Received: April 2021
DOI 10.17677/fn20714807.2021.03.02
Fluorine Notes, 2021, 136, 3-4
A SERIES OF FRAGMENT IONS OF CYCLOALKANES, PERFLUOROCYCLOHEXANE, PERFLUOROPOLYCYCLOALKANES
N.D. Kagramanov
A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 119991, GSP-1, Moscow, B-334, Vavilov St. 28
e-mail: ndkagram@gmail.com
Abstract: Analysis of the spectra of compounds containing regular [CH2]n or [CF2]n fragment groups: n-alkanes, cycloalkanes, n-perfluoroalkanes and perfluoropolycycloalkanes, it was found that they consist of a ions series arising after the abstraction of primary radicals. The choice of a radical from the possible candidates is determined by the spread of the excitation energies of molecular ions and the values of the standard enthalpies of formation of radicals. In the spectra of n-alkanes, the difference between the maximum and minimum abstraction energies of primary radicals: М+. - 2H. + .CH3 (2.52,1 + 34,8=∆H 139) end М+. -.C2H5 (∆H 26,5) 139-26,5= is about 112.5 kcal/mol. After the primary separation, the excitation energies of the parent ions of n-alkanes C5-C60 and cycloalkanes C6 –C30 are averaged, which are fragmented by successive emissions of the constant group (C2H4 m/z 28). In the spectra of perfluoroalkanes, perfluorocyclohexane upon abstraction of primary radicals, including: .F, 2 .F, 3 .F, the excitation energies of parent ions are averaged, fragmented by successive group ejections (CF2 m/z 50). As a result of primary separations of different energetics, quasi-equilibrium fragmentation processes acquire an equilibrium character. In this communication, along with the ions series of cycloalkanes and perfluorocycloalkanes, the previously described ions series of n-alkanes, n-perfluoroalkanes and perfluorotributylamine are discussed. For all series, primary radicals, abstraction topology, formed primary ions, and decay sequences were determined. An increase in the intensity of a single peak of an ion or a radical cation is usually the result of its structural stabilization by rearrangement or cyclization. In the spectra of higher homologues of n-alkanes and cycloalkanes, after the abstraction of primary radicals, there is a gradual increase in the intensities of all peaks of fragment ions, which is difficult to explain by their synchronously increasing structural stabilization. Fragmentation ends with the formation of the 6 most intense spectral peaks with m/z 99, 97, 85,71,57,56, in which the ratio of molecular weight to ethylene weight is not more than 3.5 : 1 and not less than 2 : 1. This mass range is optimal for the most efficient redistribution of excitation energy and maximum intensities of ion peaks. Ion structures are considered using the cyclo-C30H60 spectrum as an example.
Keywords: fragmentation algorithms, ions series, ions series of cycloalkanes, perfluorocyclohexane, perfluorodecalin, perfluoroacenaphthylene, quasi-equilibrium and equilibrium fragmentation processes.
Introduction
Attempts to decipher mass spectra in order to establish fragmentation schemes have been made for a long time. It was known that there are ion peaks in the mass spectra, formed in several competing (sequential and parallel) processes of fragmentation of molecular and fragment ions. [1]. Even for the mass spectrum of a relatively simple compound, a possible scheme for the formation of most of the fragmentation ions was usually given. Analysis of the mass spectrum is a complex problem, and the unambiguous assignment of fragmentation paths with complete certainty is hardly possible[1]. At the end of the last century, simultaneously with the creation of quadrupole mass spectrometers and the establishment of a list of requirements for the recording conditions of spectra for their reproducibility [2], the emergence of commercial mass spectral libraries that allow comparing the spectrum of an unknown substance with the spectra of a database, an alternative approach to interpreting mass spectra appeared. homologues of unknown compounds based on a comparison of the spectrum with statistically processed mass spectra of ionic series of homologous series [3]. However, as a result of the rapid growth of proprietary mass spectral libraries, the ion-series method has faded into the background. The present study, which has a similar name, in contrast to the method of ionic series, does not aim at the problems of interpreting homologues of unknown compounds.
