectrum C4F8Hal2 |
Library |
Formula: |
MW and M Hal % |
I % peak +C4F8Hal |
% Hal / m/z I |
|
1,4-diiodo-octafluorobutane |
ID#:230290 DB: mainlib |
C4F8I2 |
454 -I (28 %) |
m/z 327 100% |
m/z I 127 100% |
|
1,4-dibromo-octafluorobutane |
Polaris Q |
C4F8Br2 |
358 -Br (22%) |
m/z 279 62% |
Br 79 62,2% |
|
1,4-dichloro-octafluorobutane |
Polaris Q |
C4F8Cl 2 |
270 -Cl (13%) |
m/z 235 8,3% |
Cl 35 27,6% |
|
Perfluoro n-butane |
ID#:36162 DB#: mainlib |
C4F10 |
238 -F (9%) |
m/z 219 2,6% |
F 19 15% |
It should be noted that the effect of the mass of the halide detachment also depends on the mass of the chain (alkyl or perfluoroalkyl). The greater the mass of the chain and the mass of the resulting ion, the less the effect of detachment of the halide atom. In Tables 1 and 2, in the MW column, the values of the masses of the detached halogen atoms are presented in % of the molecular weight of the compounds. The percentage values of the masses of Cl and F atoms in compounds with a perfluoroalkyl chain C4 are 2.2 times less than the percentage values of their masses in compounds with an alkyl chain (Tables 1 and 2). Whereas the separation of the masses I and Br from the perfluoroalkyl chain C4 is only 1.5 and 1.7 times less than the separation from the alkyl chain, respectively. The effect of detachment from the perfluoroalkyl chain of halides with minimum masses (Cl and F) compared to the detachment of halides with maximum masses (Br and I), respectively, should be less. The presented examples of the detachment of Hal atoms from the perfluoroalkyl chain confirm the influence of the mass of the detached radical and the mass of the chain on the intensity of the formed ion.
Bromine atom detachment effects in mass spectra of n-alkyl bromides and α,ω-dibromoalkanes
The effects of the mass of the bromine atom detachment on the intensities of the alkyl and bromoalkyl peaks can be estimated by comparing the spectra of n-alkyl bromide and α,ω‑dibromoalkane containing the same number of carbon atoms.
Compared to the mass spectrum of 1-bromo dodecane in the spectrum of 1,12-dibromo dodecane (Fig. 7), the intensity of the +C4H8Br peak with m/z 135 decreases more than twofold to (42.5%), and the intensity of the +C5H10Br peak with m/z 149 remains almost unchanged.
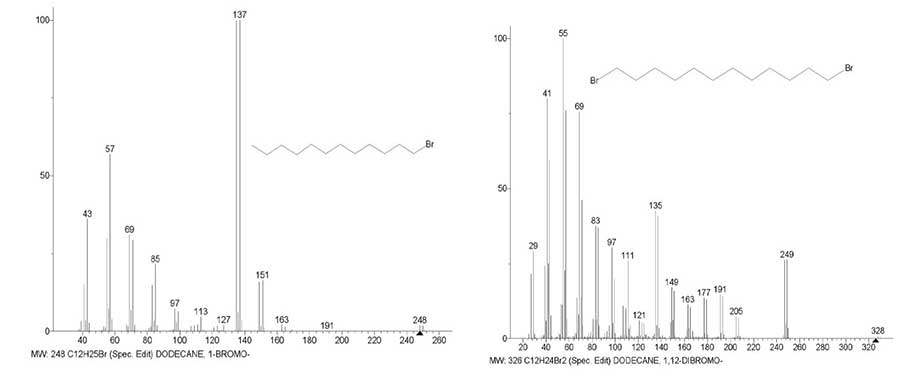
Figure 7. Mass spectra of 1-bromo dodecane C12H25Br MW: 248 ID #: 183675 DB #:
wiley_nist 98 and 1,12-dodecane dibromo C12H24Br2 MW: 326 ID #: 276501 DB: wiley_nist 98.
The weak peak of +C6H12Br with m/z 163 (11.3%) is enhanced, which can appear in the spectrum of C12H24Br2 during the decay of M/2, and bromine-containing peaks appear that did not appear in the spectrum of 1-bromo dodecane. The peak +.M -.Br (26.1%) with m/z 247 acquires the highest intensity. Ions with m/z 135 and m/z 149 are formed in two different ion series, for this reason, a change in the intensity of one of the peaks cannot lead to a change in the peak intensity of another series. In contrast to the asymmetric molecule of 1-bromo dodecane, a twofold decrease in the peak intensity with m/z 135 in the spectrum of 1,12-dibromo dodecane, is the result of a twofold increase in the probability of detachment of the second atom. The twofold decrease in the peak intensity with m/z 135 is probably the result of the opposite direction of the same detachments occurring during the fragmentation of a symmetric molecule, in contrast to the asymmetric molecule of 1-bromo dodecane. Detachments of two bromine atoms occurring in four series of the spectrum (Fig. 6) reduce the probability of the +C4H8Br peak formation and increase the intensities of the series of alkyl and alkenyl ions (Fig. 7). An increase in the intensity of bromine-containing fragment peaks with m/z 247, 205, 191, 177 is the result of the stabilizing effect of the masses of two terminal atoms, which reduce the vibrational excitation of +.M twice as strong as one terminal Br atom.
