Preparation by the interaction of fluorinated alcohols with diazomethane, addition of polyfluoroalkyl iodides to alkenes, intermolecular dehydration..."
Received: October 2021
DOI 10.17677/fn20714807.2021.05.04
Fluorine Notes, 2021, 138, 7-8
FLUORINATED ETHERS.
COMMUNICATION 2. PREPARATION BY THE REACTION OF FLUORINATED ALCOHOLS WITH DIAZOMETHANE,
ADDITION OF POLYFLUOROALKYL IODIDES TO ALKENES, INTERMOLECULAR DEHYDRATION, ADDITION OF PERFLUOROALKYL
HYPOHALOGENITES TO ALKENES AND PYROLYSIS OF DERIVATIVES OF PERFLUORO-2-ALKOXYPROPIONIC ACIDS
S.V. Vershilova, V.V. Kornilov, A.S. Tsyrulnikovaa,b, L.M. Popovaa,b, N.V. Lebedevа
a S.V. Lebedev Scientific Research Institute of Synthetic Rubber, Gapsalskaya str. 1, St. Petersburg, 198035, Russia
b Peter the Great St.Petersburg Polytechnic University, Novorossiyskaya str. 48, St. Petersburg, 194021, Russia
Abstract: The preparation of fluorinated ethers by the reaction of fluorinated alcohols with diazomethane, addition of polyfluoroalkyl iodides to alkenes, intermolecular dehydration, addition of perfluoroalkylhypohalogenites to alkenes, and pyrolysis of derivatives of perfluoro-2-alkoxypropionic acids are considered in the second part of the review. The synthesis conditions and possible mechanisms of reactions are presented for individual methods.
Key words: diazomethane, perfluoroalkyl iodides, fluorinated alcohols, intermolecular dehydration, perfluoroalkyl halides, perfluoro-2-alkoxypropionic acids, perfluoroalkyl vinyl ethers.
Introduction
Two general methods for the preparation of fluorinated ethers were described in the first part of the review [1] - the alkylation of alcohols (alkyl and alkenyl halides, alkyl sulfates and alkyl sulfonates) and the addition of alcohols to alkenes and alkynes. In this article, the authors discuss a number of other methods for the preparation of fluorinated ethers, namely:
- reaction of fluorinated alcohols with diazomethane
- addition of polyfluoroalkyl iodides to alkenes
- intermolecular dehydration reactions
- addition of perfluoroalkyl hypohalogenites to alkenes
- pyrolysis of derivatives of perfluoro-2-alkoxypropionic acids
Some of these methods are mainly used in laboratory. Others, such as pyrolysis of derivatives of perfluoro-2-alkoxypropionic acids, are widely used in industry.
1. Preparation of methyl-fluoroalkyl ethers by the reaction of fluorinated alcohols with diazomethane
One of the first mentions of the reaction of fluorinated alcohols with diazomethane is found in the A. Henne and M. Smook’s paper in 1950 [2]. The authors carried out the reaction of 2,2,2-trifluoroethyl ether with diazomethane, which was dissolved in petroleum ether. The reaction was carried out at -10°C to the yellow colour of the solution disappearance. The reaction gave the corresponding methyl-2,2,2-trifluoroethyl ether in 27% yield.
CF3CH2OH + CH2N2 → CF3CH2OCH3.
The addition of Al(O-i-Pr)3 (aluminium isopropoxide) to the process as an acid catalyst made it possible to increase the yield to 75% but the resulting product contained a difficult-to-separate hydrocarbon impurity. The use of other solvents, in particular dicyclohexyl, allowed only a slight increase in the ether yield (30%).
H.G. Adolph, M.J. Kamlet [3] described the reaction of a diazomethane solution in ether with 2,2-dinitro-2-fluoroethanol. The reaction product was the expected 2-fluoro-2,2-dinitroethyl methyl ether. The authors noted that 2,2-dinitro-2-fluoroethanol actively reacted with diazomethane in ether only in the presence of boron trifluoride etherate (BF3) as a catalyst, while the alcohol conversion and the yield of the target methyl ether (bp 45°C, 3 mmHg) were low.
The reactions of perfluoropinacone with diazomethane, dissolved in diethyl ether at room temperature, are considered in the paper by A. Eleev and V. Cherstkov et al. [4]. The authors obtained both monomethyl (bp 135°C, yield 85%) and dimethyl (mp 50-51°C, yield 60%) perfluoropinacone ethers.
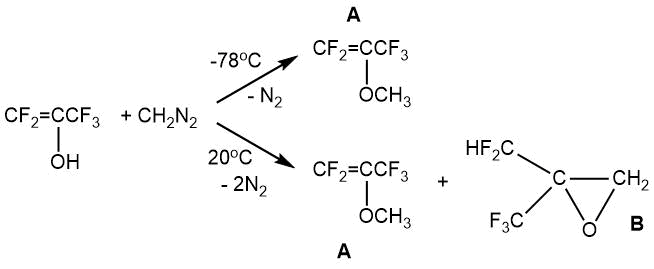
According to I.L. Knunyants et al. [5] the reaction of pentafluoropropen-2-ol (in the enol form) with diazomethane in dibutyl ether led to various products depending on the synthesis conditions. Pentafluoro-iso-propenyl methyl ether «A» (bp 32°С) thus was obtained at -78°С in 45% yield, and a mixture of pentafluoro-iso-propenyl methyl ether «A» and oxirane «B» was obtained at 20°С ( 60 and 40%, according to the GLC data).
