Received: November 2021
DOI 10.17677/fn20714807.2021.06.02
Fluorine Notes, 2021, 139, 3-4
α-FLUOROALKYLAMINES, THEIR PROPERTIES AND APPLICATION AS SOURCES OF NUCLEOPHILIC FLUORIDE ION
V.E. Boyko a,b, V.L. Don a,b
aA.N. Nesmeyanov Institute of Organoelement Compounds of the Russian Academy of Sciences,28 Vavilova St., 119991 Moscow, Russia.
bP@M-Invest Scientific Production Association, 119991, Leninsky ave. 47, Moscow, Russia.
Abstract: This mini-review is devoted to α-fluoroalkylamines, including compounds containing more than one nitrogen atom in the α-position to fluorine, their preparation, properties, and application as sources of nucleophilic fluoride ion in the synthesis of fluoroaliphatic and fluoroaromatic compounds.
Keywords: Fluoride ion α-fluoroalkylamines, fluoromethyl(dimethyl)amine, difluoromethyl(dimethyl)amine, trifluomethyldimethylamine, bis-(dimethylamino)difluoromethane, formamidinium hydrofluoride, huanidinium fluoride, tris-(dimethylamino)fluoromethane.
The fluoride ion plays an important role in organofluorine chemistry. It is not only able to substitute other halogens and various leaving groups with fluorine, but, most importantly, it is capable to generate fluorine-containing anions from electrophilic unsaturated compounds. The nucleophilicity of the fluoride ion cardinally depends on the medium. In protic solvents, particulary in water, the fluoride ion, due to its small radius and high charge density, forms strong hydrogen bonds and, as a result, is a weak nucleophile. The inner sphere of a hydrated fluoride ion contains 5 tightly bound water molecules, in contrast to chloride (three) bromide (two) ions. [1]. This explains the order of nucleophilicity of halide ions in water I- <Br- <Cl- >> F- [2, 3]. In polar aprotic solvents, the order of the nucleophilicity is reversed: F- >> Cl-> Br-> I- [1]. Nucleophilicity of fluoride ion in polar aprotic solvents depends, inter alia, from the cation. The dependence is mainly caused by the radius of the cation which determines the ability to dissociate [4, 5].The sources of fluoride ion for most reactions are primarily alkali metal fluorides. Solubility of inorganic fluorides in polar aprotic solvents is low. The nucleophilicity of fluoride ion can be increased by specific solvation of the cation, such as complexation with crown ethers [6]. Solubility of tetraalkylammonium salts is much higher, and they are very effective sources of fluoride ion for many reactions, however, they are extremely difficult to prepare in anhydrous form, and the presence of water reduces the activity of fluoride ion [7]. For economic reasons, for large-scale industrial syntheses in the 70-80s, the choice of fluoride ion was limited to potassium fluoride. The problem of searching for new alternative sources of fluoride ion has been highly relevant in 70-80 years of the last century when I. L. Knunyants payed his attention to alpha-fluoroalkylamines, and continues to remain actual.
1. α-Fluoroalkylamines
A number of α-fluoroalkylamines are known, these are fluoromethyl(dialkyl)amines, including fluoromethyl(dimethyl)amine
(1) [8], difluoromethyl(dimethyl)amine (2) [9-10], trifluoromethyl(dialkyl)amines
(3) [11-14],

polyfluoroalkylamines the most studied of these, obtained by the reaction of fluoroolefins with dialkylamines, also called fluoroalkylamino reagents (FAR)s (4a-c) [15-17], as well as perfluorotrialkylamines (5).

Like other tertiary amines, α-fluoroalkylamines, with the exception of perfluoroalkylamines lacking basic properties [23], form quaternary iminium salts under the action of alkylating agents. The crystalline salt of fluoromethyl (trimethyl) ammonium iodide is formed by mixing fluoromethyl(dimethyl)amine (1) with methyl iodide [8]; similar salt is obtained from difluoromethyltrimethylamine (2) [24].
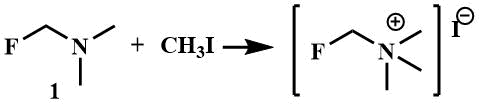
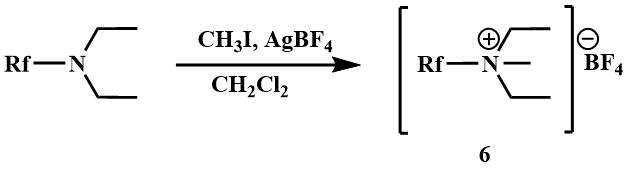
The quaternary salts of trifluoromethyl(dimethyl)amine and polyfluoroalkylamines (6) are prepared by reacting with methyl iodide in the presence of silver tetrafluoroborate [23].
The general property of α-fluoroalkylamines is the high mobility of α-fluorine atoms. The reason for the mobility of α-fluorine atoms is the stabilization of the cation formed upon the abstraction of the fluoride ion, by the lone pair of nitrogen atom [25].
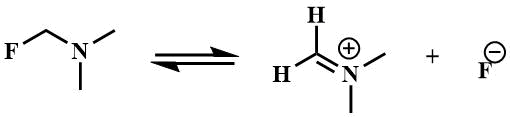
All the α-fluoroalkylamines hydrolyze readily with water and react vigorously with various hydroxyl-containing compounds. This property is widely used to replace hydroxyl groups with fluorine.
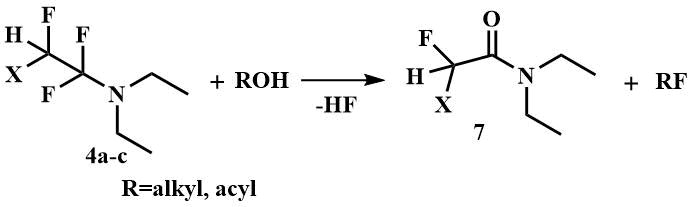
FARs are most commonly used for this purpose: the reagents of Yarovenko (4a) and Ishikawa (4b), prepared by the reaction of chlorotrifluoroethylene and perfluoropropylene, respectively, with diethylamine, and the product of the reaction of tetrafluoroethylene with diethylamine, also called Petrov's reagent (4c).
Monofluoroalkanes are obtained from alcohols by reaction with FARs [15-17, 28], and acyl fluorides and sulfonyl fluorides are obtained from acids and sulfonic acids [15, 26-28]. The second product of these reactions is corresponding amide (7), in the case of Yarovenko's reagent - chlorofluoroacetic acid diethylamide, Ishikawa's - 2,3,3,3-tetrafluoropropionic acid diethylamide, and difluoroacetic acid diethylamide when Petrov's reagent is used.
α-Fluoroalkylamines, in particular FARs, form iminium salts with Lewis acids (8)
[29].
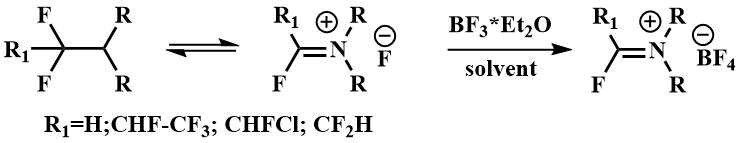
Such iminium salts are used in the synthesis of fluorine-containing heterocycles [29-31].
A number of reviews are devoted to the reactions of FARs used as fluorinating reagents for replacing of hydroxyl by fluorine: Yarovenko's, Ishikawa's, and Petrov's reagents, and the use of iminium ions formed by the interaction of FARs with Lewis acids in the synthesis of heterocycles [30-38].
