Preparation of fluorinated ethers by addition of alcohols to epoxides, interaction of fluoroolefins and alcohols with formaldehyde, transesterification...."
Received: February 2022
DOI 10.17677/fn20714807.2022.01.01
Fluorine Notes, 2022, 140, 1-2
Fluorinated ethers. Communication 3.
Preparation of fluorinated ethers by addition of alcohols to epoxides, interaction of fluoroolefins and alcohols with formaldehyde, transesterification, electrochemical fluorination and a number of other methods.
S.V. Vershilova, V.V. Kornilov, A.S. Tsyrulnikovaa,b, L.M. Popovaa,b, N.V. Lebedeva
aS.V. Lebedev Scientific Research Institute of Synthetic Rubber, Gapsalskaya str. 1, St. Petersburg, 198035, Russia
bPeter the Great St.Petersburg Polytechnic University, Novorossiyskaya str. 48, St. Petersburg, 194021, Russia
Abstract: The following methods of obtaining fluorinated ethers are described in the
final part of the review: the addition of alcohols to epoxides, the interaction of fluorinated olefins
and alcohols with formaldehyde, transetherification, and electrochemical fluorination.
In addition,
a brief description of methods for obtaining fluorinated ethers that are not included in the separate
chapters is given, namely: free radical addition of fluorine-containing alkenes to ethers, fluorination
with elemental fluorine and higher fluorides of metals of variable valence, reactions of alcohols
with ketones and carboxylic acids, reactions of perfluorinated nitrosoalkanes with alcohols and interaction
of alcohols with 2,4,6-tris-(2,2,2-trifluoroethoxy)-[1,3,5]triazine (TriTFET).
Key words: fluorinated ethers, epoxides, formaldehyde, transesterification, electrochemical fluorination, cobalt trifluoride, TriTFE.
Introduction
The most well-known methods for the synthesis of fluorinated ethers have already been considered in the first two parts of the review, namely: Williamson reaction, addition of alkenes and alkynes to alcohols, interaction of fluorinated alcohols with diazomethane, addition of polyfluoroalkyl iodides to alkenes, intermolecular dehydration, addition of perfluoroalkyl hypohalogenites to alkenes, and pyrolysis of derivatives of perfluoro-2-alkoxypropionic acids.
The third part of the review describes the preparation of fluorinated ethers by addition of fluorine-containing alcohols to epoxides, interaction of fluorinated olefins and alcohols with formaldehyde, transesterification, and electrochemical fluorination.
A brief description of methods for obtaining fluorinated ethers that are not included in the separate chapters is given, namely: free radical addition of fluorine-containing alkenes to ethers, fluorination with elemental fluorine and higher fluorides of metals of variable valence, reactions of alcohols with ketones and carboxylic acids, reactions of perfluorinated nitrosoalkanes with alcohols and interaction of alcohols with 2,4,6-tris-(2,2,2-trifluoroethoxy)-[1,3,5]triazine (TriTFET).
1. Addition of alcohols to epoxides
Both reactions of fluorine-containing alcohols with hydrocarbon epoxides and reactions of fluorine-containing epoxides with alcohols can be used for the synthesis of partially fluorinated ethers.
1.1. Interaction of fluorine-containing alcohols with epoxides
The interaction of fluorine-containing alcohols with epoxides in the presence of acidic or basic catalysts leads to the formation of one ether bond like hydrocarbon analogs.
Polyfluorinated alcohols of the general formula RF(CH2)nOH (n ≥ 1) react with ethylene oxide and propylene oxide to form fluorine-containing diol ethers.
In 1949 I.L. Knunyants et al. [2] reported the preparation of ethylene glycol 2-fluoroethyl ether (bp 172–174°C) by heating a mixture of 2-fluoroethanol with ethylene oxide at 170–180°C for 54 h.
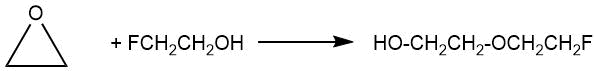
In 1957 M. Brey and P. Tarrant described the addition of polyfluoroalkanols of the general formula RFCH2OH (RF = CF3, C2F5, C3F7) with ethylene oxide at 70°C in the presence of potassium hydroxide in an autoclave [3].
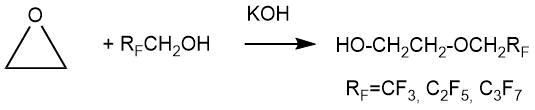
The procedure for the addition of polyfluoroalkanols with ethylene oxide was the following: a cooled solution (5 g of KOH in 1.5 mol of fluorine-containing alcohol) was loaded into a 300 ml rocking autoclave. The autoclave was then cooled with a mixture of dry ice and acetone and then 1 mol of ethylene oxide was downloaded. The mixture was kept for 4 h at 70°C. The product was isolated by distillation. The characteristics of the obtained ethers and their yields are given in Table. 1-1.
Table 1-1.
|
Ether |
Yield, % |
bp °С (mm Hg) |
nD25 |
d425,g/cm3 |
|
CF3CH2OCH2CH2OH |
50 |
84(80) |
1,3502 |
1,2902 |
|
C2F5CH2OCH2CH2OH |
32 |
87 (84) |
1,3370 |
1,3806 |
|
C3F7CH2OCH2CH2OH |
62 |
91-92 (54) |
1,3300 |
1,4965 |
|
CF3(CH3)2COCH2CH2OH |
35 |
92 (77) |
1,3749 |
1,1931 |
|
(CF3CH2OCH2)2CHOH |
19 |
86 (16) |
1,3528 |
1,3890 |
|
(C3F7CH2OCH2)2CHOH |
24 |
112-115 (15) |
1,3338 |
1,5569 |
A procedure similar to that one described by M. Bray and P. Tarrant is given in the book by I.L. Knunyants and G.G. Jacobson [4]. Reducing the loading of reagents by 2 times (for a reactor with a volume of 250 ml), cooling with liquid nitrogen, and evacuating the reactor before loading ethylene oxide made it possible to increase the yield of the desired 2-hydroxyethyl-1',1'-dihydroperfluorobutyl ether to 70%.

V. Grakauskas described the interaction of ethylene and propylene oxides with 2-fluoro-2,2-dinitroethanol in aqueous sodium hydroxide to obtain the corresponding monoethers in 1957 [5].
2-Fluoro-2,2-dinitroethyl 2-hydroxyethyl ether was obtained in up to 68% yield by adding 2-fluoro-2,2-dinitroethanol and ethylene oxide to an aqueous solution of sodium hydroxide at 0÷5°C. The reaction mixture was kept for 16 hours at 0°C.
2-Fluoro-2,2-dinitroethyl 2-hydroxypropyl ether was obtained under similar conditions in about 25% yield. The yield of the target ether rases to 63% as the molar ratio of the starting 2-fluoro-2,2-dinitroethanol to propylene oxide increases to 4:1.
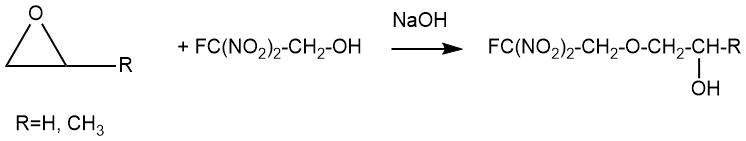
The reaction of 2-fluoro-2,2-dinitroethanol with propylene oxide under acid catalysis (SnCl4) led to the low yields of ether (12%).
The article by A.V. Fokin et al. [6] describes the interaction of 2,2-difluoro-2-nitroethanol (0,15 mol) with ethylene oxide and propylene oxide (0,2-0,5 mol) in 5% aqueous sodium hydroxide (0°C, 16 h ). 2,2-Difluoro-2-nitroethyl-2-hydroxyethyl and 2,2-difluoro-2-nitroethyl-2-hydroxypropyl ethers were obtained in 32 and 67% yields, respectively as a result of these reactions. The formation of diethylene glycol 2,2-difluoro-2-nitroethyl ether (about 4%) was noted in reactions with ethylene oxide.
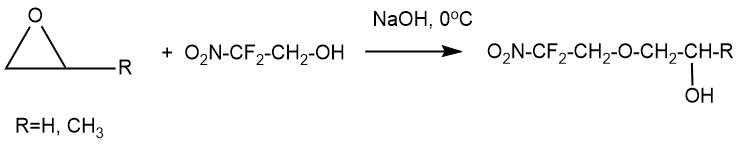
The patents by E. Banitt [7], [8] and R. Chang, E. Banitt, R. Joos [9] show that monoadducts can be obtained by reacting of fluorine-containing alcohols (CF3CH2OH, C3F7CH2OH) with ethylene oxide (molar ratio of alcohol : ethylene oxide = 1 : 0.7-0.8) in the presence of catalytic amounts of sodium hydroxide (in the form of a 50% aqueous solution) for 5-7 hours with heating (70°C or boiling). The target ethers contained about 10% of the initial fluorine-containing alcohols after distillation (the yields are not indicated).
R.N. Thompson, M.F. Hoover [10] obtained ethylene glycol 1,1-dihydroperfluoroheptyl ether (bp 212-213°C) by heating 1,1-dihydroperfluoroheptanol (2.99 mol) with ethylene oxide (1.63 mol) at 80°C. The substitution of the hydroxyl group with bromine (ZnBr2, Br2, H2SO4, 100-110°С) gave 1-bromoethyl ether.