2. Addition of polyfluoroalkyl iodides to alkenes
The use of perfluoroalkyl iodides in the reactions with aliphatic unsaturated ethers makes it possible to introduce a perfluoroalkyl moiety into a substrate that already has an ether bond. The addition reaction of n-perfluorobutyl iodide (C4F9I) to nonfluorinated vinyl ether was studied in the paper by C. Dapremont and C. Amatore [6]. This reaction gave an 89% yield of the corresponding ether during electrochemical activation.
n-C4F9I + CH2=CHOC4H9 → n- C4F7CH2CHIOC4H9
M. Knell and N. Brace's patent [7] describes the addition of 1-iodoperfluoroheptane to allyl propyl ether to obtain n-propyl 2-iodo-1,1,2,3,3-pentahydroperfluorodecyl ether in the presence of azobisisobutyronitrile (AIBN) as an initiator. The treatment of the reaction mixture for 12 hours at 70°C led to the target product in 71% yield.
n-C7F15I + CH2=CHCH2OC3H7 → n-C7F15CH2CHICH2OC3H7
In a similar way, N. Brace [8] obtained the addition product of 1-iodoperfluorobutane to allyl perfluoroisopropyl ether (initiator AIBN) in 75% yield.
n-C4F9I + CH2=CHCH2OCF(CF3)2 → n- C4F9CH2CHICH2OCF(CF3)2.
The addition of perfluoroalkyl iodide to the allyl fragment of polyethylene glycols was carried out by H. Meinert et al at a higher temperature (170°C) [9].
C6F13I + CH2=CHCH2OR → C6F13CH2CHICH2OR
R = CH3(OC2H4)m, where m=7,12,17
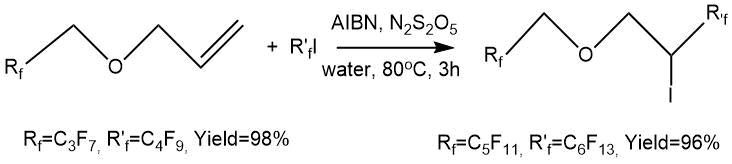
D. Lazzari et al. [10] synthesized a number of fluorinated multiblock molecules, in particular 2,2,3,3,4,4,4-heptafluorobutyl 4,4,5,5,6,6,7,7,7-nonafluoroheptyl and 4,4,5,5,6,6,7,7,8,8,9,9,9-tridecafluorononyl 2,2,3,3,4,4,5,5,6,6,6-undecafluorohexyl ether. These ethers were obtained by the reaction of allyl polyfluoroalkyl ethers with perfluoroalkyl iodides (C4F9I, C6F13I) followed by deiodination. The reactions were carried out in an aqueous medium in the presence of the initiating system (Na2S2O5, AIBN) at a moderate temperature (80°C). The synthesis time was 3 hours and the yield of adducts was high (95–98%).
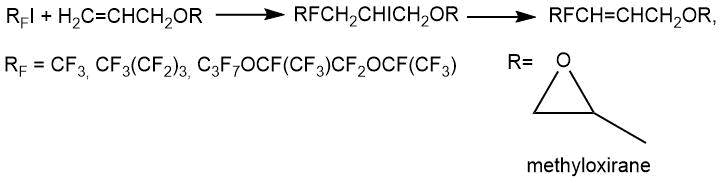
V.I. Saloutin et al. [11] performed perfluoroalkylation of glycidyl ether with perfluoroalkyl iodides in aqueous acetonitrile in the presence of Na2S2O4 (argon atmosphere, 0-5°C, 1h, yield 68-75%).
3. Preparation of fluorinated ethers by the method of intermolecular dehydration of alcohols
Fluorinated alcohols are less apt to ether formation by dehydrating agents than their fluorine-free analogues. A. Henne and M. Smook in their paper reported on an unsuccessful attempt to obtain fluorinated ethers by intermolecular dehydration of alcohols using 2,2,2-trifluoroethanol and 3,3,3-trifluoropropanol as examples [2].
In the article by V.A. Komarov et al. [12], it was also noted that the action of sulfuric acid or phosphoric anhydride on 2,2-difluoro-2-nitroethanol did not lead to the formation of the corresponding ether. However, in some cases fluorinated alcohols can react with other alcohols in the presence of dehydrating agents such as concentrated sulfuric acid (H2SO4), methanesulfonic acid (CH3SO3H), p-toluenesulfonic acid or glacial acetic acid.
H. Muramatsu et al. [13] thus obtained ethyl ethers of mono- (bp. 75-76°C), di- (bp. 66-67°C) and trifluoroethanol (bp. 50-51°C) with moderate yields by heating ethanol and the corresponding fluorinated alcohol in the presence of sulfuric acid.
RFCH2OH + C2H5OH → RFCH2OC2H5,
RF = H2FC- (yield 30%), HCF2- (yield 55%), CF3- (yield 18%).
The reaction of 2,2-dinitro-2-fluoroethanol with 1-bromo-1,1,4-trinitro-4-azapentanol-5 in the presence of concentrated sulfuric acid (94%) (0-10°С, 2 h) is described in the paper [14]. The reaction gave unsymmetrical ether (mp 50-53°C) in 70% yield.
FC(NO2)2CH2OH + R-N(NO2)CH2OH → FC(NO2)2CH2OCH2N(NO2)-R,
R- = BrC(NO2)2CH2CH2-.
As noted by the authors of the cited paper, the predominant formation of the asymmetric ether occurred when using an excess of 2,2-dinitro-2-fluoroethanol and 80-90% sulfuric acid. In the case of equimolar amounts of reagents, a mixture of symmetric and unsymmetrical ethers was formed.
In the paper of V.V. Korshak et al. [15], it was noted that tertiary vinyl ethynyl carbinols in the presence of mineral acids formed ethers with alcohols more readily than primary alcohols. It was shown that the reaction of dimethyl(vinyl)ethynylcarbinol with a number of polyfluorinated alcohols (H2SO4, 20-40°C, 20 h) gave the corresponding polyfluoroalkyl ethers in moderate yields (40-60%).
CH2=CH-C≡C-C(CH3)2OH + HOCH2(CF2)nH → CH2=CH-C≡C-C(CH3)2OCH2(CF2)nH.