The aim of this work is a detailed analysis of all spectral series, search and establishment of general patterns of fragmentation based on a comparative analysis of the series of fragment ions common to related classes of chemical compounds, such as n-alkanes and cycloalkanes, or n‑perfluoroalkanes and perfluorocycloalkanes. First of all, to solve this problem, key algorithms for the fragmentation of compounds, such as n-alkanes and n-perfluoroalkanes, were identified [4]. Previously, using the examples of the spectra of n-alkanes, as well as n-carboxylic acids and their esters, the same fragmentation algorithm was applied, which proved to be universal both in the analysis of the spectra of n-alkanes and carboxylic acids and their esters under conditions of Mac-Lafferty rearrangements [4-5]. The analysis of the cycloalkane ion series was carried out in order to extend the n-alkane fragmentation algorithm and evaluate its applicability for cycloalkanes
Comparison of the ions series of n-perfluoroalkanes, perfluorotributylamine PFTBA [7], and perfluorocycloalkanes makes it possible to assess their similarities and differences necessary to establish general rules for fragmentation of perfluoro compounds.
Cyclohexane and Cyclotriacontane Ions Series
Mass spectra of C6-C30 cycloalkanes, (NIST LIBRARY), consist of 5-6 series of fragment ions. In this communication, the spectra of C6 cyclohexane and C30 cyclotriacontane are considered as examples of fragmentation. It is known that in the spectrum of cyclohexane after the rupture of the cycle, the stabilization of the radical cation with separated cation and radical centers occurs by the migration of a hydrogen atom from a position adjacent to the cation center [6]. Five series of fragment ions of cyclohexane are presented in Table 1.
The mother ions of the first three series 1-3 are ions: M -15 [M * -CH3]+ ; M-28 [M -C2H4]+; M -29 [M * -C2H5]+, fragmented by successive C2H4 elimination. Along with series 1–3, there is a rather intense decay of M+./2→+C3H6 + .C3H6 (series 4), as well as a two times less intense asymmetric decay of M+. → +C3H7 + .C3H5 (series 5).
Table 1. Five series of fragment ions of cyclohexane C6H12 MW 84 NIST #: 291493 ID # 22612 DB: mainlib.
|
Series |
C6H12 |
M 84 82,0% |
-C2H4 |
-.C2H3 |
- C2H2 |
-.C2H3 |
- 3.H |
∑%= 280,5 |
100 % I current |
|
1 |
M*-.CH3 |
69 31.1% |
41 50.2% |
15 0.6% |
101,6% |
36,2% |
|||
|
1 |
M*-.CH3 |
6 31.1% |
42 20,3% |
15 0.6% |
|||||
|
2 |
M-C2H4 |
56 100% |
28 3.5% |
103,5% |
36,9% |
||||
|
3 |
M*- .C2H5 |
55 9,9% |
27 10.9% |
20,8% |
7,4% |
||||
|
4 |
M/2 M- C3H6 |
42 20,3% |
39 23,8% |
44,7% |
15,9% |
||||
|
5 |
M*- .C3H5 |
43 9,3% |
15 0,6% |
9,9% |
3,5% |
* - rearrangement ion
The basic ion of the C6 spectrum is the +C4H8 m/z 56 ion, which is formed in series 2 upon the abstraction of C2H4. With the abstraction of the allyl radical (series 5), the intensity of the resulting alkyl ion +C3H7 m/z 43 is only 9.3%.
The close values of the total ionic current of series 1 and 2 (Table 1) allow us to conclude that the opening of the C6H12 ring without rearrangements of terminal groups, followed by the abstraction of C2H4 (series 2 - 36.9%) and opening of the ring with rearrangement, with the formation of terminal groups of methyl and vinyl , (series 1 - 36.2%) occur with close and high efficiency.
A small additional contribution of 7.4% to the rearrangement of the ring opening is made by series 3, which begins with the elimination of the ethyl radical.