In contrast to the spectrum of 1,12-dibromo-dodecane (Fig. 7) containing seven bromoalkyl ions, in the spectrum of 1,12-dibromo-hexadecane (Fig. 8) the series consists of eleven bromoalkyl ions, but their intensities are half as much. A decrease in the intensities of halogen-containing peaks with an increase in the chain mass occurs as a result of a decrease in the stabilizing effect of a fixed mass of a halide atom, which is distributed over the entire chain from +.M to +C4H8Hal.
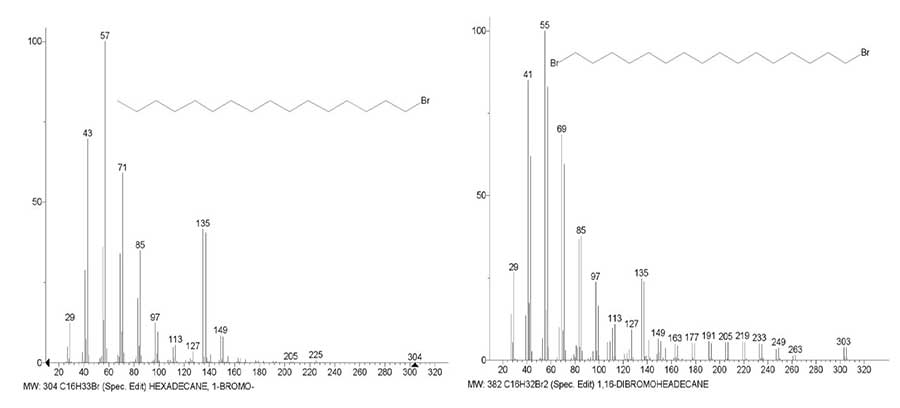
Figure 8. Mass spectra of 1-bromo hexadecane C16H 33Br MW: 304 NIST #: 228693 ID #: 24350
DB: mainlib and 1,16-dibromo hexadecane C16H32Br 2 MW: 382 NIST #: 133768 ID #: 19147
DB: mainlib.
With an increase in the alkyl chain by four methylene groups from C12 to C16, a decrease in the intensity of the peak with m/z 135 (24.7%) in the spectrum of 1,16-dibromo hexadecane as compared to the spectrum of 1-bromo hexadecane (41.6%) (Fig. 8) Is almost the same as in the spectrum of 1,12-dibromo dodecane (Fig. 7).
Ionic series of mass spectra of α, ω-dihaloperfluoroalkanes
Three series of ions: perfluoroalkyl, perfluoroalkenyl and perfluoroolefin of the mass spectrum of perfluoroeicosane NIST #: 239239 ID #: 36518 DB: mainlib were considered by us earlier [2,4]. The mass spectra of α, ω-dihaloperfluoroalkanes consist of four ion series (Fig. 9-12) of perfluoroalkyl (+CF3 m/z 69), perfluoroalkenyl (+C3F5 m/z 131), halogenoperfluoroalkyl (+CF2Hal) and halogenoperfluoroalkenyl series (+C3F4Hal). Ions of the halogenoperfluoroalkenyl series arising from the detachment of a halide atom (Cl or Br) and the emission of two fluorine atoms can have both linear and cyclic structures.
However, the successive detachments of CF2 occurring in the haloalkenyl series are incompatible with its linear structure and the -CF = +CF end group. A consistent increase in the peak intensities of this series, with a maximum at m/z 147 +C3F4Cl (10%) (in the spectrum of C16Cl2F32 MW: 870 Polaris Q), the intensity of the peak with m/z 147 (39%), suggests that all ions except the terminal linear ion CF2=+CCl m/z 97 have a cyclic structure (Fig. 9).
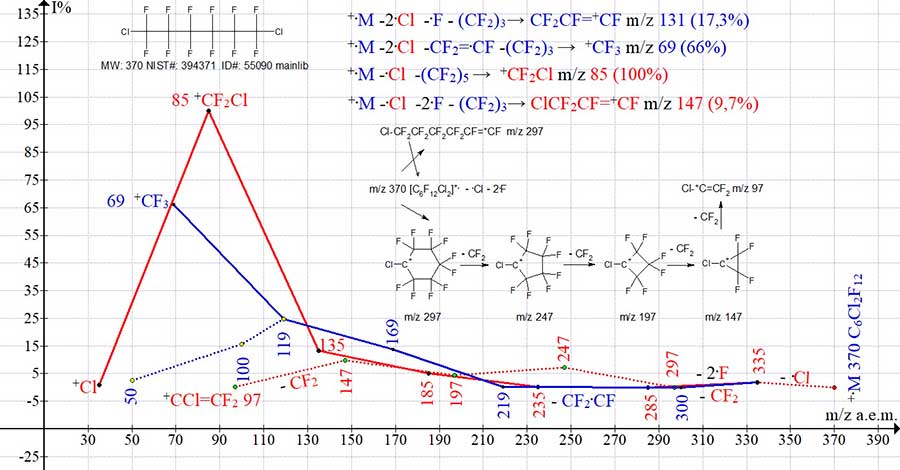
Figure 9. Four series of ions of the mass spectrum of 1,6-dichloro perfluorohexane C6Cl2F12
MW: 370 NIST #: 394371 ID #: 55090 DB: mainlib. Alkenyl series (M-2.Cl - .F- (CF2)n m/z 131, not shown in this figure).