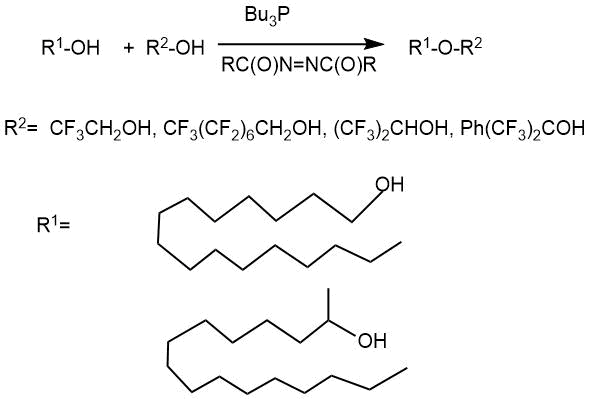
Mitsunobu condensation variant, modified by J. Falk et al. [16], opened the possibility of synthesis asymmetric polyfluorinated ethers by condensation of alcohols with polyfluorinated primary, secondary and tertiary alcohols under relatively mild conditions. Condensation of non-fluorinated and polyfluorinated alcohols in anhydrous benzene in the presence of 1,1'- (azodicarbonyl)dipiperidine (ADDP) and tri-tert-butylphosphine (Bu3P) led to the target ethers with a medium (33-55%) to high yield (83-96%) depending on the structure of non-fluorinated alcohol.
4. Addition of perfluoroalkyl hypohalogenites to alkenes
One of the first reports on the reaction of perfluoroalkyl hypohalogenites with alkenes is the article by R. Porter and G. Cady, published in 1957 [17]. The attempts to obtain trifluoromethyl pentafluoroethyl ether by the reaction of trifluoromethyl hypofluorite with tetrafluoroethylene were unsuccessful. A solid polymer or a mixture of carbonic oxide and tetrafluoromethane were obtained as a result of the reaction. At the same time, the use of the less reactive perfluorocyclopentene led to the production of the desired perfluoro(methoxycyclopentane) in almost quantitative yield.

The reaction of trifluoromethyl hypofluorite with ethylene to obtain trifluoromethyl 2-fluoroethyl ether was described in 1959 in the article by J. Alison and D. Cady [18]. A fourfold dilution of ethylene with nitrogen and UV irradiation for 12 hours was required to successfully carry out the reaction with a yield close to quantitative.
CF3OF + CH2=CH2 → CF3-O-CH2CH2F
In the absence of dilution, mixing of the starting reagents led to an explosion after a short induction period.
The article by L. Anderson et al. [19] describes the reactions of polyfluoroalkyl hypochlorites with various alkenes, which led to the corresponding ethers in high yield (>90%).
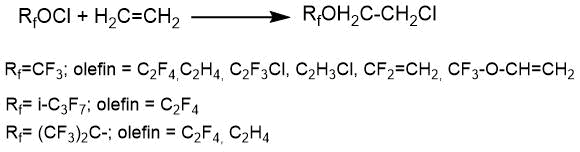
According to the authors of the cited article, the chlorine atom of the hypofluorite molecule predominantly associates to the carbon atom at the double bond, which has the highest electron density. Therefore, the reaction of trifluoromethyl hypochlorite with 1,1-difluoroethylene and vinyl chloride almost proceeds selectively.
CF3OCl + CF2=CH2 → CF3OCF2CH2Cl (изомер CF3OCF2CH2Cl - 96%)
CF3OCl + CClH=CH2 → CF3OCHClCH2Cl
The formation of two isomers is observed in the cases where the difference between the electron density on carbon atoms at the double bond was small (for example, in the chlorotrifluoroethylene molecule).
CF3OCl + CClF=CF2 → CF3OCFClCF2Cl + CF3OCF2CFCl2
This assumption was also confirmed in [20], where the results of a study of the reaction of hexafluoropropylene with trifluoromethyl hypochlorite at 0°С with the preparation of two adducts are presented.
CF3OCl + CF2=CFCF3 → CF3OCF2CFClCF3 (70%) + CF3OCF(CF3)CF2Cl (30%).
The use of the reaction of perfluoroalkyl hypofluorites with perfluoroalkenes makes it possible to synthesize completely fluorinated ethers in some cases. S. Toy and R. Stringham [21] thus obtained perfluoro-n-propyl perfluoro-tert-butyl ether by the reaction of perfluoro-tert-butyl hypofluorite with hexafluoropropene. In this case, the content of the isomer with the perfluoro-n-propyl fragment exceeded 95%.
CF3CF=CF2 + (CF3)3COF → CF3CF2CF2OC(CF3)3.
The authors of this paper suggested that the reaction could proceed through an electrophilic attack of the O-F group followed by the addition of the fluoronium ion.
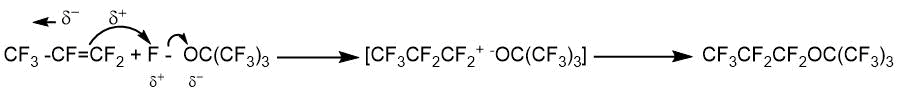
In the Italian patent [22], it was shown that the reaction of trifluoromethyl hypofluorite with 1,2-dichlorodifluoroethylene at -70°C led to the production of trifluoromethyl 1,2-dichlorotrifluoroethyl ether in 60% yield.
CF3OF + CClF=CClF → CF3-O-CClF-CClF2
The reaction of synthesis perfluoro-tert-butyl 1,2-dichlorotrifluoroethyl ether was described in the article by C. Lu et al. (-55°C, yield 93%) [23].
5. Pyrolysis of derivatives of perfluoro-2-alkoxypropionic acids
Most of the known methods for the synthesis of fluorinated ethers are not suitable for the preparation of perfluoro(alkyl vinyl) ethers (Rf-O-CF=CF2), which are the key monomers in the production of highly fluorinated copolymers with unique properties.