When the methyl radical is removed from the linear rearrangement *[M]+., The resulting cation with two terminal radical centers (1) is capable of ring formation.
*[M]+.cycle → [CH3 (CH2)3+CH-CH2.]* - .CH3 → .CH2(CH2)2+CH-.CH2 (1)
[M]+.cycle → .CH2CH2CH2CH2CH2CH2+ (2)
When the ion of the series 1 M * - .CH3 with m/z 69 +С5H9 is fragmented both with the abstraction of ethylene and with the abstraction of the vinyl radical, the cycle apparently does not form. The decay of M/2, in comparison with the abstraction of the allyl radical, is 2 times more intense.
Cyclotriacontane Ion Series
The spectrum of cyclotriacontane C30H60 MW 420 NIST #: 61729 ID #: 26169 DB: mainlib includes six ions series, and each of the series of the spectrum consists of at least 13 ions. In order to simplify the figure (Figure 1), it shows five ions series.
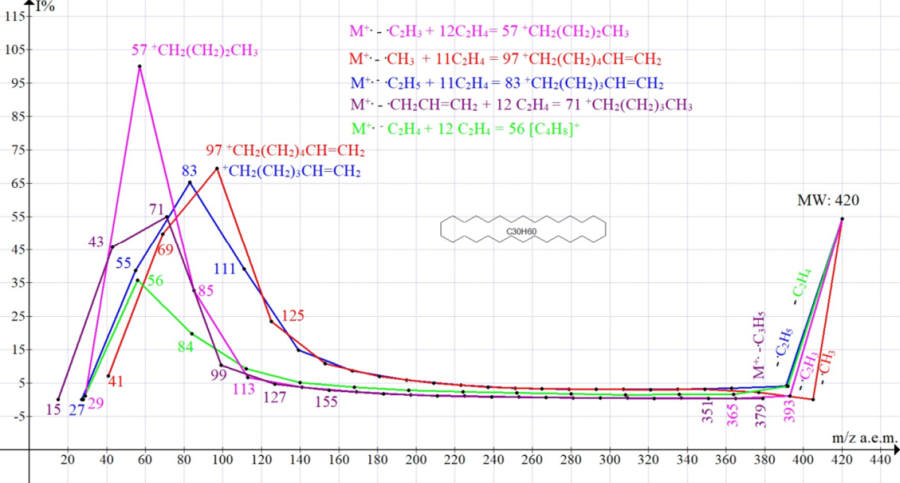
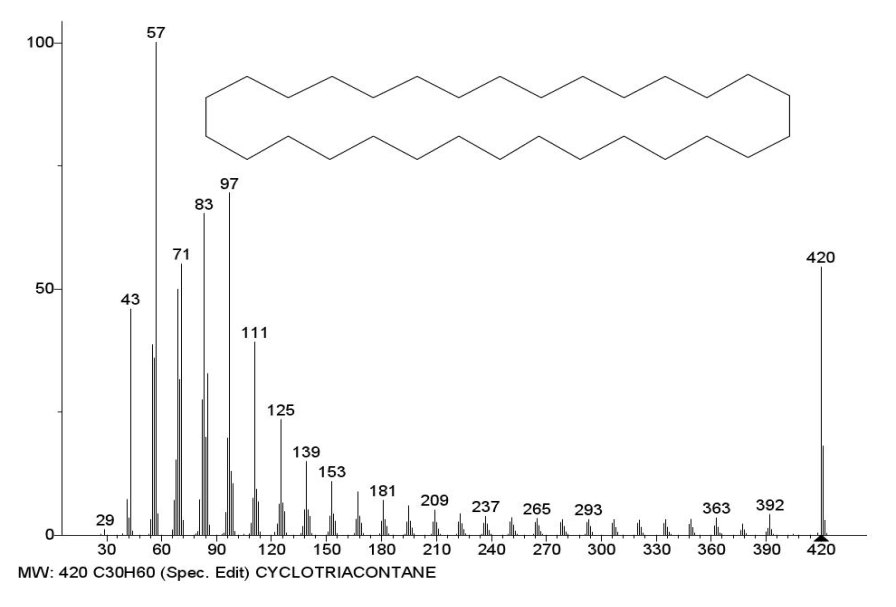
Figure 1. Five series of mass spectrum ions of cyclotriacontane NIST #: 61729 ID #: 26169 DB: mainlib.