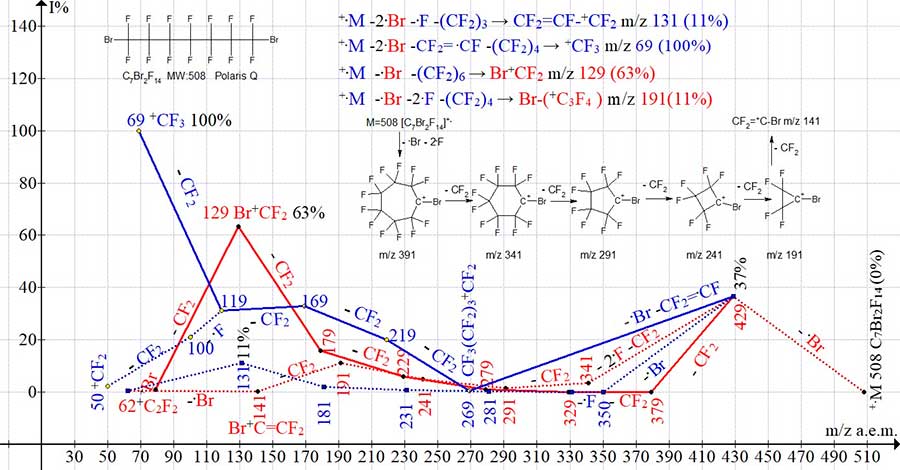
Figure 10. Four series of mass spectrum ions of 1,7-dibromo perfluoroheptane C7Br2F14
MW: 508 Polaris Q.
Similar fragmentation processes occur in the spectrum of 1,7-dibromo perfluoroheptane (Fig. 10), in which the intensity of the peak is +C3F4Br with m/z 191 (11%). However, in the mass spectrum of 1,2-dibromo tetrafluoroethane (Fig. 11), due to the too close arrangement of the terminal bromine atoms and the mutual stabilization of their masses, the detachment of the bromine atom and two fluorine atoms does not occur and the Br+C=CF2 of the haloalkenyl series with m/z 141 is not formed.
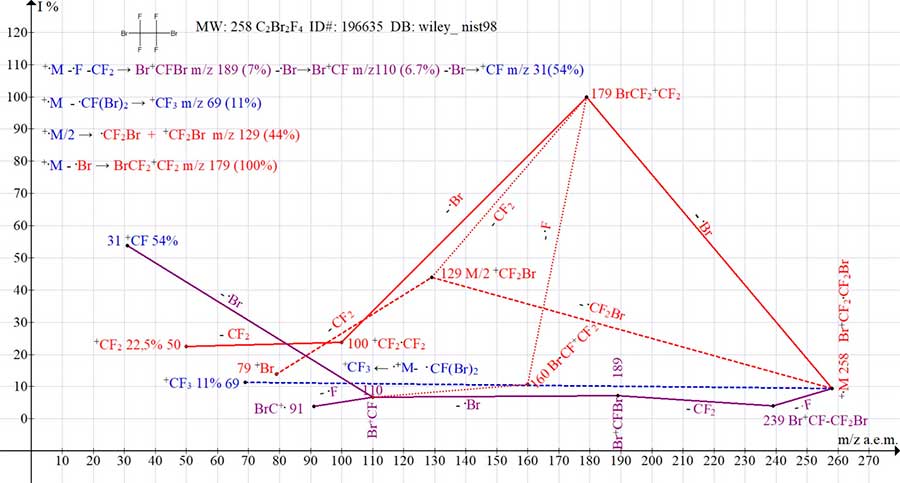
Figure 11. Four series of mass spectrum of 1,2-dibromo -1,1,2,2-tetrafluoroethane C2Br2F4
MW: 258 ID #: 196635 DB: wiley_nist98.
Instead, as a result of the detachment of one fluorine atom and the detachment of CF2, the ion Br-+CF-Br 4% m/z 189 7% appears. And with the abstraction of the Br -.CF-Br radical, the rearrangement ion +CF3 is 11% (Fig. 11). The detachment of a bromine atom from the Br-+CF-Br ion does not lead to an increase in the intensity of the resulting Br-+CF ion, since this is not a primary detachment, but a costly process that destroys the mutual stabilization of the masses of two bromine atoms. However, the final abstraction of the bromine atom with the formation of the +CF ion leads to a sharp increase in the intensity of its peak by 54%.
In the mass spectra of α,ω-dihaloperfluoroalkanes (Fig. 9-12), when the terminal halogen atom is removed, a primary haloalkyl ion is formed, the intensity of which depends on the mass of the halide and the mass of the chain. The maximum intensity is obtained by the peak of the iodine-containing ion +.M-.I (Fig. 12), and the minimum for the chlorine-containing ion +.M-.Cl (Fig. 9), which corresponds to the masses of the separated halides. Fragmentation of the primary halogen-containing ion leads to a sharp decrease in its peak intensity, however, subsequent detachments of CF2 lead to a series of increasing ion peaks, culminating in the most intense +CF2Hal peak. The minimum masses of the alkyl and perfluoroalkyl chains stabilized by halide atoms are close in their value: m/z 56 a.m.u. in the alkyl chain and m/z 50 a.m.u. in the perfluoroalkyl chain, which correspond to the masses of two ethylene (C2H4)2 groups or one CF2.