It is possible to use dehalogenation of 1,2-dichlorotrifluoroethyl perfluoroalkyl ethers to obtain a number of compounds for example perfluoro(methyl vinyl) ether. Such ethers can be obtained by the addition of trifluoromethyl hypofluorite to alkenes (see chapter 4).
In addition, Ch. Fritz and S. Selman [24, 25] mentioned the possible reaction of alcoholates of perfluorinated alcohols with tetrafluoroethylene.
RfOMe + CF2=CF2 →Rf-O-CF=CF2 + MeF
In 1965 M. Redwood and C. Willis [26] reported on the preparation of trifluoromethoxides of a number of alkali metals by the reaction of carbonyl fluoride with metal fluorides in acetonitrile:
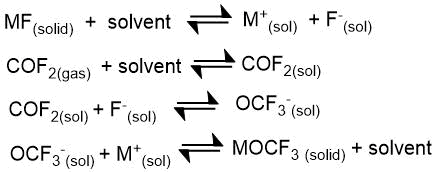
Where M=K, Rb, Cs.
Potassium, rubidium and cesium trifluoromethoxides were isolated and described as stable solid crystalline compounds. The attempts to obtain lithium or sodium alcoholates under these conditions were unsuccessful.
In 1967 M. Redwood and S. Willis also obtained fully fluorinated ethoxides, n-propoxides, isopropoxides, and n-butoxides of the heavier alkali metals (K, Rb, Cs) [27].
However, the authors of this review could not find any works describing the possible synthesis of perfluoro(alkyl vinyl) ethers by the reaction of alcoholates of perfluorinated alcohols with tetrafluoroethylene or chlorofluoroethylenes.
The only practical method for synthesis a wide range of perfluoroalkyl vinyl ethers is pyrolysis of fluoroanhydrides or salts of perfluoro-2-alkoxypropionic acids to date. A brief description of the method is given in the book by S. Ebnesajjad on the example of perfluoro(propyl vinyl) ether synthesis (PPVE) [28].
In the first stage of the process, hexafluoropropylene oxide (HFPO) reacts with perfluorinated acyl fluoride in the presence of a fluorine anion (for example, alkali metal fluorides) to form perfluoro-2-alkoxy-propionyl fluoride.
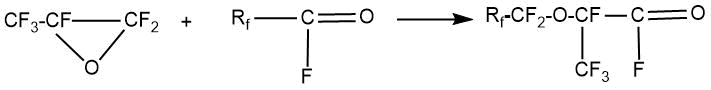
In the second stage, perfluoro-2-alkoxy-propionyl fluoride reacts with an oxygen-containing salt (carbonates, sulfates) of an alkali or alkaline earth metal at an elevated temperature. The range of used temperatures depends on the nature of the salt. In some cases, perfluoro-2-alkoxy-propionyl fluoride is converted into an alkali or alkaline earth metal salt, which is then decarboxylated. This stage of the process is often named like the pyrolysis stage.
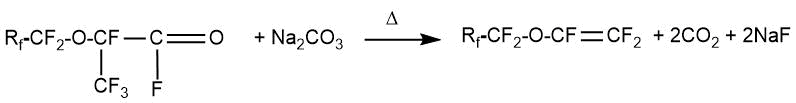
A possible mechanism for the reaction of HFPO with perfluoroacyl fluorides is presented in the work by H. Millauer et al. [29] on the example of synthesis perfluoro(2‐propoxypropionyl) fluoride. At the initial stage, perfluoropropionyl fluoride can transform into the equilibrium form of perfluoropropoxide ion 2, which interacts with one HFPO molecule to form alkoxide 3. Further, the alkoxide 3, depending on the process conditions, can either interact with the next HFPO molecule or transform into perfluoro-2‐propoxypropionyl fluoride 4 after elimination of one fluoride-ion.
The patent of C. Fritz, E. Moore and S. Selman [24] is one of the first examples of synthesis perfluoro(alkyl vinyl) ethers by pyrolysis. This patent describes the preparation of perfluoro(methyl vinyl), perfluoro(ethyl vinyl), perfluoro(n-propyl vinyl) (PPVE) and several other ethers.
The reaction of perfluoroacyl fluoride with HFPO was carried out in the presence of a catalyst in an inert polar solvent. Activated carbon, alkali metal fluorides, silver fluoride, or a quaternary ammonium salt were used as a catalyst.
The second stage of pyrolysis was carried out at temperatures from 100 to 600°C. The examples describe the use of sodium sulfate, potassium sulfate, cesium carbonate and a number of other salts.
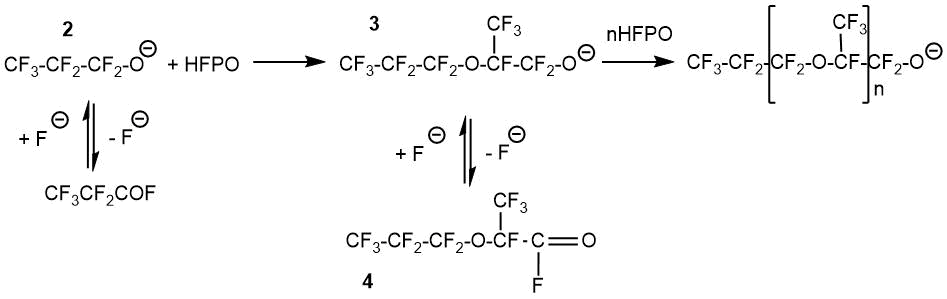
A possible two-stage mechanism for the formation of sodium salts of carboxylic acids was proposed by C. Fritz and S. Selman using sodium sulfate and carbonate as an example [25].