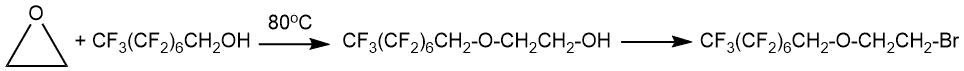
The interaction of fluoroalkanols with ethylene oxide or propylene oxide (PO) can be accompanied by the formation of oligomers (polyglycols).
According to the data of M. Hayek and R. Moody [11], the formation of fluoroalkyl ether CF3(CF2)nCH2O(CH2CH2)mOH (n=7 average value; m=10.6) occurred when fluorine-containing alcohol was heated with the calculated amount of ethylene oxide in the presence of boron trifluoride at 65-90°C for 10 hours.
Other examples are given in the patent by A. Szur [12]. Two or more propylene oxide units are attached to 2,2,3,4,4,4-hexafluorobutanol CF3CFHCF2CH2OH at a ratio of propylene oxide : alcohol = 3:1 in the presence of boron trifluoride etherate at moderate temperature (40°C).
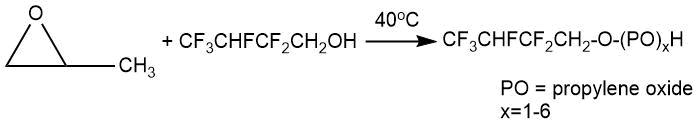
Glycidyl ethers are the final products in the reactions of fluorine-containing alcohols with 1-chloro-2,3-epoxypropane (epichlorohydrin) and 1-bromo-2,3-epoxypropane (epibromohydrin).
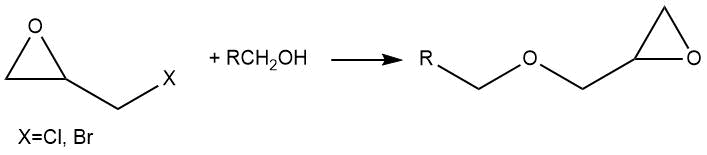
The work of M.L. Brey and P. Tarrant [3] describes the interaction of 1,1,1-trifluoroethanol with 1-chloro-2,3-epoxypropane in the presence of sodium hydroxide as a catalyst. With an excess of sodium hydroxide, the product was glycidyl ether, which could further react with alcohol to obtain diester.
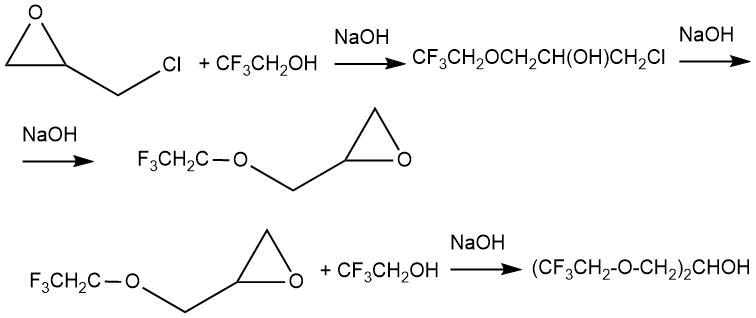
When carrying out the synthesis in a cooled sodium hydroxide solution, the reaction products included 19÷31% glycidyl ether and 19÷24% diester.
The introduction of catalytic amounts of pyridine into the reaction made it possible to obtain 2,2,2-trifluoroethyl-2-hydroxy-3-chloropropyl ether as a simple adduct in 50% yield.
V. Grakauskas [5] obtained 2-fluoro-2,2-dinitroethylglycidyl ether (bp 70-71°C at 0.1 mm Hg, yield 31%) by adding equimolar amounts of epichlorohydrin and 2-fluoro-2,2-dinitroethanol in excess of aqueous sodium hydroxide solution (0-3°C, 48 h). The action of 8% hydrochloric acid (hydrobromic, hydroiodic, and nitric) led to the opening of the oxirane ring [13] and the formation of 1-chloro-2-(2-fluoro-2,2-dinitroethoxy)-2-propanol (25°C, 16 h, yield 100%). It is noted that this substance is also a by-product in the preparation of 2-fluoro-2,2-dinitroethylglycidyl ether from 2-fluoro-2,2-dinitroethanol and epichlorohydrin. The corresponding glycidyl ethter was obtained in 95% yield when treating a solution of 1-chloro-2-(2-fluoro-2,2-dinitroethoxy)-2-propanol in methanol with 85% sodium hydroxide in methanol (22-25°C, dropwise, then another 15 min).
1.2. Interaction of alcohols with fluorine-containing epoxides
D. Sianesi and co-authors in their article [14] and patent [15] described the addition of alcohols to hexafluoropropylene oxide (HFPO), which led to the formation of 2-alkoxy derivatives of carboxylic acids.
Esters of the corresponding alcohol and 2-alkoxytetrafluoropropionic acids were obtained as a result of the interaction of methanol, ethanol, 2,2,3,3-tetrafluoropropanol and a number of other alcohols with HFPO.
2ROH + HFPO → CF3CF(OR)COOR,
R = CH3, CH3CH2, ClCH2CH2 (CH3)2CH, HCF2CF2CH2, CH2=CHCH2
The interaction of alcohols, which do not contain fluorine atoms in their structure, with HFPO easily carrying out at room temperature and atmospheric pressure. In contrast, the reaction of 2,2,3,3-tetrafluoropropanol and HFPO required more severe synthesis conditions (autoclave, 80°C).
A description of hydrolysis of synthesized methyl 2-methoxytetrafluoropropionate and ethyl 2-ethoxytetrafluoropropionate was also given to obtain the corresponding carboxylic acids in high yield.
CF3CF(OCH3)COOCH3 → CF3CF(OCH3)COOH (Yield 74%)
The article by L.W.Breed, R.L. Elliott and C.F. Key [16] describes the preparation of a series of fluorinated esters with an ether bond in their structure by adding alcohols of the general formula H(CF2)nCH2OH (n =4, 6) to HFPO at 80°C in the presence of sodium hydroxide.
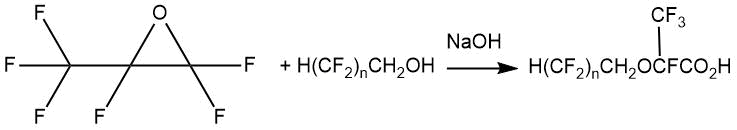
The yield of such acids with an ether fragment in their structure was 46% in the case of 1,1,2-trihydroperfluoropentanol and 67% in the case of 1,1,2-trihydroperfluoroheptanol.
Subsequently, the corresponding 2-alkoxy-2,3,3,3-tetrafluoropropanols were obtained by reduction of 2-alkoxy-2,3,3,3-tetrafluoropropionic acids with sodium borohydride in tetrahydrofuran (for n=4, the yield 32%; n=6, the yield 39%).
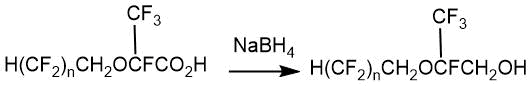
2. Formation of fluorinated ethers in reactions with formaldehyde
2.1. Interaction of fluorine-containing olefins with formaldehyde
Depending on the reaction conditions, the interaction of fluorine-containing olefins with formaldehyde, can lead to various products.
In 1949, D.D. Kofman et al. [17] showed that the reaction product of paraformaldehyde and tetrafluoroethylene (TFE) in concentrated sulfuric acid (80°С, 15 h) is 2,2,-difluoro-3-hydroxypropionic acid with a yield of about 25%.
CF2=CF2 + CH2O + H2O → [HOCH2CF2CF2OH] → HOCH2CF2COOН
The same reaction was studied by E.T. McBee et al. in 1952 [18]. The authors obtained difluorohydroxpropionic acid in 20% yield (medium - 95% sulfuric acid, 85°С).
At the same time, the interaction of fluorine-containing olefins with formaldehyde can lead both to the production of ethers of various structures and alcohols in a medium of anhydrous hydrogen fluoride (or chloride) hydrogen.
A description of the interaction of formaldehyde with fluorine-containing olefins in a hydrogen fluoride medium was given in the article by V. Weinmayr in 1963 [19].
Based on NMR spectroscopy data, V. Weinmayr found that the dissolution of paraformaldehyde in anhydrous hydrogen fluoride is accompanied by the release of water and the formation of compounds with FCH2 groups. V. Weinmayr suggested that fluoroethanol and difluoromethyl ether are the hypothetical intermediates that enter into further the next interactions with alkenes.
СН2О + HF → FH2COH
2СН2О + 2HF → FH2COCH2F
In the research process, it was found that the products of the interaction of formaldehyde with fluoroethylene and 1,1-difluoroethylene were symmetrical ethers at low temperatures. 2,2,3,3,3-Pentafluoropropan-1-ol and fluoromethyl-2,2,3,3,3-pentafluoropropyl ether were obtained as the main reaction products in the case of tetrafluoroethylene. Moreover, at low reaction temperatures (~20°C), fluoromethyl-2,2,3,3,3-pentafluoropropyl ether was obtained as the main reaction product. The main reaction product was 2,2,3,3,3-pentafluoropropan-1-ol at higher synthesis temperatures (50÷100°C).
The reaction with hexafluoropropylene required higher temperatures (above 100°C) and led to the formation of 2,3,3,3-tetrafluoro-2-trifluoromethylpropanol. The results of Weinmayr's studies are given in Table. 2.1.