Here we would like to focus in more detail on other α-fluoroalkylamines, as covalent compounds, which however can be considered as mesomeric equilibrium iminium structures that can act as sources of fluoride ions, and on ionic bis- and tris- (diaminofluoromethanes), in which the fluoride ion is coordinated to alkylimmonium cation, which are also of great interest as sources of fluoride ions.
1.1. Fluoromethyl(dimethyl)amine
Fluoromethyl(dialkyl)amines have been first obtained in 1970 by the cleavage of the corresponding aminals
(9) with acyl fluorides [8].
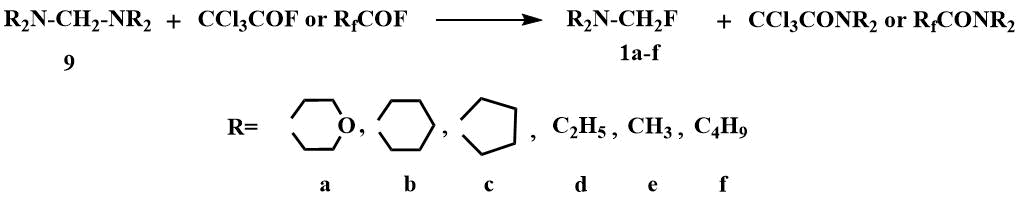
In contrast to the previously obtained chloromethyl-, bromomethyl- and iodomethyldialkylamines, which exhibited salt-like properties and were considered to be carbiminium compounds [39], the fluoromethyl(dialkyl)amines were found to be low boiling point liquids soluble in polar and nonpolar organic solvents - typical covalent compounds [8]. However, in the 19F spectrum of fluoromethyl(dialkyl)amine (1b), the spin-spin interaction FH was observed only at negative temperatures, with an increase in temperature it disappeared, in the 19F the triplet turned into a singlet and, accordingly, the doublet in the 1H spectra became a singlet, indicating a rapid exchange of fluorine between the molecules, i.e. the predominance of the ionic structure [8].
The simplest of fluoromethyl(dialkyl)amines - fluoromethyl(dimethyl)amine is a colorless liquid boiling at 46°C at normal pressure, soluble in pentane, ether and other polar and nonpolar organic solvents. At the beginning of the 2000s, fluoromethyl(dimethyl)amine was an object of studies of the molecular structure and quantum-chemical calculations, a model of the molecule in the gas phase was constructed [25].
The study of FCH2NMe2 by gas electron diffraction showed its existence in the form of a single conformer with an antiperplanar orientation of the C–F bond with respect to the lone pair of the nitrogen atom (Fig. 1).
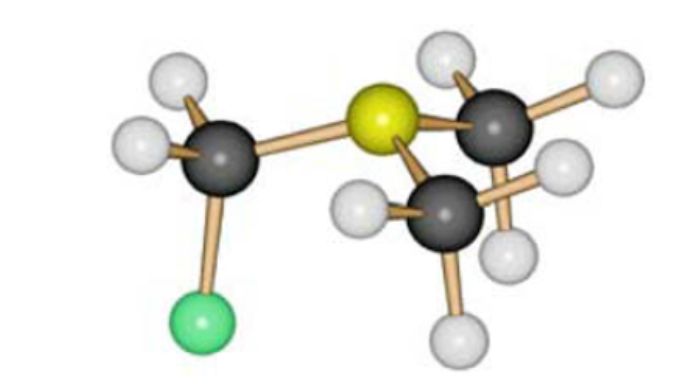
Figure 1. Molecular model of FCH2NMe2.
Donor-acceptor interaction between the lone electron pair and vicinal σ* orbitals - the so-called anomeric interaction, considered in the classical version as resonance structures amine - iminium ion leads to the expected structural consequences - shortening of the bond between the nitrogen atom and the fluorine-bound carbon atom [N– CF = 1.408Å], compared with the length of the nitrogen atom – methyl group [N – CMe = 1.466Å], the lengthening of the C–F bond to 1.410Å, and the increase in the angles of the nitrogen and carbon bonds. Quantum-chemical calculated values are close to experimental ones. The quantum-chemical calculated energy of interaction of the lone pair of nitrogen with the σ* orbital of the C-fluorine bond in the antiperplanar orientation is 28.2 kcal/mol [25].
These studies are in good agreement with the reactions discovered in the 80s in the Knunyants laboratory.
When the chemical properties of fluoromethyl(dimethyl)amine were studied in Knunyants laboratory, it was found to bind readily to perfluoroolefins such as perfluoropropylene and perfluoroisobutylene via the ionic mechanism [40].
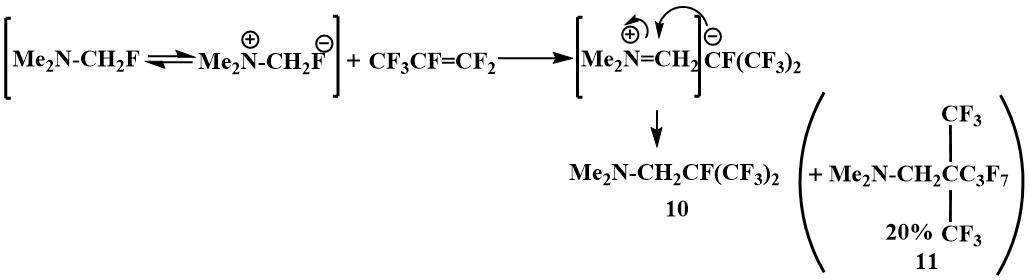
The ionic mechanism of this reaction is confirmed by the formation of characteristic by‑products. For the formation of (11), 20% of which results from the reaction of α‑fluoromethyl(dimethyl)amine with perfluoropropylene, it is necessary that a perfluoropropylene dimer is first formed, the formation of which is initiated by the F-ion [40].
Similarly, fluoromethyl(dimethyl)amine reacts with perfluoroisobutylene to give perfluoro(tert-butyl)trimethylamine,
Me2NCH2C(CF3)3, but this is readily hydrolyzed with hydrochloric
acid, unlike the product of the reaction with perfluoropropylene, perfluoro(isopropyl)trimethylamine
(10), which forms a stable chlorohydrate.
The reaction with perfluoroazapropene (12), which
is exclusively electrophilic, also apparently begins with the abstraction of the fluoride ion from
fluoromethyl(dimethyl)amine [40].
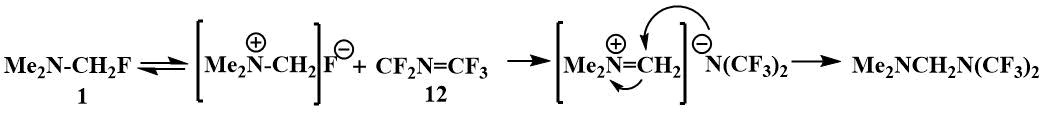
Fluoromethyl(dimethyl)amine also reacts with fluoroketones, but in this case ionic compounds are formed - alcoholates of N,N-dimethylmethyleneiminium (13), whose ionic structure is confirmed by their physical properties and spectral data. These salts dissolve well in polar solvents (DMF, acetonitrile), but not in ethers. Some data support the reversibility of the process of addition of fluoromethyl(dimethyl)amine to fluoroketones. The signals of a single F in the spectrum of salt (13) disappear above -20°C, indicating exchange processes apparently associated with the reversibility of the addition of fluoromethyl(dimethyl)amine to polyfluoroketones [41].

These salts react readily with perfluoropropylene and perfluoroisobutylene to form the addition products of the perfluoroalkoxy anion and the methyleneiminium cation on the multiple bond of olefin. Since the ease of addition of perfluoroisobutylene to perfluoropropylene and tetrafluoroethylene (which was not involved in this reaction at all) decreases, the authors proposed a nucleophilic mechanism of salt addition in which the perfluoroalkoxy anion first attacks the olefin and then the resulting β-alkoxycarbanion covalently bonds to the methyleneiminium cation to form perfluoro(alkoxyalkyl)amine (14).