In the spectrum of the homologue C4F8Br2 (Fig. 13), due to the remoteness of the masses of the C4F8Br2 terminal bromine atoms, the Br-CF2+CFCF2-Br ion with m/z 289 is absent. In the spectrum of dichlorotetrafluoroethane MW:170 C2Cl2F4 ID#:72335 (wiley_nist98) ion Cl +CFCl m/z 101 (13%). But in the spectrum of 1,2-diiodotetrafluoroethane MW: 354 C2F4I2 ID #: 301040 DB: wiley_nist98, the I+CFI ion is not formed. Instead, an intense peak I2 m/z 254 (48%) +.M-C2F4 appears.
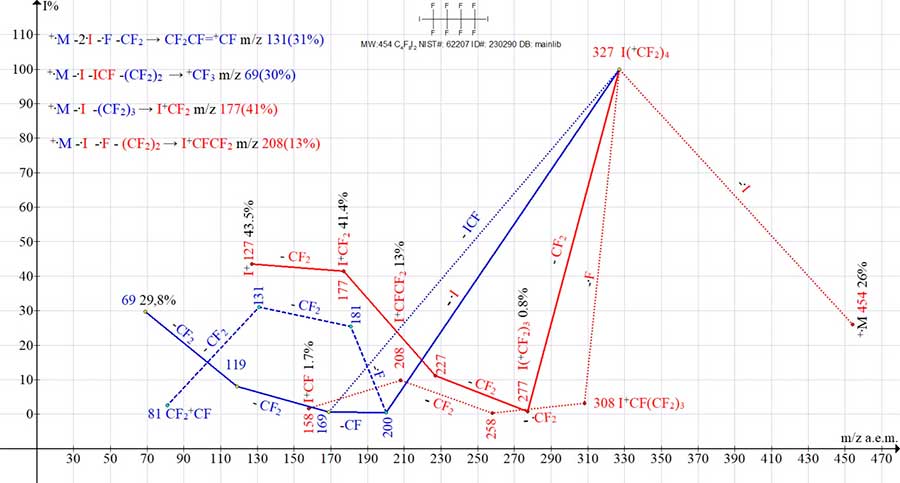
Figure 12. Four series of mass spectrum ions of perfluoro 1,4-diiodoperfluorobutane C4F8I2
MW: 454 NIST #: 62207 ID #: 230290 DB: mainlib.
In the spectrum of 1,4-diiodoperfluorobutane, ion +I2 m/z 254, as well as I+CFI m/z 285 are not formed, and after the detachment of iodine and fluorine radicals, appears a peak ion I+CF(CF2)3 m/z 308 (3%). As a result of the fragmentation of which, a peak with m/z 208 (13%) I+CFCF2 is formed. Due to the mutual stabilizing effect of the masses of the most closely spaced two halide atoms In the mass spectra of 1,2-dihaloperfluoroethanes, fragmentation processes occur, which are not characteristic of the higher homologues C3-C7.
The effect of the detachment of the mass of the bromine atom on the intensities of fragmented perfluoroalkyl and bromoperfluoroalkyl peaks can be estimated by comparing the spectra of 1-bromo nonafluoro and 1,4-dibromo octafluoro butane (Fig. 13), containing the same number of carbon atoms.
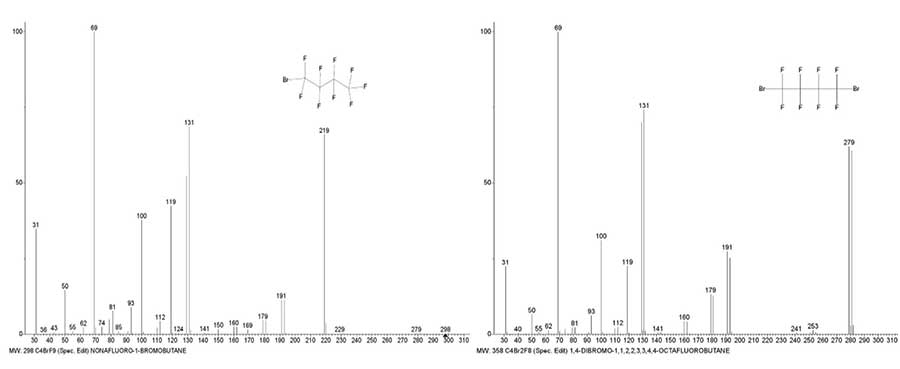
Figure 13. Mass spectra of 1-bromo nonafluorobutane C4BrF9 MW: 298 NIST #: 222723
ID #: 36291 DB: mainlib and 1,4-dibromo octafluorobutane C4Br2F8 MW: 358 Polaris Q.
In the mass spectra of 1-bromo nonafluoro and 1,4-dibromo-octafluoro butanes (Fig. 13) during the primary detachment of the fluorine atom and, accordingly, the bromine atom, the same Br(+CF2)4 ion with m/z 279 is formed. The intensity of the Br(+CF2)4 peak arising upon the detachment of .F is (0.2%), which is 310 times less than the peak intensity upon detachment of the .Br atom (62%). Based only on the ratios of the separated masses Br and F, without considering the inversely proportional effect of the mass of the perfluoroalkyl chain, the peak intensity of the ion with m/z 279 should be 15%. Detachment of the bromine atom from 1-bromo nonafluoro butane and 1,4-dibromo octafluorobutane lead to ions +C4F9 and +C4F8Br, the peaks of which have the same intensity of 64%. In the spectrum of +C4F8Br2, the intensities of the peaks of bromine-containing ions: Br +CF(CF2)2 m/z 191 (27%), +(CF2)2Br m/z 179 (13%), Br+CFCF2 m/z 160 (4%), more than twice the peak intensities of these ions in the spectrum of +C4F9Br. The peak intensities of the Br+CF2 with m/z129 in the spectra (Fig. 13) differ by a factor of 1.3.