Table. 2.1. The results of studies of the interaction of paraformaldehyde with fluorine-containing alkenes in a hydrogen fluoride medium.
|
Alkene |
Alkene /Н2С=О/HF |
T, °С; τ, h |
Products |
|
FCH=CH2 |
160/30/200 |
minus 40 ÷ 100°С; |
(HCF2CH2CH2)2O |
|
F2C=CH2 |
271/120/560 4,2/4,0/excess |
0÷ 10°С; |
(CF3CH2CH2)2O |
|
F2C=CF2 |
Excess /6/excess |
50°С, 24 h |
CF3CF2CH2OCH2F CF3CF2CH2OH (CF3CF2CH2O)2CH2 (CF3CF2CH2OCH2)2O |
|
20°С, 24 h |
CF3CF2CH2OCH2F CF3CF2CH2OH |
||
|
CF3CF=CF2 |
160/30/200 |
160°С, 8 h |
CF3CF(CF3)CH2OH |
The results of the V. Weinmair’s work were confirmed by L.S. German and I.L. Knunyants. They obtained 3,3,3-trifluoropropyl ether (3,3,3-trifluoropropyl ether) under similar conditions using 1,1-difluoroethylene and formaldehyde in 47% yield [20].
СН2=CF2 + CH2O + HF → О(CH2CH2CF3)2
In another work, L.S. German and I.L. Knunyants [21] proposed a possible mechanism of the reaction. This mechanism is that the ionic dissociation of the molecule of the intermediate fluoromethanol (FH2COH) occurs at the C–F bond with the formation of an oxymethyl cation under the action of anhydrous HF. In the next step, the hydroxymethyl cation carries out an electrophilic attack on the multiple bond of the olefin (scheme 2.1).
The reaction does not stop at the stage of obtaining fluoromethyl-3,3,3-trifluoropropyl ether in the case of 1,1-difluoroethylene. The second molecule of olefin is added to form di-3,3,3-trifluoropropyl ether. The authors of the article explained it by the fact that the trifluoromethyl group is at a fairly large distance from the oxygen atom in fluoromethyl-3,3,3-trifluoropropyl ether. The inductive effect of such group is weak, so dissociation easily occurs along the C-F bond (scheme 2.2).
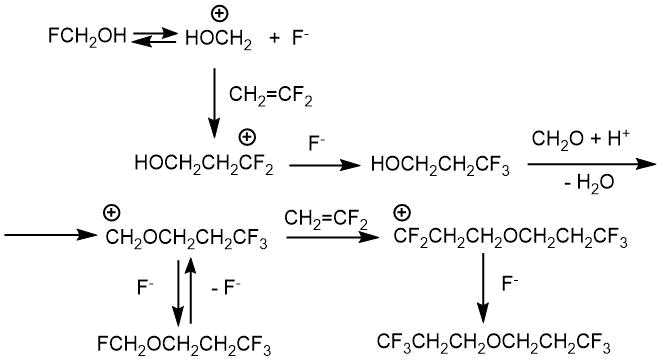
Scheme 2.1.
When interacting with tetrafluoroethylene, the main reaction products are fluoromethyl 3,3,3,2,2-pentafluoropropyl ether and 2,2,3,3,3-pentafluoropropanol. The difference between the interaction of formaldehyde with tetrafluoroethylene is that the strong inductive effect of the pentafluoropropyl group prevents dissociation C-F bonds in the fluoromethyl group. The addition of the second molecule of olefin to fluoromethyl-3,3,3,2,2-pentafluoropropyl ether does not occur for this reason (scheme 2.2).
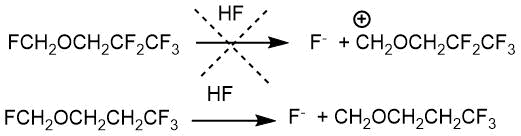
Scheme 2.2
In addition, the authors gave a description of the interaction of paraformaldehyde with trifluoroethylene at 0÷-5°C. In this case, the reaction products contained both symmetrical di-2,3,3,3-tetrafluoropropyl ether in 20% yield and 2,3,3,3-tetrafluoropropanol (32% yield).
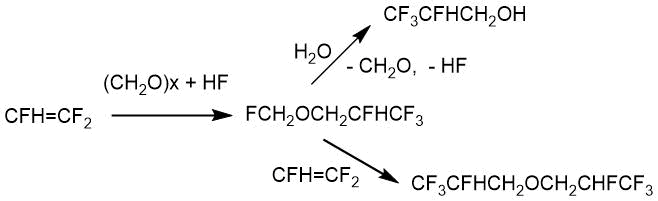
Composition relations of the reaction products of TFE with paraformaldehyde on the water content in hydrogen fluoride were presented in the patent by V. Weinmayr [22]. The main products were trifluoromethyl-2,2,3,3,3-pentafluoropropyl ether and 2,2,3,3,3-pentafluoropropan-1-ol in anhydrous hydrogen fluoride. As the water content in hydrogen fluoride increased, the ether content decreased. The results are given in Table. 2.2.
Table. 2.2. The ratio of the reaction products of paraformaldehyde with TFE depending on the water content in hydrogen fluoride [19].
|
Content HF |
Temperature, °С |
Pressure, MPa |
Ratio CF3CF2CH2OCH2F : CF3CF2CH2OH |
|
Anhydrous HF |
50 |
2,07 |
181:125 |
|
Anhydrous HF |
75 |
2,07 |
104:156 |
|
90% HF, 10% H2O |
50 |
2,76 |
28:222 |
|
80% HF, 20% H2O |
75 |
2,76 |
Only for CF3CF2CH2OH |
The patent by H.L.Yale [23] describes the interaction of tetrafluoroethylene with formaldehyde in the presence of cesium fluoride (triethylene glycol, 100°C, 5 h) to obtain 2,2,3,3,3-pentafluoropropanol in high yield.
CF2=CF2 + CH2O → CF3CF2СН2OH
2.2. Interaction of fluorinated alcohols with formaldehyde
The interaction of fluorinated alcohols with formaldehyde in the presence of acid catalysts (HCl, HF, H2SO4) can lead to the formation of ethers.
The reaction of 2-fluoroethanol with trioxane (trioxymethylene, formaldehyde trimer) was considered in the article by I.L. Knunyants [24]. It was shown that different products are formed depending on the ratio of the reactants and the reaction conditions.
Сhloromethyl-2-fluoroethyl ether was obtained with a yield of 60% by passing dry HCl into a mixture of 0,3 mol of 2-fluoroethanol and 0,1 mol of trioxane until the second reagent was completely dissolved (with ice cooling and further keeping for 24 hours at room temperature).
FCH2CH2OH + (CH2O)3 → FCH2CH2OCH2Cl
At the same time, bis-(2-fluoroethoxy)methane was obtained with a yield of 50% (bp 162–164°C) by reacting 0,47 mol of 2-fluoroethanol and 0,05 mol of trioxane (blowing with dry HCl until the complete dissolution of the second reagent under ice cooling and keeping for 72 hours at room temperature):
FCH2CH2OH + (CH2O)3 → FCH2CH2OCH2OCH2CH2F
Gy. Olah et al. [25] also studied the interaction of trioxane with 2-fluoroethanol. A suspension of trioxane (4.5 g) in 2-fluoroethanol (30 g) was blowed with anhydrous hydrogen chloride to give bis(2-fluoroethoxy)methane (9.5 g). In another example, the authors used acetaldehyde trimer (Paraldehyde) and obtained 1,1-bis-(2-fluoroethoxy)ethane (1,1-bis(2-fluoroethoxy)ethane, (FCH2CH2O)2CHСН3) as a reaction product.
The examples of the synthesis of a number of chloromethyl ethers of fluorinated alcohols under similar conditions are given in the patent by G.C. Tesoro and R.N. Ring [26]. A mixture of alcohol and paraformaldehyde in dimethoxyethane and/or petroleum ether was treated with dry hydrogen chloride with stirring. Then the mixture was kept for 5-10 hours at a moderate temperature (5-20°C). The results are presented in Table. 2.3.
Table. 2.3.
|
Alcohol |
Ratio (mol) Alcohol /(CH2O)n |
T, °С; τ, h |
Target products, yield (%) |
|
CF3CH2OH |
1,05/4,3 |
5 ÷ 10°С |
CF3CH2OCH2Cl (50%) |
|
CF2H(CF2)5CH2OH |
1,4/7,7 |
5°С |
CF2H(CF2)5CH2OCH2Cl (40%) |
|
CF3(CF2)6CH2OH |
0,25/1,37 |
0 ÷ 10°С |
CF3(CF2)6CH2OCH2Cl (47%) |
|
CF2H(CF2)9CH2OH |
0,86/4,7 |
5 ÷ 15°С |
CF2H(CF2)7CH2OCH2Cl (97%) |
|
OHCH2(CF2)3CH2OH |
0,3/3 |
5 ÷ 10°С |
CH2ClOCH2(CF2)3CH2OCH2Cl |
V.A. Komarov et al. [27] showed that the reaction of 2,2-difluoro-2-nitroethanol with formaldehyde and hydrogen chloride (ZnCl2, minus 10°С, 2 h) leads to the formation of 2,2-difluoro-2-nitroethylchloromethyl ether (bp 54°C/15 mm Hg) with a yield of 54.5%.
O2NCF2CH2OH + CH2O + HCl → O2NCF2CH2OCH2Cl
The reaction product of the same reagents is hydroxymethyl-2,2-difluoro-2-nitroethyl ether (bp 116°C/3 mm Hg) with a yield of 76, 5% at a higher temperature (from room (rt) to 60°C) [28].