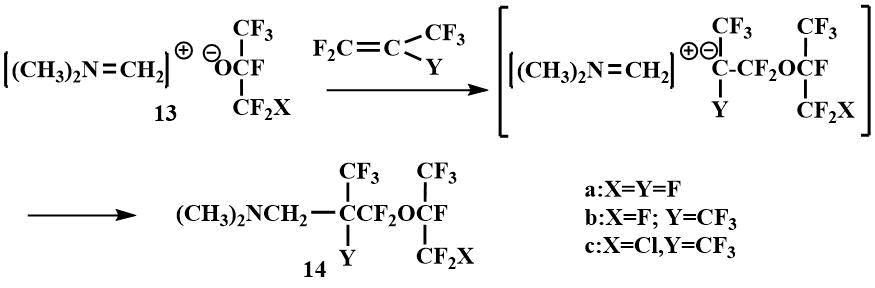
The reversibility of the addition of fluoromethyl(dimethyl)amine to ketones is also confirmed by the formation of Me2NCH2C(CF3)3 as a by-product together with (14) in the addition of salt (13) to perfluoroisobutylene at temperatures above -10°C [41].
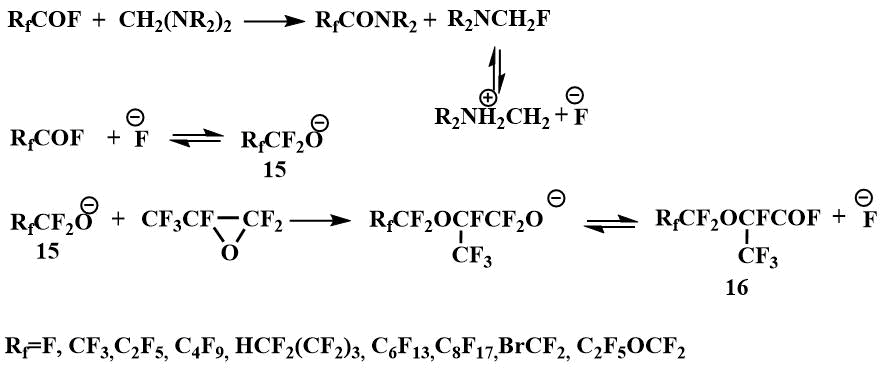
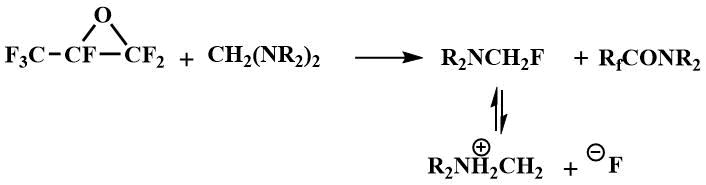
The formation of dialkylaminofluoromethanes capable of generating a fluoride ion in the reaction of tetraalkyldiaminomethanes (bis-(dimethylamino)methane, bis-(diethylamino)methane, bis-morpholino- and bis-piperidinomethanes) with perfluorocarboxylic acid fluorides, enabled the use of tetraalkyldiaminomethanes as catalysts for the condensation of perfluoropropylene oxide with perfluorocarboxylic acid fluorides to produce perfluoroalkoxypropanoic acid fluorides (16) - starting materials for preparing perfluoroalkoxyvinyl monomers - raw materials for obtaining polymers with special properties [42].
Condensation in the presence of a catalytic amount of N,N,N',N'-tetraalkyldiaminomethanes proceeds with
the intermediate formation of dialkylaminofluoromethane, which generates an fluoride ion that initiates
the formation of perfluoroalkoxyanion (15) by interaction with perfluorocarboxylic
acid fluoride.
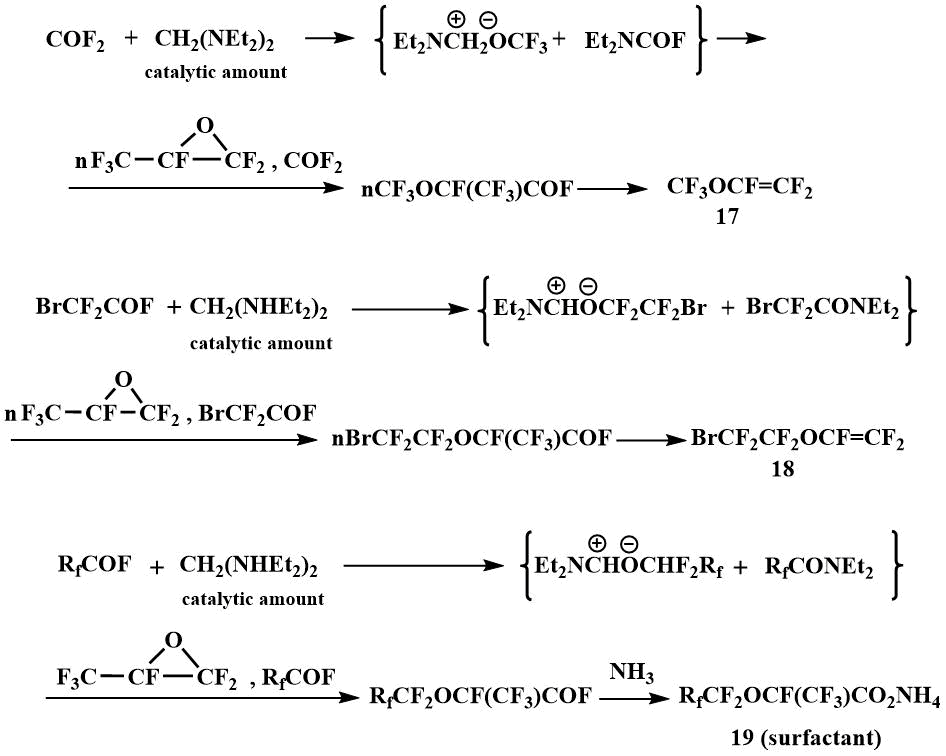
Subsequently, such reactions formed the basis for the pilot production launched by Igumnov in the Perm branch of the Russian Center of Applied Chemistry to obtain perfluorovinyl ethers, in particular, perfluoromethyl vinyl ether (17) and perfluorobromoethyl vinyl ether (18), as well as surface-active materials based on perfluoroalkoxypropanoic acids (19) [43]. N, N, N', N' -tetraethylmethylenediamine has been used in catalytic amounts for these processes. The use of this catalytic method allowed many processes with the participation of O-anions to be carried out in the temperature range from -10°C to room and atmospheric pressure, while the use of other F-ion resources in similar processes requires elevated temperatures (90-150°C) and pressure (5-200 atm) [44].
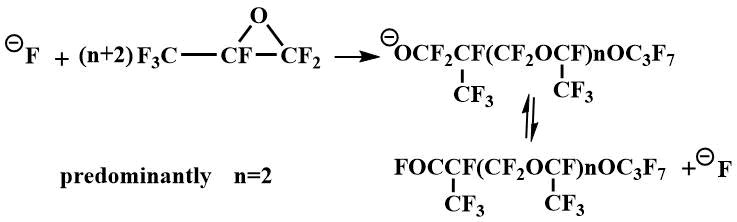
Tetraalkyldiaminomethanes have also been used as catalysts for the oligomerization of perfluoropropylene oxide (PFPO). When the oligomerization is catalyzed by tetraalkyldiaminomethanes, predominantly tetramer of PFPO is obtained.