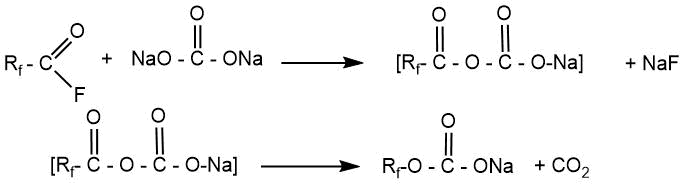
The authors of this patent noted that oxygen-containing salts used for pyrolysis must be thoroughly dried since residual moisture leads to the formation of hydrogen-containing compounds.
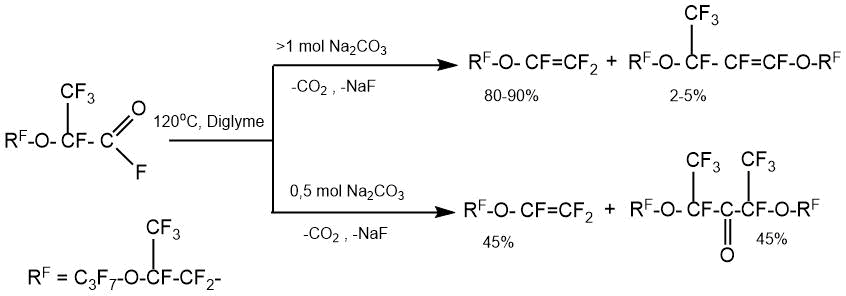
By the example of the pyrolysis of perfluoro-2-(2-propoxypropoxy)propanoyl fluoride it was shown that, depending on the taken amount of sodium carbonate for the reaction relative to perfluoroacyl fluoride, the direction of the pyrolysis process can lead to the predominant formation of perfluoro(alkyl vinyl) ethers or the corresponding diperfluoro-1-methyl-2-oxaalkyl ketones [30]:
In 1961 D. Anderson and S. Selman published an article [31] where applied the method of pyrolytic decarboxylation to obtain divinyl ethers. At the first stage of the process, perfluoro-2-methyl-3-oxa-adipoyl fluoride was obtained from perfluoromalonyl fluoride and HFPO.
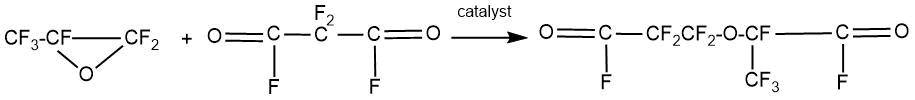
The process was carried out in a polar solvent medium (ethylene glycol dimethyl ether, acetonitrile, and dimethyl sulfoxide or N-methylpyrrolidone) with the addition of catalytic amounts of alkali metal or quaternary ammonium fluorides.
The synthesis of perfluoro(alkyl vinyl) ether from the obtained acyl fluoride was conducted by two methods:
1) When a dry oxygen-containing salt of an alkali metal or zinc oxide interacts with perfluoro-2-methyl-3-oxa-adipoyl fluoride at 250-350°C.
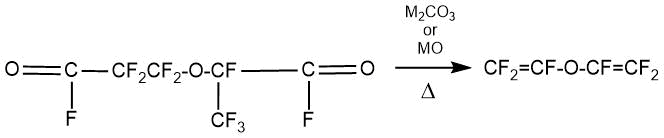
M = alkali metal or zinc.
2) Perfluoro-2-methyl-3-oxa-adipoyl fluoride was treated with an aqueous solution of an alkali metal hydroxide to give the corresponding salt. Next, the resulting salt was pyrolized at 250–270°C.
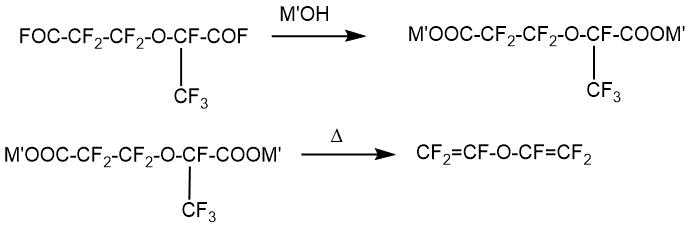
M’= alkali metal.
R. Sullivan showed the possibility of monodecarboxylation of salts of dicarboxylic acids with the formation of perfluoro(alkyl vinyl) ethers containing a carboxyl group [32]. The unsymmetrical diacyl fluorides were obtained as a result of the addition of HFPO to perfluoroglutaryl and succinyl fluorides.
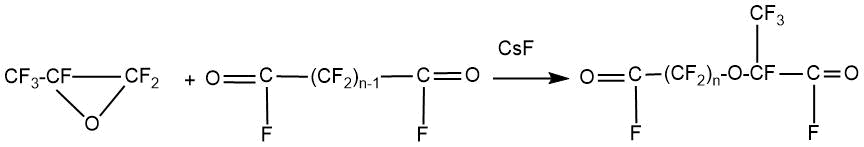
n = 3,4; yield ~70%
When studying the pyrolysis process of the obtained salts, it was found that at a low temperature of 185-200°C, monodecarboxylation occurs with predominant decarboxylation of a carbonyl group having a trifluoromethyl group in the α-position. The author attributed this to the stabilizing role of the α-CF3 group in the intermediate carbanion formation.