The maternal ions of the 6 series of cyclotriacontane are ions: M * -15 [M -.CH3]+; M * -27 [M- .C2H3]+; M-28 [M-C2H4]+.; M * -29 [M- .C2H5]+; M * -41 [M- C3H5]+; M-42 [M -C3H6]+ fragmented by successive emissions of C2H4. A sharp drop in the intensities of primary fragment ions, compared with the intense peak M+. - the result of the destabilization that occurs during the rupture of the cycle, its rearrangements with the abstraction of primary radicals and the formation of six linear fragment ions.
Immediately after the formation of linear ions and successive emissions of ethylene, a gradual increase in the intensities of the peaks of all fragment ions begins, which is difficult to explain by their synchronously increasing structural stabilization. A similar increase in the intensities of the peaks of all fragment ions is observed in the spectrum of hexacontane n-C60H122 [4].
An increase in the intensities of successively fragmented ions occurs synchronously with a decrease in their excitation energies, as a result of ethylene emissions and a decrease in masses. Abstraction of radicals: - .CH3 and - .C2H5 confirm the rearrangement of the molecular cation of the cyclotriacontane radical with the formation of linear rearrangement ions, in which one of the terminal CH2CH2 groups of the broken cycle loses a hydrogen atom, turning into a vinyl group, and the other acquires a hydrogen atom, turning into a terminal group CH3.
[M]+.cycle → CH3 (CH2)26CH2 +CH .CH2 - .CH3 → .CH2(CH2)25CH2+CH .CH2 (3)
When the methyl radical is removed from the linear rearrangement radical cation, a biradical cation (3) is formed, which is capable of cyclization. Ion +C7H13, fragments by successive emissions of ethylene with the formation of ions with smaller cycles (4).
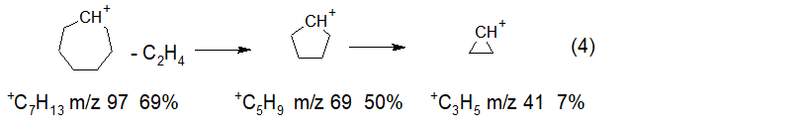
As a result of eleven separations of ethylene from ions with terminal vinyl groups with m/z 405 (M * -.CH3 graph in red) and with m/z 391 (M * - .C2H5 graph in blue), two series with increasing peak intensities arise, with maxima at m/z 97 +C7 H13 69% and m/z 83 +C6H11 65%. When ethylene is removed from them, peaks of less intense ions with m/z 69 50% and m/z 55 39% are formed. The subsequent release of ethylene leads to a sharp decrease in the intensity of the +C3H5 peak with m/z 41 7.2%. Two other rearrangement processes of cyclotriacontane begin with the abstraction of vinyl -.C2H3, as well as allyl .C3H5 radicals, as a result of which one of the methylene groups of the broken ring acquires an additional hydrogen atom, turning into a CH3 group. As a result of twelve and, accordingly, eleven detachments of ethylene from alkyl ions with m/z 393 (M-.C2H3 graph of light violet color) and m/z 379 (M-.C3H5 graph of dark violet color) two series with increasing intensity are formed peaks of alkyl ions with maxima at m/z 57 +C4H9 100% and m/z 71 +C5H11 55%. When ethylene is removed from them, ions are formed with peaks of lower intensity with m/z 29 1.1% and m/z 43 46%. The subsequent separation of ethylene from the ion with m/z 43 does not occur, since the intensity of the peak with m/z 15 +CH3 is 0.0%. Two more non-rearranged series of cyclotriacontane ions begin with the primary abstraction of ethylene, as well as propylene, and subsequent emissions of ethylene. As a result, two series of olefin ion peaks are formed, with maxima at m/z 56 +(CH2)4 36% and m/z 70 + (CH2)5 32%.