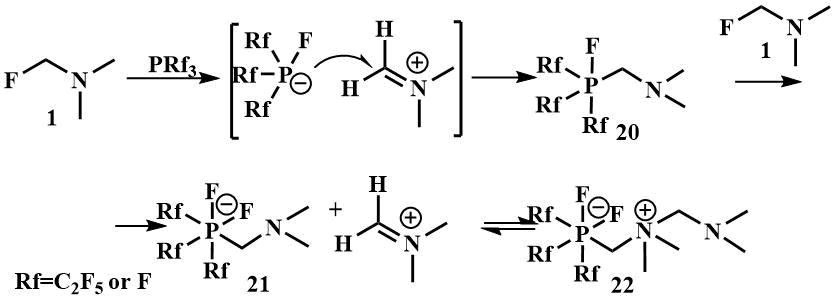
An example of the interaction of fluoromethyl(dimethyl)amine with phosphorus electrophiles was studied by Röschentaller and Hoge; as a result of the reaction of fluoromethyl(dimethyl)amine with phosphorus trifluoride or trialkylphosphines, a solid was obtained which is a zwitterion (22). The proposed reaction mechanism involves the initial transfer of the fluoride ion from fluorotrimethylamine to PRf3 with the formation of the [Rf3PF] anion and the iminium cation, the attack of the anion on the iminium carbon atom with the formation of the phosphorane Rf3PFCH2NMe2 (20), then the abstraction of the fluoride ion from the second Me2NCH2F molecule by phosphorane to form phosphate (21), which coordinates with the remaining iminium ion and forms the zwitterion (22) [45].
Treatment of the reaction product with P(C2F5)3 –[(C2F5) 3PF2 (CH2NMe2CH2NMe2)] (18) with aqueous sodium hydroxide leads to the formation of sodium phosphate Na+ [(C2F5)3PF2 (CH2NMe2)]ˉ (19) [45].
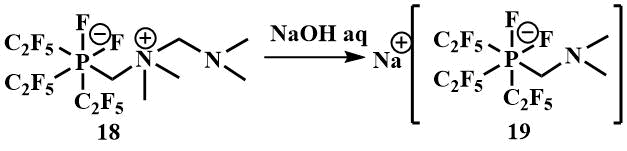
Perfluoroalkyl fluorophosphates of alkali metals are used in the electronics industry. They are characterized by higher thermal stability and hydrolysis resistance compared to the traditionally used hexafluorophosphates and are also used as ionic liquids [46].
1.2. Difluoromethyl(dimethyl)amine
Difluoromethyl(dimethyl)amine (2), first obtained in 1962 [2] by the reaction of carbonyl fluoride with dimethylformamide, is a low-boiling liquid (bp 49-51°C) in whose spectrum and at 20°C a spin-spin interaction occurs between fluorine and hydrogen atoms, which clearly indicates the covalent nature of the bond, while the analogous chlorine derivatives have a purely ionic character [(CH3)2N=CHCI]+Cl-, and is a hygroscopic, non-volatile salt [47].
A 1986 X-ray study of the quaternary salt (CH3)3NCHF2 showed that the length of the N-CHF2 bond (1.497Å) is shorter than that of the N-CH3 bond (1.508Å) [24].
Difluoromethyl(dimethyl)amine also exhibits chemical properties due to the mobility of fluorine atoms - it decomposes exothermically with water, reacts with boron trifluoride to form N,N-dimethylfluoromethyleneiminium tetrafluoroborate, reacts with carboxylic acids to form acyl fluorides at 0°C and with benzaldehyde to form α,α-difluorotoluene in good yields [10].
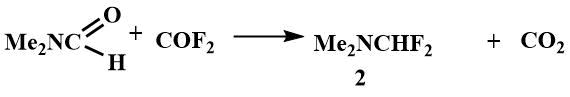
Difluoromethyl(dimethyl)amine, like fluoromethyl(dimethyl)amine, has been shown to be a source of fluoride ion when it reacts with highly electrophilic compounds such as bistrifluoromethylketene and trifluoromrthylisocyanate to give hexafluoroisobutenyl(dimethyl)amine (20) and N-trifluoromethyl-N',N'-dimethylformamidine (21). It is believed that the amine is initially attached to a multiple bond of the unsaturated compound and the intermediates then undergo dephosgenation [48].
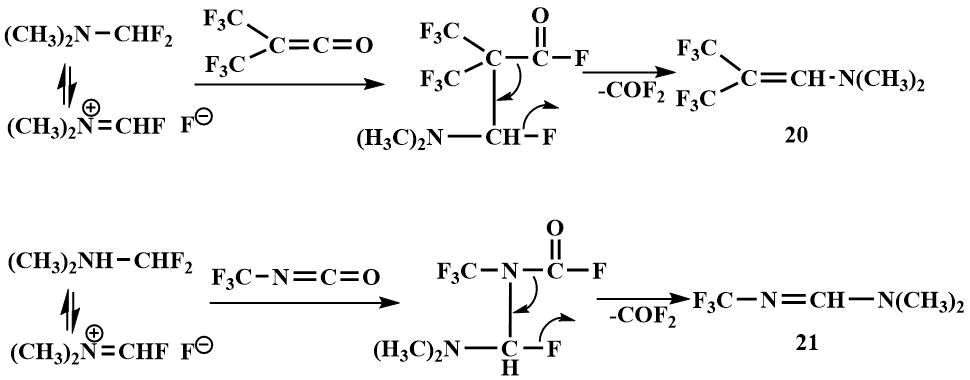
On reaction with hexafluoroacetone, an ether-insoluble crystalline product is formed; analogous to the reaction of fluoromethyl(dimethyl)amine, the formation of a salt (22) may be suspected, but it has not been identified because it is probably decomposed at temperatures above -30°C by dimethylaminofluorocarbene (23), which forms dioxolane (24) with an excess of hexafluoroacetone. (The product of dioxolane hydrolysis has been isolated and identified) [48].
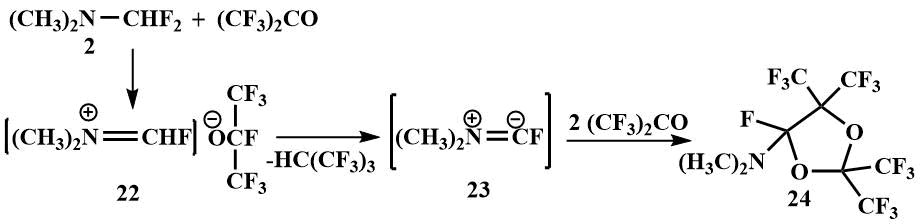
Difluoromethyl(dimethyl)amine (2) is added to perfluoroisobutylene to form an iminium
salt stabilized by the tert-butyl anion (25), which is in equilibrium with salt
(2а). At 20°C, the tert-butyl anion attacks the carbon of the cation of the iminium
salt (25) to form 1-fluoro-1-perfluoro(tert-butyl)trimethylamine (26),
and at 80°C, the tert-butyl anion of the salt (25) deprotonates the cation to form
hydroperfluoroisobutane and dimethylaminofluorocarbene (23) which reacts with perfluoroisobutylene
partially in equilibrium (2а) ↔ (25) to form 1-dimethylamino-perfluoro((3-methyl)butene-2)
(27) in 80% yield [48].
Recently, interest has arisen in the use of difluoromethyl(dimethyl)amine as a fluorinating agent. It has been successfully used as a fluorinating agent for the replacement of hydroxyl by F in the synthesis of the intermediate (28) for the drug fluorophenicol; the fluorination was carried out in dichloromethane. The authors reported that the use of difluoromethyl(dimethyl)amine allowed to reduce the waste as the by-product dimethylformamide is reused to obtain difluoromethyl(dimethyl)amine, lower the temperature required for the reaction compared to fluorination with Ishikawa reagent and increase the yield to 97% [49].