The effect of the mass of the perfluoroalkyl chain (inversely proportional) to the effect of the mass of the detached halide is illustrated by the dependences of the peak intensities of the +.M and +.M -.I of perfluoroalkyl iodides presented in (Fig. 14).
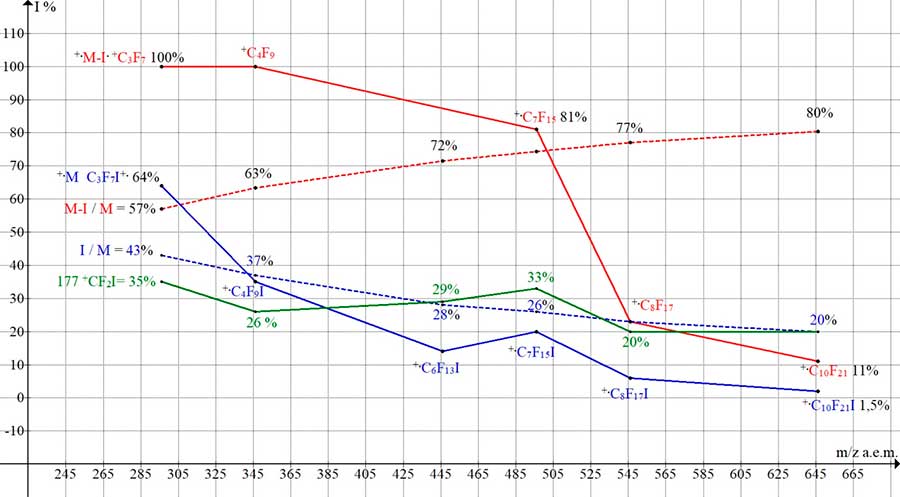
Figure 14. Dependences of the intensities (I rel.%) of the peaks in the spectra of perfluoroalkyl iodides on the molecular weight of homologues (C3-C10 296-646). (C3F7I MW: 296 NIST #: 235506 ID #: 157700; C4F9I MW: 346 NIST #: 376328 ID #: 195259; C6F13I MW: 446 NIST #: 239055 ID #: 36897; C7F15I MW: 496 NIST #: 118317 ID # : 36933; C8F17I MW: 546 NIST #: 238726 ID #: 36511; C10F21I MW: 646 NIST #: 239284 ID #: 36183; DB: mainlib.
With an increase in the molecular weight of n-pefluoroiodide homologues, the intensity of the +.M peak (blue graph) decreases from 64% to 1.5%, in proportion to a decrease in the relative fraction of the mass of the iodine atom (from 43% to 20% in the n-perfluoroiodide molecule. With an increase in the molecular weight of n-pefluoroiodide homologues, the intensity of the +.M-.I peak decreases in proportion to the increase in the weight of the perfluoroalkyl chain. The higher the molecular weight of the perfluoroalkyl chain, the less effect on fragmentation is exerted by the detachment of the iodine atom (red graph and red dotted line). The intensity of the +CF2I m/z 177 fragment ion in the C3-C10 perfluoroalkyl iodides homologues changes insignificantly, from 35 to 20% (green graph).
Gradual growth of peaks of sequentially fragmented ions
Concerning the problem of a gradual increase in the peak intensity of sequentially fragmented ions, in the spectra of eicosane C20H42, hexacontane n-C60H122, cyclotriacontane C30H60, perfluoroeicosane C20F42, it should be noted that we usually do not notice an insignificant, gradual and sequential increase in peaks. The attention of researchers, as a rule, is drawn to the peaks with the maximum intensity, as well as rearrangement peaks of ions. The high intensity of the peaks is explained only by the energy advantage, structural isomerization or cyclization [7]. Indeed, the calculated values of the enthalpies of formation of alkyl ions containing a tertiary carbon atom are 18-30 kcal / mol less than the enthalpies of the corresponding linear ions [8].
Enthalpies of formation ΔHf (g) 298K hydrocarbon ions (kcal /mol), [8, page 199].
|
Alkyl ions |
M/Z |
∆Hf 0 (kcal/mol) |
|
CH3CH2+CH2 +CH(CH3)2 |
43 |
207 189 207-189=18 |
|
CH3CH2CH2+CH2 +C(CH3)3 |
57 |
201 168,5 201- 168,5=32,5 |
|
CH3CH2CH2CH2+CH2 +CH(CH2CH3)2 |
71 |
no data available 172,6 |
Nevertheless, all the most intense alkyl peaks c m/z 99, 85, 71, 57 of n-alkanes are fragmented only by successive C2H4 detachments, which does not fully agree with the assumption about their rearrangements and differs from the high stability of +C(CH3)3 in mass-spectra with notorious tertiary groups. In contrast to the spectra of n-alkanes, consisting of 6 series of peaks (Fig. 1), in the mass spectra of linear alkanes with a terminal group C(CH3)3, the number of main series decreases to three. The main pathways of their fragmentation are: emission of a linear radical with the formation of the peak of the base ion +C(CH3)3, emission of the radical .C(CH3)3 with the formation of an alkyl ion including the entire alkyl chain, as well as detachments of .C(CH3)3 and .H to form an alkyl ion with a terminal vinyl group. A gradual increase in the peak intensities of all fragment ions is difficult to explain by their synchronously increasing structural stabilization, however, with a decrease in the chain length of a linear ion, the probability of cyclizations or rearrangements certainly increases. In two different series of halogen-containing ions of the mass spectra of n-alkyl halides, only two ions +C4H8Hal and +C5H10Hal, completing fragmentation, the peak intensities of which are maximum compared to the intensities of the remaining peaks of linear Hal-containing ions - are rearrangement onium ions, the cyclic structure of which is confirmed experimentally [9].