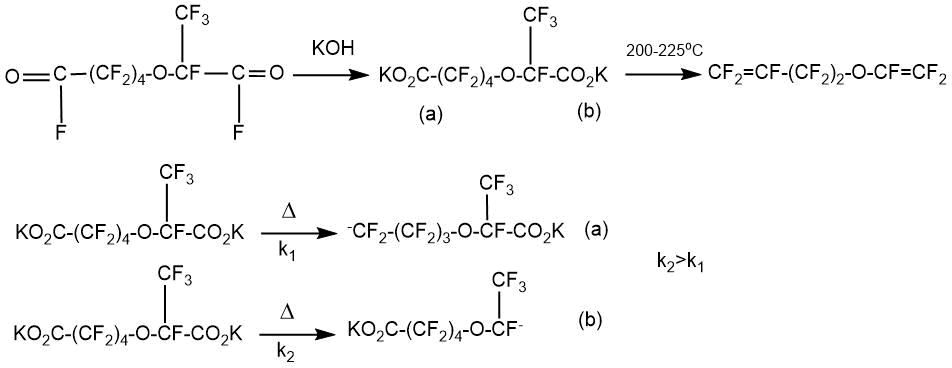
It should be noted that the yield of potassium perfluoro-6-oxa-7-octenoate obtained in this way was low (~ 25%):
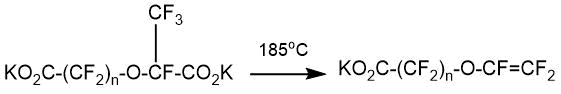
It was noted that in the case of perfluoro-oxa-alkanedicarboxylic acids having 6-7 atoms in the chain, pyrolysis of their salts proceeds predominantly with the formation of cyclic compounds [33]. During the pyrolysis of diacyl fluorides, obtained as a result of the addition of HFPO to perfluoromalonyl and succinyl fluorides, the formation of perfluoro-2-methyloxalan-3-one and perfluoro-2-methylpyran-3-one was observed, respectively:
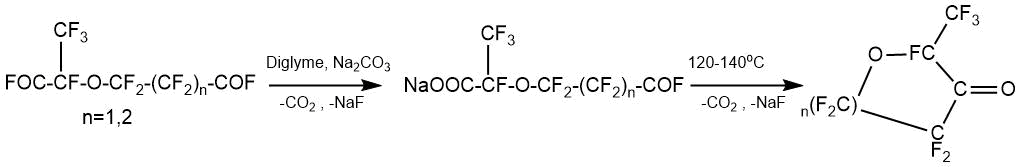
The process of monodecarboxylation, which avoids cyclization processes in the preparation of vinyl ethers by the pyrolytic method, is described in the article by M. Yamabe [34].
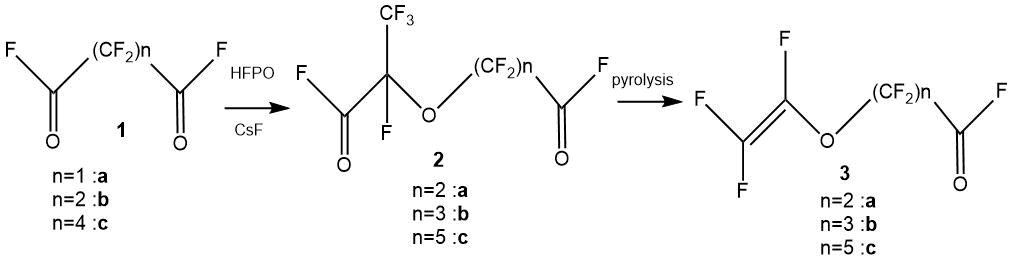
Acyl fluorides 1(a-c) were treated with HFPO in the presence of anhydrous cesium fluoride to obtain the intermediate 2-(perfluoroalkoxy)-2,3,3,3-tetrafluoropropinonyl difluorides 2(a-c).
The pyrolysis stage was carried out in the gas phase by passing dilute vapors of asymmetric diacyl fluorides 2(a-c) through the pyrolysis zone. The temperatures, diluent composition, and other reaction parameters are shown in the table.
|
# |
Starting compound 2 |
Temperature (°C) |
Diluter |
Gas volume ratios |
Volumetric flow rate (min. -1) |
Conversion (%) |
Selectivity 3a-c (%) |
|
|
1 |
2a |
295 |
n2 |
1/6 |
2.2 |
18.5 |
3a |
79 |
|
2 |
2a |
300 |
N2/SO2 (4:1) |
1/6 |
2.2 |
14.3 |
3a |
83 |
|
3 |
2a |
300 |
Air/H2O (1000:5) |
1/20 |
6.5 |
22.5 |
3a |
92 |
|
4 |
2b |
295 |
N2 |
1/6 |
2.2 |
19.5 |
3b |
85 |
|
5 |
2b |
300 |
N2/SO2 (4:1) |
1/6 |
2.2 |
15.0 |
3b |
85 |
|
6 |
2b |
300 |
Air/H2O (1000:5) |
1/6 |
6.5 |
20.0 |
3b |
85 |
|
7 |
2b |
370 |
N2 |
1/6 |
2.2 |
80.0 |
3b |
85 |
|
8 |
2c |
290 |
N2 |
1/6 |
2.2 |
20.3 |
3c |
83 |
|
9 |
2c |
300 |
N2/SO2 (4:1) |
1/6 |
2.2 |
14.5 |
3c |
81 |
|
10 |
2c |
300 |
Air/H2O (1000:5) |
1/6 |
6.5 |
25.0 |
3c |
89 |
|
11 |
2c |
370 |
N2 |
1/6 |
2.2 |
78.5 |
3c |
32 |
V. Bardin and H. Frohn's article describes a typical procedure for the preparation of perfluoro(alkyl vinyl) ethers using PPVE (C3F7OCF=CF2) as an example. This procedure includes the pyrolysis process (300-350°C) of the sodium salt of the corresponding acid, which was pre-dried for 4 hours at 150°C [35].
C3F7OCF(CF3)CO2Na → C3F7OCF=CF2 (bp 35°С, Yield 46%).
The preparation of perfluoro-2-methoxypropionyl fluoride and the synthesis of various perfluoro(alkyl vinyl) ethers are described in the patent by J. Harris [36]. These substances were obtained from the corresponding perfluoro-2-alkoxypropionyl fluorides by pyrolysis while passing through a potassium sulfate layer. Another method for producing ethers consisted in heating the potassium salts of perfluoro-2-alkoxy-propionic acids previously obtained from perfluoro-2-alkoxypropionyl fluorides
As an example, this patent shows the synthesis of perfluoro-2-methoxypropionyl fluoride (bp 10-12°C) by the reaction of carbonyl fluoride with HFPO (CsF, diglyme, autoclave, -80°C, 75°C, 4 h) with a yield about 70%.