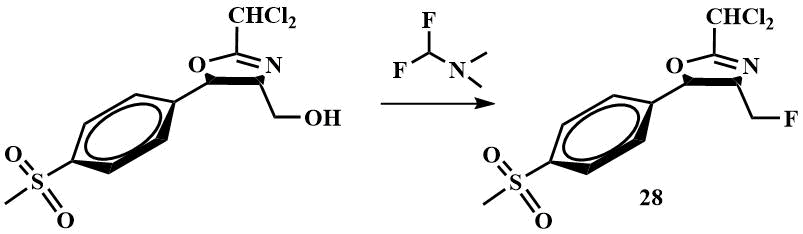
Other authors report that it can be used to fluorinate secondary alcohols. 1-Fluoroethylbenzene was obtained from the corresponding alcohol with a yield of more than 80% [50].

1.3. Trifluoromethyl(dimethyl)amine
Trifluoromethyl(dialkyl)amines, first obtained in 1957, are covalent compounds [51].
Trifluoromethyl(dimethyl)amine is a low boiling liquid with a boiling point of 20°C, trifluoromethyldiethylamine boils at 71°C.
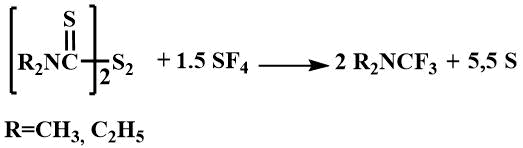
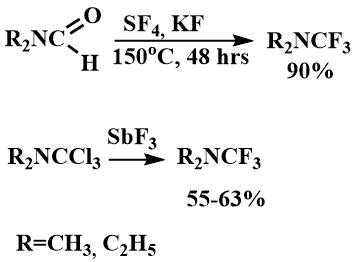
They have also been obtained by fluorination of dimethylformamide with sulfur tetrafluoride in the presence of potassium fluoride [14] or by fluorination of the corresponding trichloromethyldialkylamine with antimony trifluoride [52].
A 1986 X-ray study of the quaternary salt of trifluoromethyldimethyl ammonium iodide showed that the difference in the interatomic distances of N-CF3 (1.491Å) and N-CH3 (1.514Å) is even greater than for the corresponding salt of difluoromethyl(dimethyl)amine [24].
Trifluoromethyl(dialkyl)amines are highly reactive compounds, fumed in air, and decompose rapidly with water to the corresponding formylamines (29).
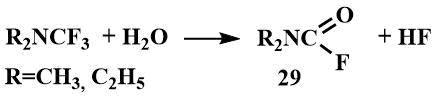
Dmovsky and his co-workers investigated the possibility of using trifluoromethyldiethylamine as a fluorinating agent and suggested that it may be useful for the fluorination of secondary and tertiary alcohols, while its reactions with primary alcohols yield complex mixtures of products. [53]. For example, fluorination of isopropanol gave isopropyl fluoride in 75% yield, and fluorination of tert-butanol gave 3.5: 1 tert-butyl fluoride and 2-methylpropene, while the by-product predominated when Yarovenko's reagent was used [53].
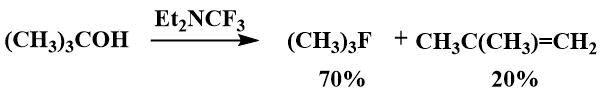
Trifluoromethyldiethylamine has not been added to perfluoroolefins; moreover, it does not catalyze the oligomerization of hexafluoropropene even at elevated temperatures [53].
2. Compounds containing more than 1 nitrogen atom in the α-position to fluorine
2.1 N,N,N'N'-Formamidinium bifluoride
It is well known that the stability of iminium cations increases in the series:
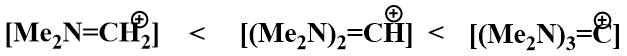
In the search for a fluorine ion source that would increase the stability of the iminium cation and thus its ability to stabilise carbanions, attempts were made in Knunyants' laboratory in the 1980s to obtain compounds containing more than one nitrogen atom in the α-position to fluorine. The first aim of the research was to obtain bis-(dimethylamino)fluoromethane. The substitution of a mobile fluorine atom in difluoromethyl(dimethyl)amine by a dimethylamino group already occurs at room temperature. However, bis(dimethylamino)fluoromethane resulting from the reaction of difluoromethyl(dimethyl)amine with dimethylamine has only been isolated in the form of hydrofluoride, tertamethylformamidinium bifluoride (TMFBF) (30) with a distinct ionic character.
Apparently, the fluoride ion of the resulting bis(dimethylamino)fluoromethane splits off HF from the dimethylamine hydrofluoride, leading to the formation of a salt (30).
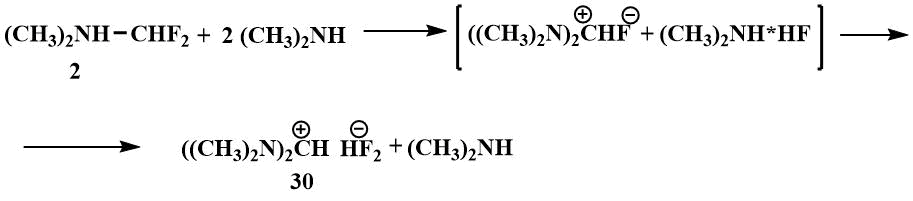
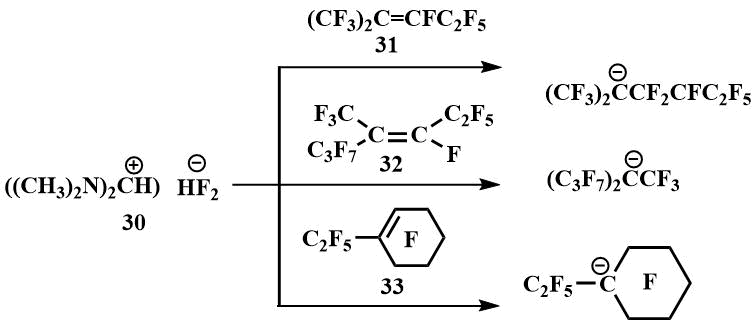
In the presence of TMPBF (30), Igumnov was able to obtain carbanions from a number of perfluoroolefins and fix them by low-temperature NMR, including those from which it had not previously been possible to obtain carbanions with CsF and KF - from perfluoro(2-methylpent-2-ene) (31), perfluoro(4-methylhept-3-ene) (32) and perfluoro(ethylcyclohex-1-ene) (33) [54].
The perfluoroalkylcarbanions thus obtained were acylated with acyl fluorides to give perfluoroalkyl ketones and their enol derivatives [55].
It can be noted that perfluoropropylene reacts with benzoyl fluoride in the presence of TMPBP already at room temperature [56], while in the presence of potassium fluoride this reaction is carried out under pressure at 120°C [57].
Acylation of perfluoropropene with benzoyl fluoride gives perfluoroisopropylphenyl ketone (34), while acylation with acetyl fluoride gives a mixture of ketone (35) with acetate of its enol form (36) [55].

Using TMPBP, it was also possible to acylate perfluoro-tert-butyl anion. When perfluoroisobutylene is
acylated with acetyl fluoride, only acetate (37) and perfluoroisobutylenolate (38)
of the corresponding enol are formed.