O2NCF2CH2OH + CH2O + HCl → O2NCF2CH2OCH2ОН
The article by H. Adolph and M. Kamlet [29] describes the reaction of 2-fluoro-2,2-dinitroethanol with polyformaldehyde in acid medium. Bis-(2-fluoro-2,2-dinitroethoxy)methane is obtained with a yield of 80% when using concentrated sulfuric acid and the reaction temperature of 0-10°C.
(O2N)2CFСН2ОН + (СН2О)n → (O2N)2CFСН2ОCH2OCH2C(NO2)2F
When using the excess of formaldehyde and a lower concentration of sulfuric acid (80-90%), the formation of a homologous series of products (O2N)2CFСН2О(CH2O)nCH2C(NO2)2F (n=2-4) is observed. The interaction of formaldehyde with 2,2,2-trifluoroethanol and 2,2,3,3-tetrafluoropropanol in sulfuric acid is described in the patent by M.E. Hill and K.G. Shipp [30]. The authors of the cited work determined the dependence of the ether yield on the concentration of sulfuric acid (from 80% H2SO4 to 10% oleum). It was found that the maximum yield of ether is achieved using 96% H2SO4. The syntheses were carried out for 1 hour at room temperature under stirring conditions. As a result, bis-(2,2,2-trifluoroethoxy)methane was obtained with a yield of 68% and bis-(2,2,3,3-tetrafluoropropoxy)methane with a yield of 63,4%.
Zapevalov et al. [31] considered a method for obtaining bis(2,2,3,3,4,4,5,5-octafluoropentyloxy)methane with a yield of 73-91% by heating a mixture of 2,2,3,3,4,4,5,5-octafluoropentanol and formaldehyde (molar ratio 2:1) in o-xylene (p-xylene) in the presence of toluenesulfonic acid.
CF2H-CF2CF2CF2CH2-OH + CH2O → CF2H-CF2CF2CF2CH2-O-CH2-O-CH2CF2CF2CF2CF2H
The production of 2-fluoro-3-hydroxy-n-propyl methyl ether through the intermediate synthesis of bis-(2-fluoro-3-chloropropoxy)methane is described in the article by L.S. Boguslavskaya et al. [32]. Bis-(2-fluoro-3-chloropropoxy)methane is formed by the reaction of 2-fluoro-3-chloro-1-propanol with paraformaldehyde in the presence of p-toluenesulfonic acid in benzene in 76% yield.
CH2ClCHFCH2OH + (CH2O)n → (CH2ClCHFCH2O)2CH2 → (CH3OCH2CHFCH2O)2CH2 → CH3OCH2CHFCH2OH
M.E. Hill and L.O. Ross reported [33] on the preparation of bis-(2,2-difluoro-2-nitroethyloxy-)methane in 60% yield by heating 2,2-difluoro-2-nitroethanol (0,07 mol) with paraformaldehyde (0,016 mol) in 90% H2SO4 medium.
O2NCF2CH2OH + HCHO → O2NCF2CH2OCH2OCH2CF2NO2
A.H. Albrecht [34] obtained polyfluoroalkylchloromethyl ethers by treating a mixture of polyfluorinated alcohol with a slight excess of paraformaldehyde in benzene with dry hydrogen chloride, followed by distillation of volatile components.
C8F17(CH2)nOH +(CH2O)m → C8F17(CH2)nOCH2Cl, n= 5, 11.
3 Ttransetherification
D.W. Codding's patent [35] describes the synthesis of heptafluorobutyl vinyl ether with a yield of 32% by the interaction of heptafluorobutanol with vinyl acetate in the presence of mercury acetate in sulfuric acid medium.
Another example of the synthesis of vinyl ether by the reaction of 2,2-dinitro-2-fluoroethanol with vinyl acetate in the presence of mercury acetate and sulfuric acid (0°C, 16 h, 51%) is given in the patent by H.G. Adolph [36].
(O2N)2CFCH2OH + СН2=СНОС(О)СН3 → (O2N)2CFCH2OСН=СН2
The patent by L.S. Croix [37] includes the example of the reaction of 2,2,2-trifluoroethanol with vinyl acetate to obtain 1,1-bis-(2,2,2-trifluoroethoxy)ethane (HgO, BF3·Et2O, 30-50°С, 2 h, yield 61,9%). Subsequent pyrolysis of 1,1-bis-(2,2,2-trifluoroethoxy)ethane (catalyst - AgNO3, Fe2O3, Pt-asbestos, 340-390°C) leads to the production of 2,2,2-trifluoroethylvinyl ether with a yield of about 88 %.
СF3CH2OH + СН2=СНОС(О)СН3 → СH3CH(OCH2CF3)2 → СF3CH2OСН=СН2
The reactions of fluorinated alcohols of the general formula H(CF2)nCH2OH (n=4, 6, 8) with vinyl acetate in the presence of Hg (II) salts as a catalyst were considered in [38]. They led to the production of a mixture of products: vinyl ether, its acetyl derivative and acetal.
H(CF2)nCH2OH + СН2=СНОС(О)СН3 → H(CF2)nCH2OCH=CH2 + CH3CH(-OC(O)CH3)OCH2(CF2)nH + CH3CH(OCH2(CF2)nH)2
The article by B.K. Mandal [39] and the patent by V. Tortelli [40] describe the production of 2,2,2-trifluoroethyl 2-hydroxyethyl ether by the interaction of ethylene carbonate with 2,2,2-trifluoroethanol in the presence of sodium hydroxide (tetraglyme , 150°C, 4 h, 76% yield).
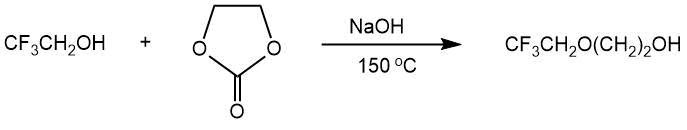
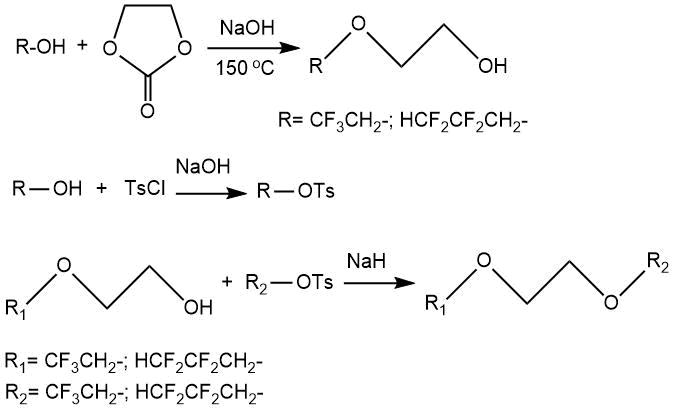
The preparation of ethylene glycol monoethers CF3CH2O(CH2)2OH and CHF2CF2CH2O(CH2)2OH via reactions of the corresponding alcohols with ethylene carbonate was considered in the work [41]. The further treatment of the obtained monoethers with tosylates of the starting fluorine-containing alcohols led to the formation of 1,2-bis-(2,2,2-trifluoroethoxy)ethane (CF3CH2O(CH2)2OCH2CF3) and 1,2-bis-(2,2,3,3- tetrafluoropropoxy)ethane (CHF2CF2CH2O(CH2)2OCH2CF2CHF2) in 28-42% yields.
B. Boutevin and B. Youssef studied the reactions of 1,1,2,2,-tetra-H-perfluorooctan-1-ol with ethyl vinyl ether in the presence of mercury acetate (or Pd (II) complex [diacetato-(1,10-phenanthroline) palladium (II)]), which lead to the formation of 1,1,2,2,-tetra-H-perfluorooctyl vinyl ether [42].
C6F13C2H4OH + C2H5OCH=CH2 → C6F13C2H4OCH=CH2.
H. Adolph and M. Kamlet showed that the interaction of 2-fluoro-2,2-dinitroethanol with a 5-fold excess of dimethoxymethane in the presence of boron trifluoride etherate (boiling, 3 h) leads to the formation 1,1-dinitro-1-fluoro-2-(methoxymethoxy)ethane (1-fluoro-1,1-dinitro-2-(methoxymethoxy)ethane) [29].
CF(NO2)2CH2OH + CH3OCH2OCH3 → CF(NO2)2CH2OCH2OCH3
The reaction already led to the production of bis(2-fluoro-2,2-dinitroethoxy)methane (bis-(2-fluoro-2,2-dinitroethoxy)methane) in a medium of concentrated sulfuric acid (t. room, 15 h), with a yield of 64%
CF(NO2)2CH2OH + CH3OCH2OCH3 → CF(NO2)2CH2OCH2OCH2C(NO2)2F
When studying the condensation of 2,2-difluoro-1,3-propanediol with dimethoxymethane (heating in the presence of polystyrenesulfonic acid, Amberlyst-15), G. Binsch et al. [43] noted that among the products there are linear compounds with the ether group CH3OCH2OCH2CF2CH2OH and CH3OCH2OCH2CF2CH2OCH2OCH3 in addition to the target 5,5-difluoro-1,3- dioxane.
The article by K. Petrov et al. [44] describes the interaction of a number of fluorine-containing alcohols with ortho esters to obtain ethers.
RFOH + RC(OR’)3 → RFOR’,
where RF= CF3CH(OH), (CF3)2C(OH), (ClCF2)2C(OH); R= H, CH3; R’=CH3, C2H5.