Perfluoro(methyl vinyl) ether (bp -22°С, 60%) was obtained at the next stage by passing perfluoro-2-methoxypropionyl fluoride through a layer of dry K2SO4 (300°С, the contact time 10 min).
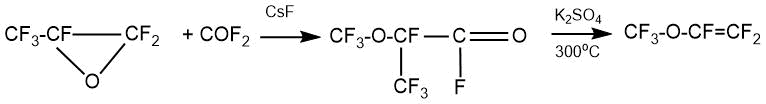
In another example of this patent, it is shown that when using a dry potassium salt of perfluoromethoxypropionic acid, the yield of vinyl ether was 80% at a lower pyrolysis temperature (185-215°C, 24 h). Perfluoro(propyl vinyl) ether (PPVE) was obtained by pyrolysis of the potassium salt in 79% yield in a similar manner.
2-Perfluoropropoxypropionyl fluoride was synthesized directly from HFPO in some cases. In these processes, perfluoropropionyl fluoride was formed in situ by isomerization of the HFPO molecule in the presence of cesium fluoride or other sources of fluoride ion [29].

Alkylamines can be used for isomerization of HFPO to perfluoropropionyl fluoride in addition to alkali metal fluorides. The example of such synthesis is given in the article by N. Ishikawa et al. [37]. HFPO was added to a mixture of tetramethylurea (Me2N)2CO) and diglyme at - 70°C. The mixture then was brought to room temperature and stirred for 3 hours. The yield of perfluoro-2-propoxypropionyl fluoride was about 70%.
Perfluoro-2-propoxypropionyl fluoride was obtained by treating HFPO with silver fluoride or tetraethylammonium iodide in acetonitrile at 0°C in E. Moore's patent [38].
As noted above, the absence water traces during pyrolysis is an important factor for the preparation of perfluoro(alkyl vinyl) ethers. Perfluoromonohydroalkanes, formed in the presence of water traces, are by-products in the production of vinyl ethers which reduce their yield [32]. At the same time, some researchers consider the pyrolysis of salts or fluoroanhydrides of perfluorinated ether acids in the presence of moisture (or another source of hydrogen ions) as a method for synthesis hydro-derivatives [39, 40]. The treatment of sodium or potassium salts of perfluorinated mono- and dicarboxylic acids, most often in ethylene glycol at 120-170°C, leads to the decarboxylation products and the formation of terminal mono- and dihydro-compounds.
References
- S.Vershilov, V.Kornilov, A.Tsyrulnikova, L.Popova, N.Lebedev, Fluorinated ethers. Communication 1. Preparation of ethers by Williamson reaction and the addition of alcohols to alkenes and alkynes. // Fluorine notes, 2021, Vol. 3(136), DOI: 10.17677/fn20714807.2021.03.01.
- A.L. Henne, M.A. Smook, Fluorinated Ethers. // J. Am. Chem. Soc.,1950, V. 72, 4378-4379.
- H.G. Adolph, M.J. Kamlet, Fluoronitroalkanes. IV. Some reactions of 2-fluoro-2,2-dinitroethanol. // J. Org. Chem., 1969, V. 34, Iss., 45-50.
- A.F. Eleev, G.A. Sokol'skii, V.F. Cherstkov, I.L. Knunyants, Perfluoropinacol ethers and esters. // Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, 1980, № 2, 451-453.
- R.A. Bekker, G.G. Melikyan, B.L. Dyatkin, I.L. Knunyants, Polyfluorinated enols and their derivatives. V. Reactions of perfluoropropen-2-ol with arenediazonium salts and aliphatic diazo compounds. // Zhurnal Organicheskoi Khimii, 1976, V. 12, Iss. 7, 1377-1379.
- C.Dapremont, P.Calas, A.Commeyras, C.Amatore, Radical chain addition of iodo-perfluoroalkanes to ethylenic or acetylenic substrates. Comparison of rates of iodine atom transfer from C4F9I to σ-vinyl and σ- alkyl α-F radicals. // Journal of Fluorine Chemistry, 1992, v. 56, 249-258.
- M. Knell, N.O. Brace, US. Patent 3 843 735, 1974
- N.O. Brace, Syntheses with perfluoroalkyl radicals from perfluoroalkyl iodides. A rapid survey of synthetic possibilities with emphasis on practical applications. Part one: alkenes, alkynes and allylic compounds. / Journal of Fluorine Chemistry, 1999, V. 93, 1-25.
- H.Meinert, P.Reuter, J.Mader, L.Haidmann, N.Northoff, Synthesis, interfacial active properties and toxity of new perfluoroalkylated surfactants. // Biomater., Artificial Cell & Immob. Biotech., 1992, V. 20, Iss. 1, 115-124.
- D.Lazzari, M.C.Cassani, G.Solinas, M.Pretto, Fluoroalkyl allyl ethers: Useful building blocks for the synthesis of environmentally safer fluorinated multiblock molecules. // Journal of Fluorine Chemistry, 2013, V. 156, 34-37.
- D.N.Bazin, T.I.Gorbunova, A.Ya.Zapevelov, V.I.Saloutin, One-step Synthesis of Epoxy(perfluoroalkyl)alkenes. // Russ. J. Org. Chem., 2009, V. 45, Iss. 4, 491-495.
- V.A. Komarov, A.V. Fokin, K.V. Frosina, Kh. A. Abdulganieva, Reactivity of some difluoronitro alcohols. // Zhurnal Obshchej Khimii, 1967, V. 37, Iss. 3, 684-686.
- H.Muramatsu. H.Kimoto, K.Inukai. The addition reactions of fluoroalkyl ethyl ethers to perfluoropropene. // Bull. Chem. Soc. Japan, 1969, V. 42, Iss. 4, 1155-1158.