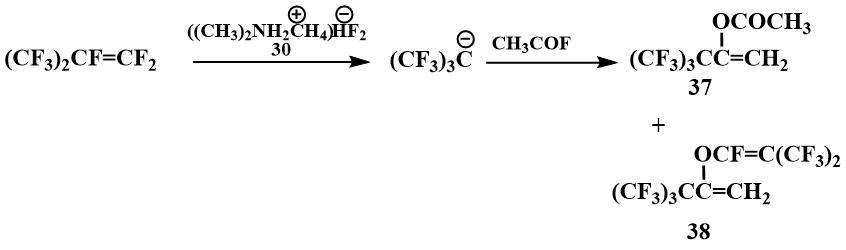
Acylates of enols (37) are converted to the corresponding ketones in a quantitative yield under reflux with H2SO4 [55].

2.2 Bis-dimethylaminodifluoromethane (39) and its cyclic analogue 2,2-difluoro-1,3-dimethylimidazoline (40)
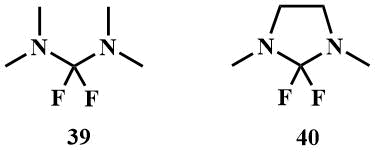
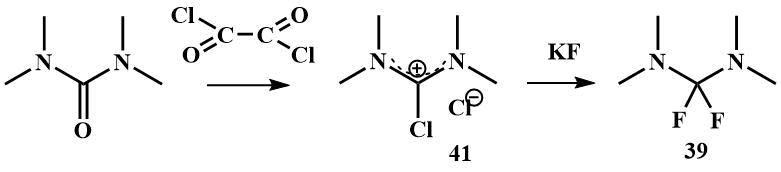
Bis(dimethylamino)difluoromethane (39) can be obtained by fluorination of tetramethylchloroformamidinium chloride (41) with KF, (41) is readily available by chlorination of tetramethylurea with any chlorinating agent. 2,2-Difluoro-1,3-dimethylimidazoline -cyclic analogue of (39) can be obtained in a similar manner [58, 59].
2,2-Difluoro-1,3-dimethylimidazoline (40) has been used as a mild fluorinating agent; it reacts with alkylcarbinols similarly to Yarovenko's reagent and the rest of FARs, as well as with carbonyl compounds. Difluoromethylbenzene has been obtained from benzaldehyde in good yield, while side reactions leading to the formation of alkylvinyl fluorides are observed in reactions with carbonyl compounds containing hydrogen atoms on the adjacent carbon atom [59].
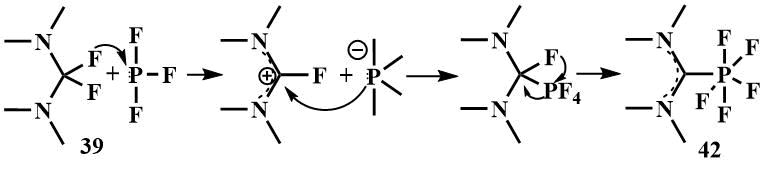
By reacting bis-(dimethylamino)difluoromethane (39) with phosphorus trifluoride, Röschentaller obtained a hexa-coordinated carbene complex of P(V)-fluoride (42) and proposed the mechanism of its formation in which, in the first stage, the fluoride ion is transferred from the amine (39) to phosphorus trifluoride, to form a phosphorus anide ion and a 2-fluoroamidinium cation, then the cation is attacked by the phosphorus anide ion and a C-P bond is formed, whereupon phosphorus cleaves another fluoride ion and forms a complex of phosphorus (V) with a carbene derived from amine (39). The proposed mechanism was confirmed by fixing the tetrafluorophosphoranide anion in solution by low temperature NMR [60]. The analogous complex was obtained from the cyclic analogue (40) [60].
The resulting hexacoordinated phosphorus complexes are colorless solids, stable and slightly soluble in water.
The same authors obtained similar complexes with elements of the main group IV – germanium and tin –
compounds (43) and (44) by oxidative addition of bis-(dialkylamino)difluoromethanes
to the corresponding halides [58].
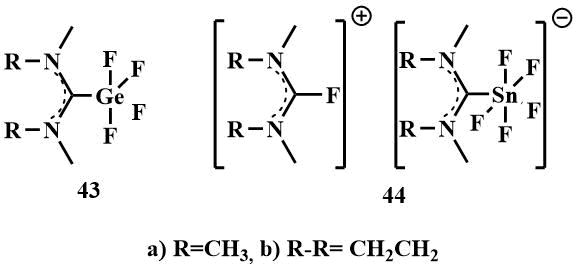
While the solid complexes (43a and b), which are insoluble in THF, were isolated for germanium, the corresponding neutral tin complexes were not obtained. The reaction of (39) and (40) with SnF2 leads to a solution of the salt (44), which is probably due to the fact that tin (IV) tetrafluoride is a stronger Lewis acid and is capable of removing an additional F-ion. from bis‑(dimethylamino)difluoromethane (39) or 2,2-difluoro-1,3-dimethylimidazoline (40) to form hygroscopic salts (44 a and b) with 2-fluoroamidinium as the counterion [58].
2.3 Tris-(dimethylamino)fluoromethane (guanidinium fluoride) (46)
Tris-(dimethylamino)fluoromethane (46) is of particular interest for the generation of fluoride ions. It was first obtained by S. Igumnov by the reaction of hexamethylguanidinium pivalate (45) with dimethylaminotrifluorosulfuran and isolated in the form of hygroscopic crystals [61].
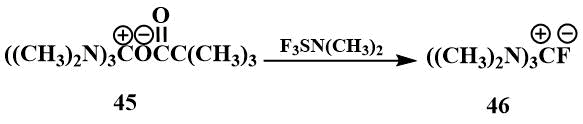
The physical properties of tris-(dimethylamino)fluoromethane (46) were found to be similar to those of the known guanidinium salts: The resulting salt was readily soluble in polar solvents such as DMF and acetonitrile, but did not dissolve in ether. In the IR spectrum of the compound, a band corresponding to the C=N bond in other guanidinium salts was observed. In the 1H NMR spectrum, there was only one signal corresponding to the CH3N group in guanidinium salts, and in the 19F spectrum, there were two signals - a singlet at 25 ppm. (from CF3COOH) corresponding to a fluoride ion not bound by strong hydrogen bonds, and a doublet at 71 ppm (from CF3COOH) indicating that this salt is partially in the form of hydrofluoride. (Such shifts and a constant are characteristic of HF2) [62]. Like tetramethylformamidinium bifluoride, hexamethylguanidinium fluoride reacts with perfluoroisobutylene to form an ionic compound (47), where the carbanion is stabilized by hexamethyltriaminomethane cation [61].
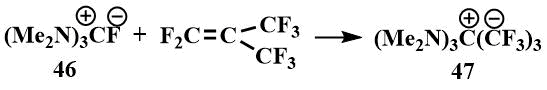
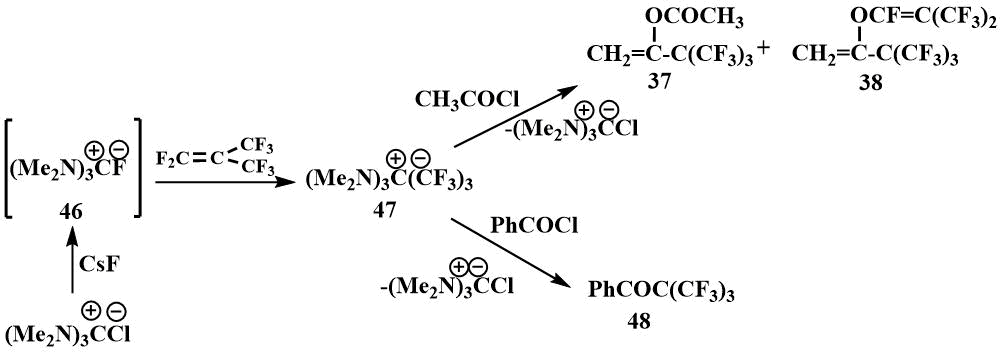
The same compound (47) was obtained by the reaction of guanidinium chloride with cesium
fluoride and perfluoroisobutylene in diglyme. The formation of salt (47) in this
case was also confirmed by its acylation with acetyl chloride to give the enol derivatives (37)
and (38) and benzoyl chloride, giving the ketone (48) [61]. The
preparation of phenyl perfluoro tert-butyl ketone (48), which decomposes in the
presence of traces of a fluoride ion into perfluoroisobutylene and benzoyl fluoride [63], demonstrates
the irreversibility of the addition of perfluoroisobutylene to hexamethylguanidinium fluoride [61].