4. Production of fluorinated ethers by electrochemical fluorination
Fully fluorinated ethers are formed in moderate yield by electrochemical fluorination (ECF) of the corresponding hydrocarbon analogues:
CnH2n+1OCmH2m+1. → CnF2n+1OCmF2m+1
By-products are compounds that are formed due to the breaking of C-O and C-C bonds. Moreover, the content of by-products increases with an increase in the molecular weight of the original dialkyl ether.
Joseph H. Simons developed the ECF method in the late 1940s [45]. J. Simons gave the first mention of obtaining fluorinated ethers by this method in his patent in 1950 [46]. A description of the production of the following ethers was given: perfluorodimethyl, perfluorodiethyl, perfluoro-n-dibutyl, perfluoro-n-diamyl, perfluoro-n-dihexyl, and a number of other ethers from the original hydrocarbon precursors, as well as perfluoro-1,2-bis-(methoxy)ethane from 1,4-dioxane.
The preparation procedure was the following: the initial hydrocarbon ether was dissolved in anhydrous hydrogen fluoride and the ECF process was carried out in an electrochemical cell with a nickel anode and an iron cathode at atmospheric pressure, a temperature of 0°C, a voltage of 4–6 volts and amperage of about 20 A/ ft2. New portions of raw materials were added to the cell as the initial hydrocarbon ether was consumed during the process. The yield of the target products was not indicated.
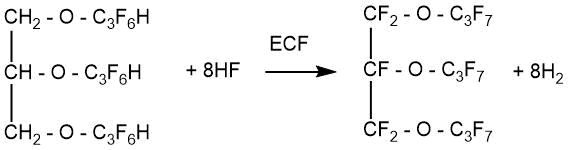
S. Benninger’s patent [47] describes the process of obtaining perfluorinated ethers of polyhydric alcohols.
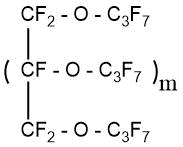
m=1-4
The process consisted of two stages:
- The synthesis of partially fluorinated ethers from polyhydric alcohol and hexafluoropropylene was carried out at the first stage,
- the obtained partially fluorinated ether was subjected to ECF in the Simons cell to obtain perfluorinated compounds.
The article by Japanese researchers [48] included a description of production of perfluorocarboxylic acids and perfluorinated ethers with low yields by ECF of diethyl, dipropyl, and a number of other ethers.
The work by K. Okazaki et al. [49] describes ECF in the medium of hydrogen fluoride of chloromethyl methyl ether, trichloromethyl methyl ether, 2,2,2,-trichloroethyl methyl ether, and a number of other chlorine-containing ethers. It was found that if the chlorine atom was located at the α-carbon atom, then it was replaced by fluorine during ECF. If the chlorine atom was in the β-position, then it remained unchanged to obtain β-chlorinated polyfluoroethers. Each synthesis resulted in a complex mixture of products with a predominance of degradation products.
The patent by J.C. Hansen [50] describes ECF of methyl 1,1,3,3,3-pentafluoro-2-(trifluoromethyl)propyl ether (CF3)2CHCF2OCH3 and ethyl 1,1,3,3,3-pentafluoro-2-(trifluoromethyl)propyl ether (CF3)2CHCF2OC2H5). Both of these ethers are waste products of toxic perfluoroisobutylene (PFIB). It is formed as a by-product in the synthesis of hexafluoropropylene.
Table 4.1 shows the composition of the products after ECF of methyl 1,1,3,3,3-pentafluoro-2-(trifluoromethyl)propyl ether. The ratio of fully fluorinated ether and ether with the remaining hydrogen atom in position 2 of the propyl fragment could be changed from 10:1 to 1:1 by varying the concentration of hydrogen fluoride in the electrically conductive solution.
Table 4.1.
|
Component |
bp °C |
Yield, % of theory |
|
(CF3)2CFCF2OCF3 |
33 |
35÷40 |
|
(CF3)2CHCF2OCF3 |
46 |
25÷30 |
|
(CF3)2CFCOF |
0 |
5÷10 |
|
(CF3)2CHCOF |
15 |
5 |
|
(CF3)2CFCF3 |
0 |
5÷10 |
|
(CF3)3CH |
12 |
10÷15 |
|
(CF3)2CHCF2OCHF2 + (CF3)2CHCF2OCH2F |
- |
<5 |
|
(CF3)2CHCF2OCH3 |
69,5-70 |
5 |
The special “active additives” are used to the electrolyte to improve the parameters of the ECF process. Such aditives make it possible to stabilize the ECF process and carry it out in a continuous mode.
G.I. Kaurova presented the results of comparing the effectiveness of additives on the process of synthesis of dibutyl ether in the article [51] (Table 4.2).
Table 4.2. ECF of dibutyl ether (C4H9)2O.
|
Additive |
i, А/cm2 |
U, V |
Q, A• h/dm3 |
Raw current output, % |
Anode corrosion, g/A•h |
|
n-butylmercaptan 15% |
0.02 |
4.6-5.0 |
4000 |
64 |
<0.001 |
|
hydroquinone - 3.5 % |
0.02 |
4.6-5.5 |
1600 |
33 |
0.03 |
|
iodine (I2) - 4 % |
0.01 |
4.9-7.0 |
<1000 |
25 |
0.03 |
As can be seen from the results of the table, the best results were obtained using n-butyl mercaptan.
V.A. Matalin [52] [53] studied the effect of tertiary amines used as additives to the electrolyte in the process of electrochemical fluorination of various organic compounds. The optimal concentrations of the fluorinated compound and amine in the electrolyte were determined (5-15 wt %). The comparative results of ECF using n-butylmercaptan and triallylamine ((C5H5)3N) are shown in Table 4.
Table 4.3. ECF of ((C4H9)2O (I – 0,03 А/sm2).
|
Raw compounds |
Current output, % |
Main fluorination products, wt. % |
|
|
(С4Н9)2О |
C5F10NF2 + C5F12 |
||
|
(С4Н9)2О + C4H9SH |
49 |
- |
- |
|
(С4Н9)2О +(C5H5)3N |
46 |
50 |
40 |
Along with the production of perfluorinated derivatives of carboxylic acids, the formation of cyclic perfluorinated ethers is observed (a mixture of perfluoro-2-alkyloxolanes and perfluoro-2-alkyloxanes) during the electrochemical fluorination of linear carboxylic acid fluorides.
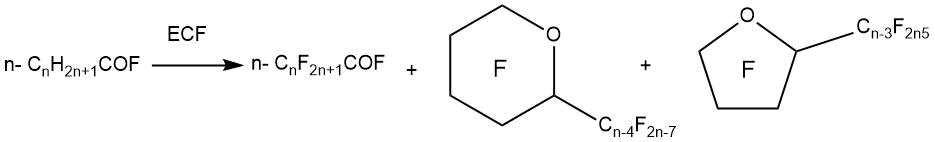
For example, G. Gambaretto et al. [54] showed that the cyclization products of octanoic acid fluoride (octanoyl fluoride) are formed in high yields (up to 60%) by ECF. It was found that both perfluorinated α-substituted oxanes (up to 69% in the mixture) and, to a lesser extent, perfluorinated α-substituted oxalanes (up to 23% in the mixture) were formed. The formation of cyclic compounds in the ECF process could be reduced by using partially fluorinated octanoyl fluorides as raw material.
On the other hand, during the ECF of cyclic derivatives of α-substituted tetrahydropyran (oxane, tetrahydro-2H-pyran) isomerization processes cannot be avoided. In the work by T. Abe et al. [55], it was shown that during the ECF process of α-substituted tetrahydropyrans, both rearrangement processes with the formation of five-membered rings and opening of the oxane ring (oxane ring) take place with the formation of linear structures.
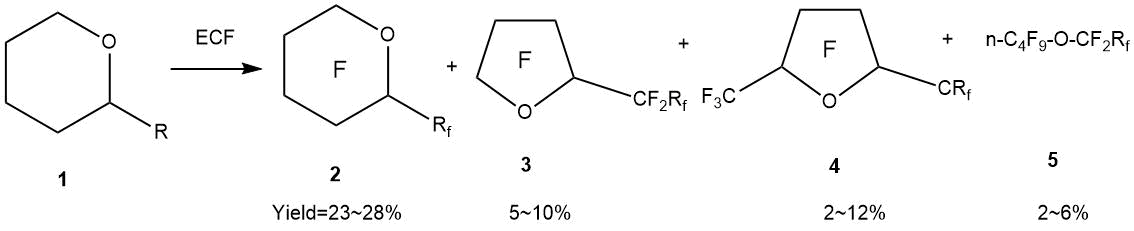
a: R=C2H5, Rf=C2F5; b: R=n-C3H7, Rf =n-C3F7; c: R=iso- C3H7, Rf =iso- C3F7;
d: R=n-C4H9, Rf =n-C4F9; e: R=n-C5H11, Rf =n-C5F11;
T. Fuchigami et al. described the ECF of some ethers and crown ethers using triethylamine complexes with hydrogen fluoride as an electrolyte [56]. The formation of a mixture of two monofluorinated products was observed during the ECF of dimethoxyethane in Et3N•5HF or Et4NF•4HF.