A gradual increase in the intensities of all sequentially fragmented peaks of n-alkane and cycloalkane ions occurs synchronously with the release of ethylene and the redistribution (transfer) of a part of its kinetic energy (according to the cannon-projectile principle) to a new alkyl ion with a lower mass. That is, in the general case, if we do not consider the cases of rearrangement or cyclization, the peak intensity depends on the ratio of the mass and energy of the formed ion to the mass and energy of the constant separated ethylene group. To describe such a system, which is a molecular radical cation, shooting out with ethylene molecules, one should take into account its two components [10]. First, the speed of movement +.M, - as the speed of movement of the center of inertia of the entire system, as well as the speed of internal movement: - the detached primary radical (or ejected ethylene), and the speed of the newly emerging ion. Accordingly, the energy of the system can be represented as the sum of the kinetic energy +.M (or the energy of any fragment ion as a whole) and kinetic energies of motion of new particles: ion and ethylene, formed as a result of a single act of decay.
In the abstraction of primary radicals with different masses, the excess energy of the molecular cation-radical of n-alkane, which is about 20eV = 460 kcal/mol, is divided in six ways between primary ions and radicals of six corresponding series of fragmentation.
The difficulty in calculating the redistribution of kinetic energy between fragment ions and ethylene is that we know the masses of ions and their peak intensities, but we do not know their velocities. For this reason, it is not possible to perform such a calculation. To assess how the energy redistribution between fragment ions and ethylene occurs during fragmentation, an assessment can be performed in a simplified form, using the values of the intensities.
The intensities of the peaks in the mass spectrum depend on the energy of the ionizing voltage and, at different values of the ionizing voltage, reflect different variants of energy redistribution. Standard conditions for recording mass spectra (70eV) minimize this dependence, but the existing relationship between the energies of ions and the intensities of their peaks is preserved. Since the peak intensity is determined by the ionic current (the number of ions having the same structure and kinetic energy), it is proportional to the energy. The fictitious intensities of ejected neutral ethylene molecules were calculated using the formula for the conservation of momentum [11], in which the peak intensities of the two main alkyl ion series in the mass spectrum of eicosane C20H42 were taken instead of the velocities (2).
+M1I1= +m2 i2 + 28 Ifict. ethylene (2)
In contrast to the changing masses of fragment ions, the mass of ethylene is constant at 28 a.m.u. For this reason, any change in the fictitious intensity of ethylene that does not correspond to the peak intensity of a given alkyl ion indicates the possibility of its rearrangement, cyclization, or decay. The found values of fictitious intensities of ethylene, proportional to the intensities and masses of the consecutively formed ions of the mass spectrum of eicosane, are presented in (Fig. 12). To keep things simple, there are six primary fragmentation options for +.M (Fig. 1), dividing the intensity (energy) +.M six ways between the six removed radicals and the six primary ions are not shown in (Fig. 12). The two upper positive plots (+ red and blue lines) (Fig. 12) are plots of mass - intensities of the two main alkyl ion series after the detachment of .CH3 and .C2H5 and ethylene emissions. They end with an intense ion peak at m/z 71 and a base peak at m/z 57. The lower graphs are graphs of fictitious ethylene intensities (dashed lines), in the mass range from 230 to 113 and 99 a.m.u. symmetric and sequential growth is mirrored, opposite to the growth of the upper graphs of the intensities of alkyl ions. This gives grounds to conclude that there are no rearrangements in this mass range, but only a consistent increase in the ion energy and fictitious intensities is taking place.
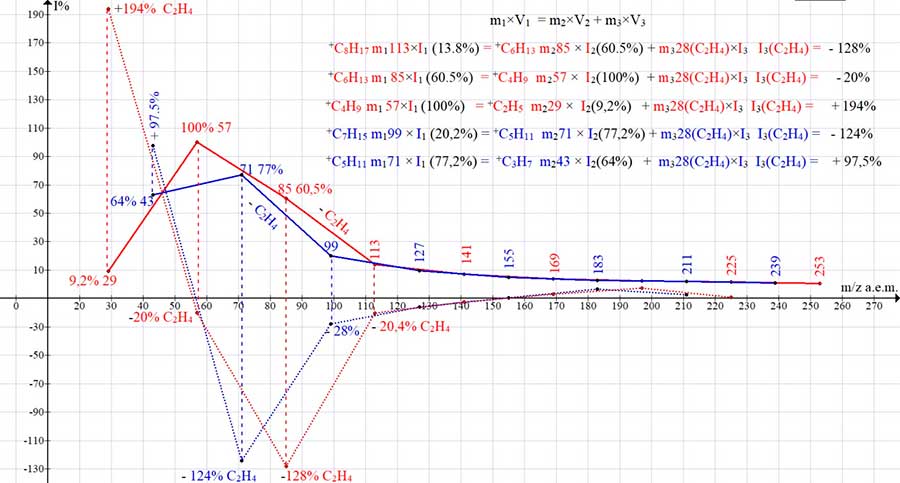
Figure 15. Two series of intensities of sequentially fragmenting alkyl ions of the mass spectrum of
eicosane C20H42 MW: 282 (+ graphs of red and blue) and two series of their fictitious intensities of
ethylene (- dashed graphs of red and blue).