Guanidinium fluoride, obtained in situ by the reaction of guanidinium chloride with cesium fluoride or potassium fluoride in dimethylformamide, can also be used as a catalyst for fluorination.
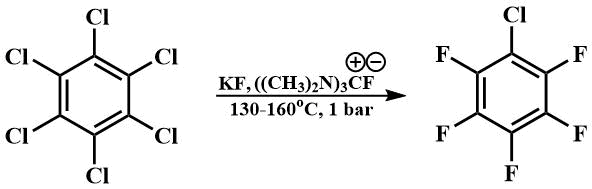
Thus, using this catalyst for fluorination, a technology for fluorination of aromatic compounds with potassium fluoride with continuous selection of target products was developed, which allowed to reduce the fluorination temperature of hexachlorobenzene from 300°C [64] to 130-160°C and to avoid the use of autoclaves. This technology once formed the basis for the production of fluorinated aromatics at the Perm branch of the Russian Research Center for Applied Chemistry [65].
With the hexamethylguanidinium fluoride thus obtained, or a similar hexaethylguanidinium fluoride, it is also possible to fluorinate pentachloropyridine in DMF at its boiling point [66], while without a catalyst a temperature of 200°C is required [67].
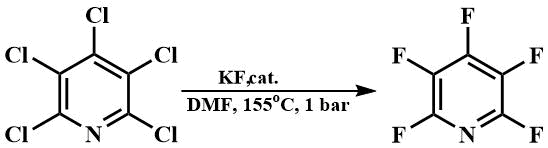
Tris-(dimethylamino)fluoromethane has subsequently attracted the attention of many researchers.
Chang attempted to obtain it by reacting guanidinium hydroxide, prepared by reacting guanidinium chloride with silver hydroxide, with an aqueous solution of hydrogen fluoride in glass, which led, as expected, to guanidinium hexafluorosilicate, which they isolated as hexahydrate and studied X-ray diffraction [68].
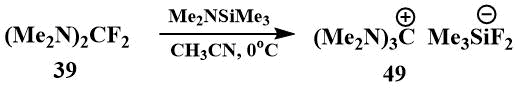
In 2000, Röschentaller reported the preparation and isolation of hexamethylguanidinium fluoride in crystalline form by the reaction of bis-(dimethylamino)difluoromethane (39) with dimethylaminotrimethylsilane [69], but later they found that the isolated salt was difluorotrimethylsilicate (49), i.e. in fact, a complex of guanidinium fluoride with fluorotrimethylsilane [70].
The hexamethylguanidinium difluorotrimethylsilicate thus obtained (in reality the complex of guanidinium fluoride with fluorotrimethylsilane) is also a source of fluorine ions. It reacts with trifluoroacetyl fluoride and hexafluoroacetone in acetonitrile to form stable salts. CF3CF2OCH3 was obtained by the reaction of the salt (49) with methyl triflate at 20°C with a yield of 95%, and CF3CF2OCH2CF3 was obtained by its reaction with trifluoroethyl triflate, these esters have recently attracted much attention because they can replace forbidden chlorine-containing freons [70].
Guanidinium fluoride was also obtained by the reaction of guanidinium borofluoride [C(NMe2)3]BF4 with spray-dried potassium fluoride in a solution of absolute methanol, but when an attempt was made to isolate it by evaporation of methanol, only the bifluoride [C(NMe2)3]HF2 was obtained. [70].
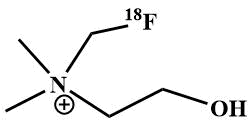
Another compound can be noted, which is α-fluoramine - [18F] fluoromethyl-dimethyl-2-hydroxyethylammonium
- [18F]fluoromethylcholine, which is used as a radiopharmaceutical to visualize cell membrane
metabolism in the diagnosis of prostate and brain tumors by PET [71]
It should be noted, then, that α-fluoroalkylamines, both covalent and ionic, have significantly expanded the choice of fluoride ion sources. They continue to find wide synthetic application in nucleophilic fluorination and perfluoroalkoxylation reactions and provide more opportunities to control the course of such reactions, in particular condensations involving fluoride ion, thus expanding the synthetic possibilities of fluorine chemistry.
Acknowledgments
This work was performed with the financial support from Ministry of Science and Higher Education of the Russian Federation.
References
- Sheppard W.A., Sharts C.M., Organic fluorine chemistry, russ. transl. edition by I.L. Knunyants, Moscow, 1972, 480 p. (in Russian)
- Puar M.S., J.Chem. Educ., 1970, 47(6), 473-474.
- Parker A.J. Q. Rev. Chem. Soc., 1962, 16, 163-187.
- Redwood M.E., Wills S J., Can. J. Chem., 1965, 43(7), 1893-1898.
- Redwood M.E.,Wills S J., Can J Chem., 1967, 45(4), 389-395
- Liotta C.L., JACS, 1974, 96(7), 2250-2252.
- Li, Hui-Yin; Sun, Haoran; DiMagno, Stephen G. e-EROS Encyclopedia of Reagents for Organic Synthesis, 2007, 1-9.
- Böhme H., M.Hilp, Chem. Ber., 1970, 103, 104 -111.
- Faucett F.S., Tullock C.W., Coffman D.D., JACS, 1962, 84(22), 4275-4285.
- Arnold Z., Collect. Czech. Chem. Commun., 1963, 28, 2047-2051.
- Harder J.R., Smith W.C., JACS, 1961, 83(16), 3422-3224.
- Tyrra W., J. Fluorine Chemistry, 2001, 109(2), 189-194.
- Pawelke G. J. Fluorine Chemistry, 1991, 52(2), 229-234.
- Dmowski W., Kaminski M., J. Fluorine Chemistry, 1983, 23(3), 207-218.
- Yarovenko N. N., Raksha M. A., J. Gen. Chem. USSR (Engl. Transl.), 1959, 29, 2125-2128. (in Russian)
- Takaoka A., Iwakiri H., Ishikawa N., Bull. Chem. Soc. Jpn., 1979, 52, 3377–3380.
- Petrov V. A., Adv. Org. Synth., 2006, 2, 269–290.
- Haszeldine R. N., J.Chem.Soc., 1951, 102-104.
- Livingston, R. L.; Vaughan, G., JACS, 1956, 78, 4866-9.
- Bürger, H.; Eujen, R.; Niepel, H.; Pawelke, G., J. Fluorine Chemistry, 1981, 17(1), 65-74.
- Satori P., Velayutham D., Ignat'ev N.; Noel M., J. Fluorine Chemistry, 1997, 83(1), 1-8.