- A.G. Korepin, R.G. Gafurov, L.T. Eremenko, Reactions of N-methylolnitramines in acid media. // Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, 1974, № 2. 474-477.
- A.M. Polyakova, M.D. Suchkova, K.A. Mager, V.V. Korshak, Synthesis of fluorine-containing dimethylvinylethynylcarbinol ethers. // Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, 1983, № 3, 625-626.
- J.R. Falck, J. Yu, H.-S. Cho, A Convenient Synthesis of Unsymmetric Polyfluoroethers. // Tetrahedron. Lett., 1994, V. 35, Iss 33, 5997-6000.
- R.S. Porter, G.H. Cady. Some Chemical Reactions of Trifluoromethyl Hypofluorite // JACS, 1957, Vol. 79, Iss. 21, 5625-5627.
- J. Allison, G. Cady, Reactions of trifluoromethyl hypofluorite with organic compounds. // JACS, 1959, Vol. 8, Iss. 5, 1089-1091.
- L.R.Anderson, D.E.Young, D.E.Gould, R.Juurik-Hogan, D.Nuechterlein, W.B.Fox, Perhaloalkyl Hypochlorites and Pentafluorosulfur Hypochlorites. IV. Reactions with Olefins. // J. Org. Chem., 1970, V. 35, 3730-3733.
- D.D. Moldavskii, V.G. Temchenko, V.I. Slesareva, G.L. Anmipenko, Addition of some fluorine-containing substances to hexafluoropropene. // Zhurnal Organicheskoi Khimii, 1973, V. 9, Iss. 4, 673-676.
- S. Toy, R.S. Stringham, Electrophilic additions involving Fluoronium ions. I. Fluoroxy additions to perfluoro-olefinic bonds // Journal of Fluorine Chemistry, 1975, V. 5, 25-30.
- G. Guastalla, G. Guglielmo, G. Fortunato, G. Gregorio, Pat. Italy IT1196519B, 1988.
- C. Lu, J.-H. Kim, D. D. DesMarteau, Synthesis of perfluoro-t-butyl trifluorovinyl ether and its copolymerization with TFE.// Journal of Fluorine Chemistry, 2010, 131, 17-20.
- Ch.Fritz, E.Moore, S.Selman, US Patent 3114778, Fluorinated vinyl ethers and their preparation, 1963.
- Ch.Fritz, S.Selman, US Patent 3291843, Fluorinated vinyl ethers and their preparation, 1966.
- M. E. Redwood, C. J. Willis, Fully fluorinated alkoxides: part I. Trifluoromethoxides of alkali metals. // Can. J. Chem., 1965, Vol. 43, 1893, DOI: 10.1139/v65-251.
- M. E. Redwood, C. J. Willis, Fully fluorinated alkoxides. Part II. Ethoxides, propoxides, and butoxides. // Can. J. Chem., 1967, Vol. 45, 389, DOI: 10.1139/v67-069.
- Sina Ebnesajjad, FLUOROPLASTICS, Volume 2: Melt Processible Fluoropolymers - The Definitive User’s Guide and Data Book, 2nd edition, William Andrew, 2015, p.72, ISBN: 978-1-4557-3197-8.
- H.Millauer, W.Schwertfeger, G.Siegemund, Hexafluoropropene Oxide – a key compound in Organofluorine Chemistry. // Angew. Chem., Int. Ed. Eng., 1985, V. 24, 161-179.
- N. V. Lebedev, V. V. Berenblit, Yu. K. Starobin, V. A. Gubanov, Pyrolytic Decarboxylation of Some Derivatives of Perfluorinated Mono- and Dicarboxylic Acids. // Russian Journal of Applied Chemistry, 2005, V 78, Iss. 10, 1640-1645.
- D.G.Anderson, S.Selman, Perfluorodivinyl ether, US pat. 3326984, 1967.
- R. Sullivan, Synthesis of Perfluoroalkyl Vinyl Ether Acids and Derivatives. // J. Org. Chem., 1969, V. 34, 1841-1844.
- N. V. Lebedev, V. V. Berenblit, Yu. K. Starobin, Cyclization processes in pyrolysis of perfluorooxaalkanedicarboxylic acid derivatives. // Russian Journal of Applied Chemistry, 2008, V. 81, Iss. 1, 95-99
- M.Yamabe, S.Munekata, I.Kaneko, H.Ukihashi, Synthesis of fluorinated vinyl ethers, having esters group. // Journal of Fluorine Chemistry, 1999. V. 94, 65-68.
- H.-J.Frohn, V.V.Bardin, The unusual reactivity of C3F7OCF=CF2 with PBu3 and the complex hydrides M[EH4] (M: Li, Na; E: B, Al); preparation of potassium perfluoro-2-propoxyeth-1-enyltrifluoroborate K[C3F7OCF=CFBF3]. // Journal of Fluorine Chemistry, 2003, V. 123, 43-49.
- J.F.Harris. Fluorocarbon ethers. Pat. USA. 3180895, 1965.
- H. Kawa, F. Yamaguchi and N. Ishikawa, Optically active perfluoro-2-propoxypropionic acid: A new chiral reagent for 19F NMR study. // Journal of Fluorine Chemistry, 1982, V. 20, 415-485, DOI: 10.1016/s0022-1139(00)82273-3).
- E.Ph. Moore, Polymerization of hexafluoropropylene epoxide, Pat. US 3322826, 1967.
- S.Selman, W.S.Smith Jr., Hydrogen capped fluorocarbon polyethers, Pat. US 3342875, 1967.
- G.Moore, R.Flynn, M.Guerra, J.Owens. Omega-hydrofuoroalkyl Ethers, Precursor Carboxylic acids and derivatives thereof, and their preparations and applications, Pat. US 6204299, 2001.
ARTICLE INFO
Received 04 October 2021
Accepted 08 October 2021
Available online October 2021
Recommended for publication by Prof. S.M Igumnov
Fluorine Notes, 2021, 138, 7-8