It is quite significant that when ethylene is separated from the ion with m/z 56 +C4H8, the intensity of the expected ion with m/z 28 is 0%. When, during fragmentation, a pair (ion-ethylene) is formed in which the mass of the detached group (ethylene) and the resulting ion are the same, the latter separation, as a rule, leads to a sharp decrease in the intensity of the resulting ion.
The first fragment ions of higher n-alkanes [4] and cycloalkanes have maximum masses close to the molecular ion and minimum intensities of 1%. According to the quasi-equilibrium theory of monomolecular reactions [8], the excitation energy of ionization by an electron for 10-12 sec. is distributed evenly over all bonds of the molecule and, therefore, during the formation of an ion and ethylene, it is divided between them in accordance with their masses. In ion series of spectra of higher homologues of cycloalkanes, as well as n-alkanes [4], the intensities of peaks with m/z 97-56 reach their maximum values in the interval when the ratio of the mass of the formed ion to the mass of ethylene is not more than 3.5: 1 and not less than 2: 1 (Table 2).
After the release of primary radicals: .CH3, .C2H5, .C3H5, as well as C3H6 (Table 2), the ion peaks with maximum intensities (m/z 97, 83, 71 and 70) arise as a result of the abstraction of the same total mass of ethylene (C2H4)11, equal to 308 a.m.u.. This fact confirms the expenditure of the same energy spent on the separation of ethylene in 4 ions series.
After the primary abstraction of .C2H3 or C2H4 (Table 2), the ion peaks with maximum intensities (m/z 57 and 56) arise as a result of the abstraction of the same total mass of ethylene (C2H4)12, equal to 336 a.m.u., which also confirms the same expenditure of energy spent on their education.
The ions with the maximum masses (m/z 97, 83, 71, 70), in contrast to the ions with the minimum masses (m/z 57 and 56), retain a high excitation energy, since the intensities of the peaks of their fragment ions (m/z 69-50%, 55-39%, 43-46% and 42-3.4%) more intensities of the peaks of fragment ions with minimum m/z masses 57 and 56 (m/z 29-1% and m/z 28-0% ). Comparison of the peak intensities allows us to conclude that there is a deficit in the energy of ions with minimum masses m/z 57 and 56, as well as the dependence of the peak intensities on their masses and energies.
Table 2. Six primary, minimum intensity, ion peaks cyclo-C30H60 MW 420, and six of their fragment ions with maximum intensities.
|
Detachments R |
Primary Ions I% |
Fragmented and maximum Ions I% |
Breakaway. weight (C2H4)n а.m.u. |
Share ion mass to the mass of the previous ion and to the mass of ethylene |
|
*M-.CH3 |
+(CH2)27CH=CH2 m/z 405 0.1% |
+(CH2)9CH=CH2 m/z 153 11% |
252 |
153/181= 0,84 153/28 = 5,5 |
|
*M-.C2H5 |
+(CH2)26CH=CH2 m/z 391 1.3% |
+(CH2)8CH=CH2 m/z 139 15% |
252 |
139/167=0,83 139/28 = 5,0 |
|
*M-.C3H5 |
+(CH2)26CH3 m/z 379 1.1% |
+(CH2)8CH3 m/z 127 5% |
252 |
127/155= 0,82 127/28 = 4,5 |
|
*M-.C2H3 |
+(CH2)27CH3 m/z 393 1.1% |
+(CH2)7CH3 m/z 113
7% |
280 |
113/141 = 0,80 113/28 = 4,0 |
|
M- C2H4 |
+(CH2)28 m/z 392 4.1% |
+(CH2)6 m/z 84 20% |
308 |
84/112 = 0,75 84/28 = 3,0 |
|
M-C3H6 |
+(CH2)27 m/z 378 1.0% |
+(CH2)7 m/z 98 13% |
280 |
98/126 = 0,78 98/28 = 3,5 |
Unlike the spectrum of cyclohexane, the direct decomposition of cyclotriacontane M+./ 2 = +C15H30 + .C15H30 probably does not occur. Ion with m/z 210 2.5%, formally corresponding to the decay of M+./2, is formed in the sixth series of M+. -C3H6, after 6 emissions of C2H4 (-168). In this series, the most intense peak 32% belongs to the +C5H10 m/z 70 ion. A gradual increase in the intensities of all peaks of fragment ions occurring with a decrease in their molecular masses is the result of a decrease in their energy spent on ethylene emissions.