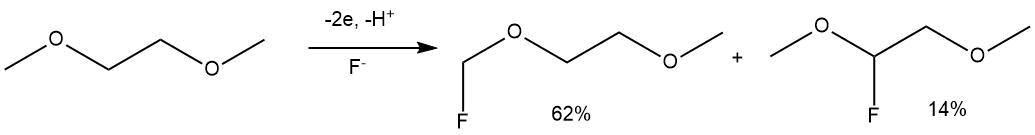
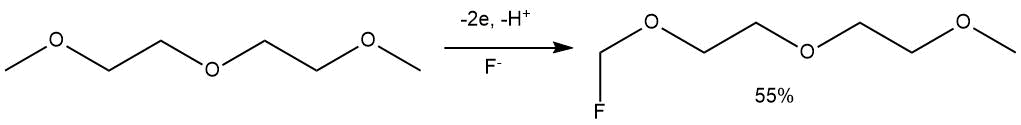
The ECF under the similar conditions of diethylene glycol dimethyl ether (diglyme) led to the production of exclusively monofluoro derivative at the terminal carbon atom (yield 55%, electrolyte Et3N•5HF).
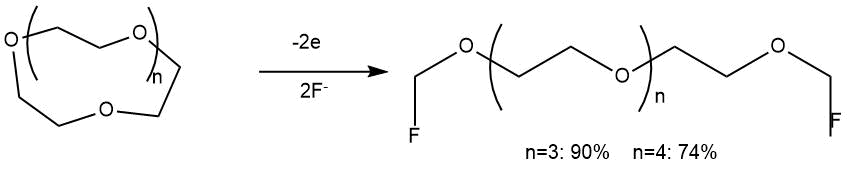
At the same time, the ECF of crown ethers led to the preferential breaking of the C–C bond with the formation of α,ω-difluoro-substituted products.
5. Other reactions for obtaining fluorinated ethers
5.1. Free radical addition of fluorine-containing alkenes to ethers
Free radical addition reactions of fluorine-containing alkenes with ethers are carried out under γ-irradiation conditions or in the presence of peroxides.
The article by H. Muramatsu et al. [57] describes the addition reaction of 1,1,2-trichlorofluoroethylene to diethyl ether. The initial mixture of alkene and ether was placed in a glass tube and subjected to γ-irradiation at room temperature for 312 hours. As a result, a mixture of 1-methyl-2-fluoro-2,3,3-trichloropropyl ethyl ether was obtained with a yield of 16% and bis(1-methyl-2-fluoro-2,3,3-trichloropropyl) ether with a yield of 26%.
In another article by the same authors [58], the addition of polyfluoroalkyl ethyl ethers to hexafluoropropene was studied. The reaction was carried out in an autoclave under γ-irradiation. Thus, 2,2-difluoroethyl 1-methyl-2,2,3,4,4,4-hexafluorobutyl ether was obtained in 64% yield as a result of the reaction (rt, 1030 hours) of 2,2-difluoroethyl ethyl ether (37 g) and hexafluoropropene (137 g).
R. Chambers et al. [59] studied the addition reactions of a number of acyclic ethers to hexafluoropropene under the same conditions (ether excess, γ-irradiation, 18°C).
Depending on the nature of the substituent in the ether, addition products of one, two or even three hexafluoropropene molecules were observed in the reaction products (Table 5.1.).
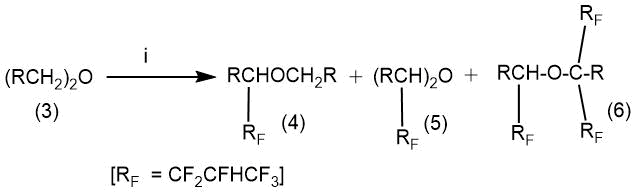
Table 5.1.
|
Products (% composition) |
Conversion % |
|||
|
(3a) R=H |
(4a) (100) |
- |
- |
70 |
|
(3b) R=Me |
(4b) (47) |
(5b) (53) |
- |
100 |
|
(3c) R=Et |
(4c) (30 |
(5c) (70) |
- |
80 |
|
(3d) R= n-Pr |
(4d) (23 |
(5d) (40) |
(6d) (40) |
70 |
5.2. Fluorination with elemental fluorine and high-valency transition metal fluorides
The method of direct fluorination with elemental fluorine has very limited use for obtaining fluorine-containing compounds, including fluorinated ethers, due to the high reactivity of elemental fluorine. In most cases, the yield of target compounds is low, and degradation products are obtained as the main reaction products.
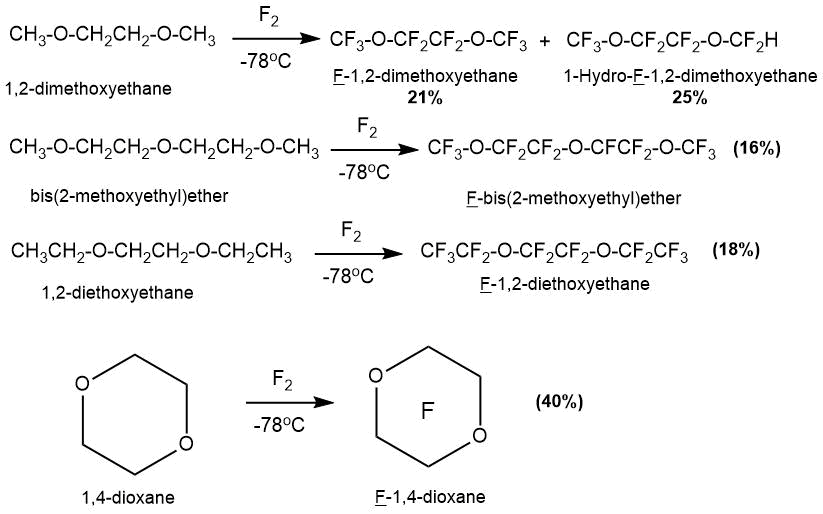
The production of a number of perfluorinated ether compounds from hydrocarbon precursors (1,2-dimethoxyethane, di-2-methoxyethyl ether, 1,2-diethoxyethane) was described in the article by R.J. Lagow in 1975 [60].
The synthesis was carried out in a multizone cryogenic reactor with a temperature gradient along the length of the reactor. The temperature of the first zone was minus 78°C, the temperatures of the subsequent zones varied for different products. It should be noted that despite the use of a specially designed reactor construction and low process temperatures, the yield of the desired products was low.
Most attempts to obtain fluorinated ethers using high-valency transition metal fluorides as fluorinating agents ended unsuccessfully [61]. In the process of fluorination, the ether group, however, like the other functional groups was subjected to destruction.
The results of fluorination of diethyl and methyl ethyl ethers using cobalt trifluoride (CoF3) at 60–80°C and potassium tetrafluorocobaltate (KCoF4) at 200°C were reported in the work by J.C. Tatlow et al. in 1974 [62]. In both cases, degradation products were obtained together with a complex mixture of polyfluorinated ethers. For example, 1,2-difluoroethane, 1,1,2,2-tetrafluoroethyl 1,2,2-trifluoroethyl ether, bis-1,2,2-trifluoroethyl ether, 1,2-difluoroethyl 1,2,2-trifluoroethyl ether were identified in the reaction of cobalt trifluoride with diethyl ether
The work by R. Chambers and B. Grievson [63] describes the preparation of perfluorinated ethers by exhaustive fluorination of free radical addition products of ethers (see 5.1.) to fluorine-containing alkenes. Perfluoroalkanes were obtained as by-products.
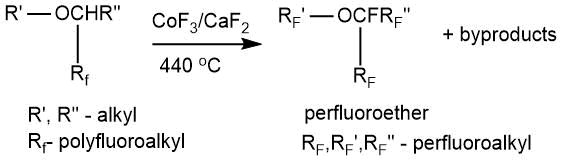
Fluorination was carried out in a horizontal nickel reactor with a stirrer at a temperature of 440°C with a mixture of cobalt trifluoride and calcium difluoride (1:1 wt.).
Thus, perfluoro-1-methylbutyl ethyl ether was obtained with a yield of 43% as a result of fluorination of 2,2,3,4,4,4-hexafluoro-1-methylbutyl ethyl ether.
Fluorination of 2,2,3,4,4,4-hexafluorobutyl methyl ether led to the formation of perfluorobutyl methyl ether in 36% yield.
J. C. Tatlow et al. described fluorination of chlorine-containing ethers with cobalt trifluoride at 110÷150°C [64]. A complex mixture of compounds was obtained as a result of fluorination of 2-chlorodiethyl ether. 2-Chloro-1,1,2,2-tetrafluoroethyl 1,2,2-trifluoroethyl ether, 2-chloro-1,2,2-trifluoroethyl 1,2,2,2-tetrafluoroethyl ether, bis-(1,2,2-trifluoroethyl) ether and a number of other fluorine-containing ethers were identified in this mixture.
5.3. Reactions of alcohols with ketones and carboxylic acids
The synthesis of 1Н,1Н,2Н,2Н-perfluorooctyl-1,3-dimethylbutyl ether (F-626, yield 92%, bp 214°С) was considered in the article by Japanese researchers H. Matsubara et al. [65]. F-626 was obtained by heating a mixture of polyfluoroalkanol C6F13C2H4OH with ketone CH3C(O)CH2CH(CH3)2 to 105°С for 8 hours in a stream of hydrogen (180 mL/min) in the presence of Pd/C with distillation of water during the reaction.
C6F13C2H4OH + CН3C(О)СH2CH(СH3)2 → C6F13C2H4OСН(СН3)СН2СН(СН3)2
The usual products of the interaction of alcohols, including fluorinated ones, with carboxylic acids are esters. However, U. Hess et al. [66] obtained ether by electrolysis of trifluoroethanol with N-acylglycine:
RC(O)NHCH2COOH + CF3CH2OH → RC(O)NHCH2OCH2CF3 + HCOOH,
R = CH3-, CF3-, C6H6-.