With a mass ratio (cannon-projectile 85:28 = 3: 1), an intense ion peak with m/z 85 (60.5%) corresponds to two times its fictitious ethylene intensity (-128%), which confirms the energetic advantage of the process. However, with the next separation of ethylene and a mass ratio of 57:28 = 2:1, a base peak with m/z 57 arises, and the fictitious ethylene intensity drops sharply to (-20%). The reason for this decrease, which differs from all the previous detachments of ethylene, may be the exothermic effect of rearrangement of a linear ion into a tertiary ion. The final release of ethylene at a mass ratio of 1:1 leads to the formation of a +C2H5 ion with a low intensity (9%), a change in polarity, and an increase in the value of the fictitious ethylene intensity (+194%).
The reason for the polarity change is probably the reverse rearrangement of the tertiary ion +C(CH3)3 m/z 57 into a linear one and its decomposition with the elimination of ethylene. At a mass ratio of 71:28 = 2.5:1, the maximum intensity of the ion +C5H11 m/z 71 (+ 77%) corresponds to the maximum of the fictitious intensity of ethylene +C2H4 (-124%).
The next detachment of ethylene lowers the intensity of the +C3H7 ion peak to 64%. At a mass ratio of 43:28 = 1.5:1, an abrupt change in the polarity of the fictitious intensity of ethylene +98% occurs, which, apparently, is the result of the reverse rearrangement of the symmetric CH3CH2+CHCH2CH3 ion into an asymmetric linear one. Indeed, for the rearrangement of the CH3CH2+CHCH2CH3 ion into the CH3(CH2)3+CH2, with one proton migration, probably requires less energy than rearranging to a linear tertiary ion +C(CH3)3. The ratio of positive values of the fictitious intensities of ethylene for ions with m/z 29 and m/z 43 is 194:98. So, the reverse rearrangement of CH3CH2+CHCH2CH3 requires less energy than rearrangement into a linear ion of the +C(CH3)3 ion.
Another example of a gradual increase and reversal of the polarity of fictitious ethylene intensities in two parallel series of ions of the mass spectrum of 1-bromo pentacosane is illustrated by the graphs of the intensities of its main alkyl series with the base alkyl ion +C4H9 m/z 57 and the main bromoalkyl series with the ion +C4H8Br m/z 135 (Fig. 16). It should be noted that the +C4H8Br ion is a rearrangement ion, the cyclic structure of which has been confirmed experimentally [9].
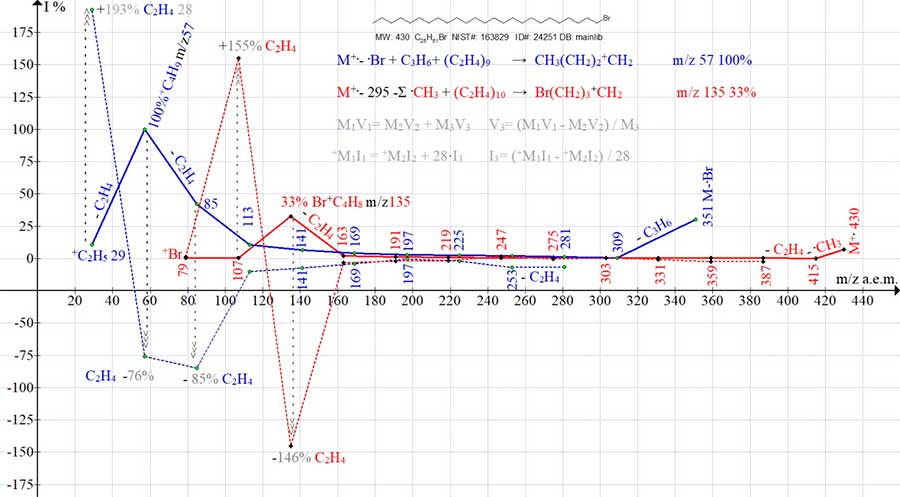
Figure 16. Two series of sequentially fragmented mass spectrum ions 1-bromo pentacosane C25H51Br MW: 430 NIST #: 163829 ID #: 24251 DB: mainlib. (+ a graph of the formation of a red +C4H8Br ion and a blue +C4H9 ion) and two series of their fictitious ethylene intensities (- dashed graphs of red and blue).
With a mass ratio m/z 135: 28 = 4.8:1, the maximum intensity of the ion +C4H8Br m/z 135 (+ 33%) corresponds to the maximum of the fictitious intensity of ethylene +C2H4 (-146%). Unlike all the preceding linear bromoalkyl ions, only the formation of the +C4H8Br ion results in an energy gain due to the cyclization of the ion. However, upon the next separation of ethylene, the intensity of the +C2H4Br m/z 107 ion peak decreases to 0.5%, and the fictitious intensity of the emitted ethylene sharply changes the polarity of +155%. Such a change in polarity corresponds to the expenditure of energy for the rupture of the cyclic ion and the detachment of ethylene from it. Despite the fact, that the ion with m/z 107 has a rather large mass in comparison with the ions of the alkyl series, the next time ethylene is detached from it with the formation of a bromine ion, an energy deficit occurs (+ 1.3%). The values of the fictitious intensities of ethylene (+155 and +193, Fig. 16) during the detachment from the cyclic halonium ion +C4H8Br [9] and the ion +C(CH3)3 confirm that the detachment of ethylene from +C4H8Br requires less energy consumption than for the reverse rearrangement of +C4H9 into a linear ion and the detachment of ethylene from it. It is obvious that the opening energy of the onium ring is certainly less than the energy required for the reverse rearrangement of the tertiary ion +C(CH3)3 into a linear one with the migration of two protons.