- Moldavskii D. D.; Furin G. G.; Shkul'tetskaya L. V.; Eifman B. Ya., Russ. J. Appl.Chem. (Translation of Zhurnal Prikladnoi Khimii) 2002, 75(6), 959-961. (in Russian)
- Yagupolsky L.M., Kondratenko N.V., Dronkina M.I., Yagupol'skii Yu. L., Zh. Org. Khim., 1980, 16(12), 2508. (in Russian)
- Brauer D. J., Bürger M, . Grunwald M., Pawelke G., J. Wilke J., Z. Anorg. Allg. Chem. 1986, 537, 63-78.
- Oberhammer H., Mendeleev Commun., 2006, 16(3), 136–137.
- Fokin A.V., Studnev Yu.N., Rapkin A.I., Sultanbekov D.A., Potarina T.M., Rus Chem Bull., 1984, 2, 372-375. (in Russian)
- Fokin A.V., Zimin V.I., Studnev Yu.N., Sultanbekov D.A., Zhurnal Obshey Khimii, 1968, 38(7), 1510-1511. (in Russian)
- V. A. Petrov, S. Swearingen, W. Hong and W. Chris Petersen, J. Fluorine Chem., 2001, 109, 25-31.
- Schmitt E., Panossian A., Vors J.-P., Funke C., Lui N., Pazenok S., Leroux F.R., Chem. A Eur. J., 2016, 22, 11239-11244.
- Takaoka A., Iwamoto K.,Kitazume T., Ishikawa N., J. Fluorine Chem., 1979,14, 421-428.
- Aribi F., Schmitt E., Panossian A., Vors J.-P., Pazenok S., Leroux F.R., Org. Chem. Front., 2016, 3, 1392–1415.
- Liska F., Chemicke Listy, 1972, 66(2),189-197.
- Dax, K. Science of Synthesis, 2006, 2005, 34, 71-148.
- Igumnov S., Kornilov V., Fluorine Notes, 2000, 1(8).
- Caster K. C., Zefirov N. S., Lermontov S. A., Filler R., N,N‐Diethyl‐2‐chloro‐1,1,2‐trifluoroethylamine, e-EROS Encyclopedia of Reagents for Organic Synthesis, 2009, 1-3 https://doi.org/10.1002/047084289X.rd184.
- Commare B., Schmitt E., Aribi F., Panossian A., Pazenok S., Leroux F.R., Molecules, 2017, 22, 977-1003.
- Filler R., Hofferberth J., Beckett J., N,N‐Diethyl‐1,1,2,3,3,3‐hexafluoropropylamine, e-EROS Encyclopedia of Reagents for Organic Synthesis, 2007, https://doi.org/10.1002/9780470842898.rd196.pub2
- Junk C.P., Petrov V. A., 1,1,2,2‐Tetrafluoroethyl‐N,N‐dimethylamine, e-EROS Encyclopedia of Reagents for Organic Synthesis, 2014, https://doi.org/10.1002/047084289X.rn01690.
- Böhme H., Hartke K., Chem. Ber., 1960, 93, p. 1305-1309.
- Knunyants I. L., Delyagina N. I., Igumnov S. M., Rus.Chem. Bull., 1981, 30(4), 637–639. (in Russian)
- Igumnov S.M., Delyagina N.I., Zeifman Yu.V., Knunyants I.L., Rus Chem Bull, 1984, 4, 762-766. (in Russian)
- Igumnov S. M., Lekontseva G. I., Shipigusev A. A. , Mukhametshin V. F., Rus. J. Appl. Chem., 2005, 78, 435–437. (in Russian)
- Igoumnov S.M., Fluorine Notes, 2006, 46, 3-4.
- Syntheses of Fluoroorganic Compounds , Edition by I.L. Knunyants (Editor), G.G. Yakobson (Editor), Springer Softcover reprint of the original 1st ed. 1985 edition (December 6, 2011), 311p.
- Allefeld N., Neumann B., Stammler H.-G., Röschenthaler G.-V., Ignat’ev N., and Hoge B., Chem. A Eur. J. 2014, 20, 7736-7745.
- Aravindan V., Gnanaraj J.,] Madhavi S., and Liu H.-K., Chem. Eur. J. 2011, 17, 14326-14346.
- Arnold Z., Coll.Czech. Chem. Comm, 1959, 24, 4048-4049.
- Knunyants I.L., Delyagina N.I., Igumnov S.M., Rus Chem Bull, 1981, 4, 762-766. (in Russian)
- Patent CN111153867A, 2020.
- Patent CN111635321, 2020.
- Patent US2957001, 1957.
- Yagupol'skii, L. M.; Kondratenko, N. V.; Timofeeva, G. N.; Dronkina, M. I.; Yagupol'skii, Yu.L. , Zhurnal Organicheskoi Khimii, 1980,16 (12), 2508-2513. (in Russian)
- Dmowski W. , Kaminski M., J Fluorine Chem. 1983, 23, 219-228.
- Delyagina N. I., Igumnov S. M., Snegirev V. F., Knunyants I. L., Russ. Chem. Bull. 1981, 30(10), 1836-1840. (in Russian)
- Igumnov S. M., Delyagina N. I. , Knunyants I. L. , Russ.Chem. Bull., 1981, 30(10), 1924-1927. (in Russian)
- Igumnov S.M., New sources of fluoride ion stabilized by organic cations: phD dissertation, Moscow, 1983, 145p. (in Russian)
- Ishikawa N., Shin S., Bull.Chem. Soc.Jpn, 1975, 48(4), 1339-1340.
- Böttcher T., Bassil B.S., Zhechkov, Röschenthaler G.-V., Inorganic Chemistry, 2012, 51(2), 763-765.
- Hidetoshi Hayashi, Hiroshi Sonoda, Kouki Fukumura and Teruyuki Nagata, Chem.Comn., 2002, 1619-1619.
- Böttcher T., Shyshkov O., Bremer M., Bassil B. S., Röschenthaler G.-V., Organometallics, 2012, 31(4), 1278-1280.
- Igumnov S. M. , Delyagina N. I. , Knunyants I. L., Rus. Chem. Bull. 1986, 35(6), 1191-1193. (in Russian)
- Fujiwara F.Y., Vartin J.S., JACS, 1974, 96(25), 7625-7626.
- Knunyants I.L., Zeifman Yu.V., Lantseva L.G., Doklady Akademii Nauk SSSR, 1980, 254(1), 117-120 (in Russian).
- Vorozhtsov N. N., V. E. Platonov V. E., Yakobson G. G. Rus.Chem. Bull ,1963, 12, 1389. (in Russian)
- Patent RU2164508, 2001. (in Russian)
- Syntheses of Fluoroorganic Compounds, (Syntezi Ftororganicheskih soedinenii), v.4, Edited by Igumnov S.M., Igymnova E.V., Moscow, ZAO PIM Invest, 2018, 222 p. (in Russian)
- Chambers K. D., Hutchinson J., and Musgrave W. K. R., Chem Soc 1964, 3573 -
- Zhang, R. Bau,J.A,Sheehy, K.O.Christe J., Fluor.Chem., 1999, 98, 121-126
- Kolomeitsev A.A., Bissky G., Kirsch P., Röschenthaler G.-V., Journal of Fluorine Chemistry, 2000, 103, 159-161.
- Kolomeitsev A. A., Bissky G., Barten J., Kalinovich N, Lork E., Röschenthaler G.-V., Inorganic Chemistry, 2002, 41(23), 6118-6124.
- Fedorova O. S., Vaitekhovich F. P., Krasikova R. N., Pharmaceutical Chemistry Journal, 2018, 52(8), 730-734.
ARTICLE INFO
Received 01 November 2021
Accepted 05 November 2021
Available online December 2021
Recommended for publication by PhD M. A. Manaenkova
Fluorine Notes, 2021, 139, 3-4