The work by R.A. Becker et al. [67] considers the preparation of unstable perfluoroisopropenyl methoxymethyl ether by the reaction of chloromethyl methyl ether with chloromercurpentafluoroacetone (0°C, 50%).
The starting chloromercurpentafluoroacetone was synthesized by reacting of perfluoropropen-2-ol with mercury trifluoroacetate (20°C, 79,2%) followed by treatment with acetyl chloride (-50°C, 97,9%).
CF2=C(OH)CF3 + (CF3COO)2Hg → CF3COOHgCF2C(O)CF3 → ClHgCF2C(O)CF3
ClHgCF2C(O)CF3 + ClCH2OCH3 → CF2=C(CF3)OCH2OCH3.
5.4. Reaction of perfluorinated nitrosoalkanes with alcohols
The article by A.V. Fokin and A.T. Uzun [68] describes the synthesis of alkyl trifluoromethyl ethers from trifluoronitrozomethane and hydroxylamine in alcohol medium.
CF3NO + H2NOH + ROH → CF3OR
The cited work shows that the reaction products of 1-nitro-2-nitrosotetrafluoroethane with hydroxylamine in methanol are 2-nitrotetrafluoroethyl methyl ether (bp 80–82°C) in 26% yield and difluoronitroacetic acid methyl ester (bp. 55 °C/100 mm Hg) with a yield of 32%.
O2NCF2CF2NO + H2NOН + CH3OH → O2NCF2CF2OCH3 + O2NCF2C(O)OCH3
5.5. Interaction of alcohols with 2,4,6-tris-(2,2,2-trifluoroethoxy)-[1,3,5]triazine (TriTFET)
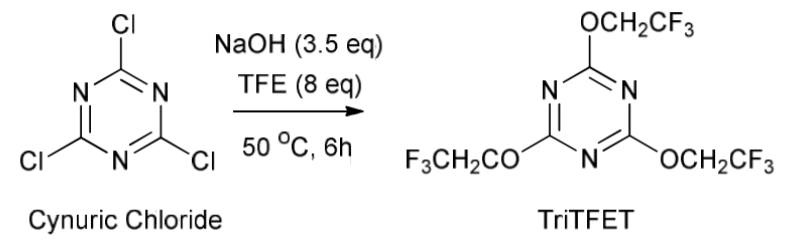
S.K. Mangava et al. [69] synthesized trifluoroethylbutyl ether by reacting n-butanol with 2,4,6-tris-(2,2,2-trifluoroethoxy)-[1,3,5]triazine (TriTFET) in the presence of p-toluenesulfonic acid as a catalyst. The TriTFET fluoroalkylating reagent was prepared by treating cyanuric acid chloride (cyanuric chloride) with trifluoroethanol (TFE) in the presence of NaOH as a base. The second stage of the synthesis is based on the interaction of n-butanol with the fluorinated alkylating reagent obtained at the first stage under the conditions of acid catalysis.
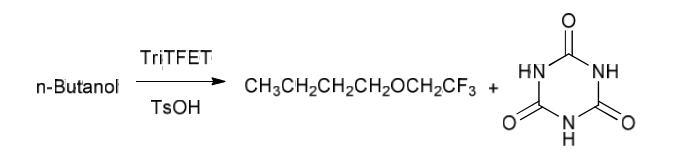
It was found that the optimal conditions for the synthesis of fluorinated ethers are the use of 0.6 equivalent of TriTFET and acetonitrile as a solvent. Under such synthesis conditions, the yield of ethers reaches 94%. The results are presented in Table. 5.2.
Table 5.2. Synthesis conditions of fluorinated ethers [55]
|
№ |
Solvent |
TriTFET (eq.) |
Yield, % |
|
1 |
CH2Cl2 |
0.6 |
20 |
|
2 |
CH2Cl2 |
0.4 |
10 |
|
3 |
CH3CN |
0.6 |
94 |
|
4 |
CH3CN |
0.4 |
68 |
|
5 |
DMF1 |
0.6 |
78 |
|
6 |
DMF |
0.4 |
60 |
|
7 |
AсOEt2 |
0.6 |
52 |
|
8 |
AсOEt |
0.4 |
40 |
|
9 |
1,4-Dioxane |
0.6 |
84 |
|
10 |
1,4-Dioxane |
0.4 |
72 |
|
11 |
Toluene |
0.6 |
45 |
|
12 |
Toluene |
0.4 |
32 |
1Dimethylformamide; 2ethyl acetate
Conclusion
The review attempts to generalize and systematize the existing methods for the preparation of fluorinated ethers. The authors did not set themselves the task of an exhaustive coverage of all the sources and individual examples of synthesis methods. Nevertheless, the authors express the hope that the presented material may be useful in research activities.
References
- S.V. Vershilov, V.V. Kornilov at all, Fluorinated ethers. Communication 1 // Fluorine Notes, Vol. 3(136), 2021, URL: http://en.notes.fluorine1.ru/public/2021/3_2021/article_1.htm; S.V. Vershilov, V.V. Kornilov at all, Fluorinated ethers. Communication 2 // Fluorine Notes, Vol. 5(138), 2021, URL: http://en.notes.fluorine1.ru/public/2021/5_2021/article_4.html
- I.L. Knunyants, O.V. Kil'disheva, I.P. Petrov, Interaction of aliphatic oxides with hydrogen fluoride. // Zhurnal Obshchej Himii, 1949, V. 19, Iss. 1, pp. 95-100.
- M.L.Brey, P.Tarrant. The preparation and properties of some vinyl and glycidyl fluoroethers // J. Am. Chem. Soc., 1957, V. 79, No 24, pp. 6533-6536.
- Syntheses of organofluorine compounds. (Monomers and intermediates). // Pod red. akad. I.L.Knunyanca i prof. G.G.Yakobsona. M. Himiya. 1977. 251 s.
- V. Grakauskas, Alkylatoin reactions of 2-fluoro-2,2-dinitroethanol // J. Org. Chem., 1970, v. 35, No. 9, pp. 3030-3036.
- A.V. Fokin, V.A. Komarov, A.N. Voronkov, V.I. Shevchenko, E.I. Lyubimova, Difluoronitroethyl ethers of 1,2-alkylene glycols and their derivatives. // Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, 1975, c. 1448-1450.
- E.H. Banitt, Fluoroalkoxyalkyl-2-cianoacrylates and polymers thereof, Patent US 3532674, 1970.
- E.H. Banitt, Fluoroalkoxyalkyl-2-cyanoacetates, Patent US 3663592, 1972.
- R.W.-H. Chang, E.H. Banitt, R.W. Joos, Fluoroalkoxyalkyl-2-cianoacrylate compositions used in tooth treatment, Patent US 3540126, 1970.
- R.N. Thompson, M.F. Hoover, Fluorocarbon containing diallyl ammonium compound, Patent US 3717679, 1973.
- M. Hayek, R.J. Moody, Fluorocarbon-containing printing ink and process for image printing, Patent US 3948668, 1976.
- A.J. Szur, Nonionic fluorochemical surfactants, Patent US 3980715, 1976; Anionic fluorochemical surfactants, process of coating and treated polymeric shapes, Patent US 4208466, 1980.
- V. Grakauskas, Polynitroalkyl Ethers // J. Org. Chem. 1973, v. 38, No. 17, pp. 2999-3004.
- D.Sianesi, A. Pasetti, F. Tarli, The chemistry of hexafluoropropene epoxide // J. Org. Chem., 1966, V. 31, No 7, pp. 2312-2316.
- D.Sianesi, A. Pasetti, F. Tarli, Derivatives of fluorinated carboxylic acids and process for their preparation, Patent US 3535369, 1970.
- L .W.Breed, R.L Elliott., C.F. Key, Fluorinated Esters Lubricants Containing Ethers Structures // Ind. and Eng. Chem. Prod. Res. and Developm., 1972, V. 11, No 1, pp. 88-91.
- D.D. Coffman, M.S. Raasch, G,W. Rigby, P.L. Barrick, W.E. Hanford, Addition reactions of tetrafluoroethylene // J. Org. Chem., 1949, V. 14, pp. 747-753.
- E.T. McBee, W.F. Marzluff, O.R. Pierce, The Ionization Constants of some fluorine-containing alcohols // J. Am. Chem. Soc., 1952, V. 74, No. 2, pp. 444-446.
- V.Weinmayr. Hydrogen fluoride as a condensing agent. VI. Reactions of fluoroolefins with formaldehyde in hydrogen fluoride // J. Org. Chem., 1963, V. 28, pp. 492-494.
- L.S. German, I.L. Knunyants. Reactions in anhydrous hydrogen fluoride. Condensation of formaldehyde with haloolefins in HF // ZHVHO im. D.I. Mendeleeva, 1966, V. 11, iss. 3, pp. 354-355.
- L.S. German, I.L. Knunyants. Reactions in anhydrous hydrogen fluoride. Synthesis of fluorine-containing ethers and esters // ZHVHO im. D.I. Mendeleeva, 1966, V. 11, iss. 3, pp. 356-358.
- V. Weinmayr, Process for preparing polyfuoro alkyl compounds, Patent US 2992276, 1961.
- H.L. Yale, Process for preparating halogenated propanols, Patent US 3415894, 1968.
- I.L. Knunyants, O.V. Kildisheva, E. Bykhovskaya, Interaction of aliphatic oxides with hydrogen fluoride. II // Zhurnal Obshchej Himii, 1949, V. 19, Iss. 1, pp. 101-113.
- Gy.Olah, A.Pavlath, Synthesis of organic fluorine compounds. IV. Derivatives of 2-fluoroethanol of insecticidal effect // Acta Chim. Acad. Sci. Hung., 1954, V. 4, p. 89.