Conclusion
Analysis of the mass spectra of eicosane C20H42, 1-bromo eicosane and 1,20-dibrom eicosane established their decay sequences - six and, respectively, eight ionic series. In the mass spectra of α, ω-dihalogen perfluoroalkanes, four ionic series are established. A graphical method of fictitious ethylene intensities is proposed, which makes it possible to distinguish between the mass spectrum zones, where there is a sequential increase in intensities, due to an increase in the energy of ions, and the zones where rearrangements of ions and their decay occur. The plots of fictitious ethylene intensities allow us to conclude that the base ion +C4H9 is the tertiary ion +C(CH3)3, which, upon the final detachment of ethylene, again turns into linear. Ion +C4H8Br - the base ion of homologues C9-C12, differs from the base ion +C4H9 in that it contains a massive bromine atom that stabilizes the entire series of bromoalkyl ions from +.M to +C4H8Br, as well as its weak fragment ions. For this reason, with an increase in the mass of the stabilized alkyl chain, the intensity of the +C4H8Br peak decreases and the probability of bromine atom abstraction increases.
Using the examples of mass spectra of α,ω-dihalobutanes and α,ω-dihalogen perfluorobutanes, it was confirmed that the peak intensity of the ion formed during the detachment of the terminal halide is directly proportional to the mass of the detached halide and inversely proportional to the mass of the alkyl or perfluoroalkyl chain.
Regardless of structural stabilization, an increase in the intensities of all n-alkanes, cycloalkanes, perfluoroalkanes and perfluorocycloalkanes ion peaks, occurs with a decrease in their masses, synchronously with the redistribution of excitation energy, as a result of successive emissions of [C2H4]n or [CF2]n. The minimum masses of the alkyl and perfluoroalkyl chains stabilized by halide atoms, which are close to m/z 56 a.m.u. in the alkyl chain, and m/z 50 a.m.u. in the perfluoroalkyl chain, which correspond to the masses of two ethylene (C2H4)2 groups or one CF2.
In general, the peak intensity depends on the ratio of the ion mass to the mass of the constant detached group. The maximum intensities of the penultimate peaks of alkyl ions completing fragmentation are due to both the optimal ratios of their masses and energies, and the increasing possibility of their cyclization or rearrangement. The presented dependences of the fictitious intensities of ethylene allow us to conclude that +C4H9, as well as +C4H8Hal, is a rearrangement ion.
Acknowledgments
This work was supported by the Ministry of Science and Higher Education of Russian Federation using scientific equipment of INEOS RAS Molecule Structure Study Center.
References
- Kagramanov N. D. and Tyutyunov A. A., Effect of eliminated radical mass and molecular weight of homologs of alkyl halides, α,ω–dihaloalkanes, and α,ω-dibromoperfluoroalkanes on their fragmentation upon electron impact ionization, Fluorine notes, 2019, 5(126), 5-6.
- Kagramanov N.D., Algorithms for fragmentation of n-alkanes and n-perfluoroalkanes, “Fluorine notes, 2020, 1(128), 3-4.
- Kagramanov N.D., A new perspective on the McLafferty rearrangement in the spectra of n-carboxylic acids and their methyl and 2,2,2-trifluoroethyl esters, Fluorine notes, 2020, 5(132), 3-4.
- Kagramanov N.D., A Series of Fragment Ions of Cycloalkanes, Perfluorocyclohexane, Perfluoropolycycloalkanes, Fluorine notes, 2021, 3(136), 3-4.
- Sheila J. Barton and John C. Whittaker Review of Factors that Influence the Abundance of ions produced in a tandem Mass Spectrometer and statistical Methods for discovering these factors. Mass Spectrometry Reviews, 2009, 28, 177-187.
- U. I. Za’horszky, Organic Mass Specrometry, 1979, 14(2), 66-75.
- Lebedev A.T., Mass-spectrometry in organic chemistry, Moscow, Binom, 2003, p. 43-46. (in Russian)
- Takhistov V.V. Organic mass spectrometry. Thermochemical description of isomerization and fragmentation of ions and radicals in the gas phase. Leningrad, Nauka, 1990, 221p., p.199, Table 71. (in Russian)
- Van de Sande, McLafferty F.W. J. Am. Chem. Soc., 1975, 97, 2298-2299.
- Landau L.D., Akhiezer A.I., Lifshitz E.M., Course of General Physics, Mechanics and Molecular Physics Second edition, revised, Moscow, Nauka, 1969, 36, 399p. (in Russian)
- Landsberg G.S., Elementary textbook of physics, volume 1, Mechanics. Heat. Molecular Physics, Moscow, Nauka, 1971, 122-127, 656p. (in Russian)
ARTICLE INFO
Received 10 January 2022
Accepted 26 January 2022
Available online February 2022
Recommended for publication by PhD O. V. Bryzgalova
Fluorine Notes, 2022, 140, 5-6