- G.C. Tesoro, R.N. Ring, Fluorinated compounds, Patent US 3420840, 1969.
- V.A. Komarov, S.M. Davydova, K.V. Frosina, A.S. USSR 269156, BI № 15. 23 (1970)
- V.A. Komarov, A.V. Fokin, K.V. Frosina, H.A. Abdulganieva, Reactivity of some difluoronitro-containing alcohols // Zhurnal Obshchej Himii, 1967, V. 38, Iss. 3, pp. 684-686.
- H.G. Adolph, M.J. Kamlet, Fluoronitroalkanes. IV. Some reactions of 2-fluoro-2,2-dinitroethanol // J. Org. Chem. 1969. V. 34. N. 1. pp. 45-50.
- M.E. Hill, K.G. Shipp, Process for acetal preparation, Patent US 3526667, 1970.
- A.Ya. Zapevalov, E.O. Zemlyakova, A.V. Pestov, A.M. Semenova, Method for producing bis(2,2,3,3,4,4,5,5-octafluoropentyloxy)methane, Patent RU 2747026, 2021.
- L.S. Boguslavskaya, V.S. Atlis, K.V. Yarovykh, A.B. Bulovyatova, Conjugated halogenation of unsaturated compounds. IV. Chlorofluorination of 2-haloallyl alcohols and some reactions of fluorochloropropanols // Journal of Organic Chemistry, 1971, V. 7. Iss. 7, pp. 1338-1343.
- M.E. Hill, L.O. Ross, Reduction of difluoronitroacetate esters. The preparation and properties of 2,2,2-difluoronitroethanol and novel formation of hemiacetals by reduction // J. Org. Chem., 1967. V. 32. N. 8. pp. 2595-2600.
- A.H. Ahlbrecht, Fluorinated Ethers, Patent US 3818074A, 1974.
- D.W. Codding, Fluorocarbon vinyl ethers and polymers, Patent US 2732370, 1956.
- H.G.Adolph, 2,2-Dinitroalkyl vinyl ethers and polymer thereof, Patent US 3808182, 1974.
- L.S. Croix, Preparation of 2,2,2-trifluoroethyl vinyl ether, Patent USA 2872487, 1959.
- V.S. Sukhinin, S.I. Mineev, Synthesis and polymerization of vinyl ethers of telomeric alcohols // ZHVHO im. D.I. Mendeleeva, 1981, V. 26, № 3, pp. 344-345.
- B.K. Mandal, Z. Yue, X. Mei, H. Dunya, Q. Ma, Synthesis and physical properties of new fluoroether sulfones // J. Fluor. Chem., 2018, V. 216, pp. 118-123.
- V. Tortelli, I. Wlassics, C. Monzani, B.L.Kent, A.Vesteroni. Hydro-fluorocompounds, WO 2012160135, 2012.
- Z. Yue, H. Dunya, S. Aryal, C.U. Segre, B. Mandal, Synthesis and electrochemical properties of partially fluorinated ethers solvents for lithium-sulfur battery electrolytes // J. Power Sources, 2018, No 401, pp. 271-277.
- B. Boutevin, B. Youssef, Synthese d’ethers vinyliques a chaine laterale fluoree // J. Fluorine Chem., 1989, Vol. 44, pp. 395-412.
- G. Binch, E.L. Eliet, S. Mager, Ring inversion barrier in 5,5-difluoro-1,3-dioxane // J. Org. Chem., 1973, V. 38, No 23, pp. 4079-4081.
- K.A. Petrov, N.A. Tikhonova, N.A. Shchekotikhina, Reactions of perhalogenated carbonyl compounds and their hydrates with orthoesters and acetaldehyde acetal // J. org. chem., 1977, V. 13, № 5, pp. 943-948.
- J.H. Simons, The electrochemical process for the production of fluorocarbons // J. Electrochem Soc., 1949, 95, pp. 47–66.
- J.H.Simons. Fluorocarbon ethers. Patent US 2500388, 1950.
- S.Benninger, T.Martini, S.Rebsdat. Aliphatic and cyclic perfluoro-alkyl ethers and process for the preparation thereof, Pat. US 3962348, 1976.
- S. Nagase, H. Baba, R. Kojima, Preparation of Perfluorocarboxylic Acids from Unsaturated Compounds by Electrochemical Fluorination // The Journal of the Society of Chemical Industry Japan, 1962, Vol.65, Iss.1, p. 1183, DOI: 10.1246/nikkashi1898.65.38.
- K. Okazaki, S. Nagase, H. Baba, K. Kodaira, Electrochemical fluorination of chlorine-containing ethers // J. Fluor. Chem., 1974, Vol. 4, p. 383.
- J.C.Hansen, Preparation of F-alkyl F-isobutyl ethers by electrochemical fluorination, Patent US 5474657, 1995.
- G.I. Kaurova, Electrochemical fluorination of organic compounds // Himicheskaya promyshlennost', 2017, V. XCIV, Iss. 4, pp. 163-207.
- V.M. Matalin, Synthesis of perfluorinated organic compounds by electrochemical fluorination in the presence of tertiary amines // Diss. na soiskanie uchenoj stepeni k.h.n., SPb, 2008.
- V.G. Barabanov, V.A. Matalin, G.I. Kaurova, D.D. Moldavskij, Perfluorinated organic compound production process, Patent RU 2221765, 2004.
- M. Napoli, A. Scipioni, G. P. Gambaretto, F.M. Carlini, M. Bertola, Yield improvement in the electrochemical production of perfluoro-octanoic acid // J. Fluorine Chemistry, 1994, V. 67, pp. 261-264.
- T. Abe, E. Hayashi, H. Baba, K. Kodaira, S. Nagase, Fluorination of 2-alkyl-substituted oxanes. The synthesis and purification of perfluoro(2-alkyl-substituted oxane)s // J. Fluorine Chem., 1980, V. 15, pp. 353-380.
- H. Ishii, Y. Hou, T. Fuchigami, Electrolytic Partial Fluorination of Organic Compounds. Part 41. Highly Selective Electrolytic Fluorination of Dimethoxyethane, its Homologues, and Crown Ethers // Tetrahedron, 2000, V. 56, p. 8877.
- H. Muramatsu, K. Inukai, T. Ueda, The Radiation-Induced Addition Reaction of Ethers to Chlorofluoroolefins // Journal of Organic Chemistry, 1964, V. 29, Iss. 8, p. 2220-2223.
- H.Muramatsu. H.Kimoto, K.Inukai, The addition reactions of fluoroalkyl ethyl ethers to perfluoropropene // Bull. Chem. Soc. Japan, 1969, V. 42, No 4, pp. 1155-1158.
- R. Chambers, B. Grievson, N. Kelly, Free radical chemistry. Part 3. Substituent Effects in Additions of Ethers to Fluorinated Alkenes // J. Chem. Soc. Perkin Trans. I, 1985, pp. 2209-2213.
- J.L. Adcock, R.A. Beh, R.J. Lagow, Successful direct fluorination of oxygen-containing hydrocarbons // Journal of Organic Chemistry, 1975, Vol. 40, pp. 3271-3275, https://doi.org/10.1021/jo00910a024.
- M. Stacey, J. C. Tatlow and A. G. Sharpe (Eds.): Advances in Fluorine Chemistry. London: Butterworths 1960 (vol. 1), 1961 (vol. 2), 1963 (vol. 3).
- M. Brandwood, P.L. Coe, C.S. Ely, J. C. Tatlow, Polyfluoro diethyl and ethyl methyl ethers: their preparation using cobalt (III) fluoride and potassium tetrafluorocobaltate (III) and their dehydrofluorination // Journal of Fluorine Chemistry, 1975, Vol. 5, pp. 521-535.
- R. Chambers, B. Grievson, Free radical chemistry. Part 5. A new approach to the synthesis of perfluorinated ethers // Journal of Fluorine Chemistry, 1985, Vol. 29, pp. 323-339.
- P. L. Coe, M. S. Lennard, J. C. Tatlow, Chloropolyfluorodiethyl ethers // Journal of Fluorine Chemistry, 1996, Vol. 80, pp. 87-90.
- H. Matsubara, S. Yasuda, H. Sugiyama, I. Ryu, Y. Fujii, K. Kita, A new fluorous/organic amphiphilic ether solvent, F-626: execution of fluorous and high temperature classical reactions with convenient biphase workup to separate product from high boiling solvent // Tetrahedron, 2002, V. 58, pp. 4071-4076.
- U. Hess, T. Gross, R.Thiele, Zur Kolbe-Reaction von Aminosauren // Zeitschrift fur Chemie, 1979, 19, N. 5, pp. 195-196.
- A.V. Fokin, A.T. Uzun, Study of the reactivity of 1-nitro-2-nitrosotetrafluoroethylene. I. Interaction with hydroxylamine // Zhurnal obshchej himii. 1966. V. 36. № 1. pp. 117-119.
- S.K. Mangawa, C. Sharma, A.K. Singh, S.K. Awasthi, Expedient and efficient one pot synthesis of trifluoroethyl ethers from metal free 2,4,6-tris(2,2,2-trifluoro-ethoxy)-[1,3,5] triazene // The Royal Society of Chemistry, 2012, V. 1, pp. 1-5.
ARTICLE INFO
Received 11 February 2022
Accepted 24 February 2022
Available online February 2022
Recommended for publication by Prof. S.M. Igumnov
Fluorine Notes, 2022, 140, 1-2