On the other hand, the process of ion fragmentation with the separation of ethylene can be considered in accordance with the law of conservation of the momentum of the system with a change in the ethylene-ion mass ratio. The impulse conservation law is a consequence of the second and third laws of Newton [9-10]. The formation of a ions series during successive detachments of ethylene can be represented as a “gun-projectile” system in which the projectile is ethylene with a mass of 28, and the ion formed during the separation of ethylene is the gun. An excited primary ion-“gun” is capable of repeatedly ejecting ethylene (12-13 times in the C30H60 spectrum), as a result of which its mass and excitation energy decrease, and the “rollback of the gun” - the kinetic energy and intensity of the peak of a new ion at this, increase accordingly.
The cannon receives a speed that is as many times less than the speed of the projectile, how many times the mass of the cannon is greater than the mass of the projectile. This happens until both the “projectile” and the “gun” have similar masses, and the excitation energy of the ion is completely consumed. The termination of further abstraction of ethylene occurs with a decrease in the molecular weight of the final fragment ions as a result of the lack of the necessary excitation energy, or as a result of the structural rearrangement of the ion.
In 6 series of the spectrum of cyclotriacontane, 6 of the most intense peaks of fragment ions are formed, more precisely, 3 pairs of ions, the masses of which differ by 14 a.m.u.:
- 2 ions with vinyl group +C7H13 m/z 97 69%, +C6H11 m/z 83 65%,
- 2 alkyl ions +C5H11 m/z 71 55%, +C4H9 m/z 57 100%;
- 2 olefinic ions +C5H10 m/z 70 32%, +C4H8 m/z 56 36%;
The intensities of the peaks of olefin ions are the same 32% and 36%. The peaks of vinyl ions +C7H13 and +C6H11 have similar intensities of 69% and 65%, but twice the intensities of the peaks of olefin ions. This can be both a consequence of their large masses and the result of their cyclization (3-4), in comparison with the linear ions +C5H10 and +C4H8. In contrast to vinyl and olefinic ions, the intensities of the peaks of the alkyl ions of 55% and 100% differ greatly. That is, with a difference in masses of only 14 a.e.m. the intensity of the peak with m/z 57 is twice as high as that of the peak with m/z 71. It is possible that this is the result of isomerization of the linear ion +C4H9 to the cation +C (CH3)3 with a tertiary carbon atom (symmetry C3V). However, in the spectrum of n-C60H122 hexacontane, the intensity of the peak with m/z 71 increases to 87% (m/z 57 100%), which may be associated with the stabilizing effect of its molecular weight [4].
Compared to alkyl and vinyl ions, the possibilities for isomerization, cyclization and stabilization of olefin ions are minimal. Nevertheless, in the ions series +.M- C2H4 and +.M- C3H6 (Figure 1, Table 2), a consistent increase in the intensities of the peaks of all olefin ions is observed.
In the spectra of C6-C30 cycloalkanes (as in the spectra of the lowest homologues of n-alkanes C1-C11 [4]), with an increase in the molecular weight of the homologues, depending on the even or odd number of carbon atoms, a change or “shift” of the base ion occurs.
Thus, in C6-C8 cycloalkanes, the base ion +C4H8 with m/z 56, in C10-C12 +C4H7 m/z 55, in C13 +C3H5 m/z 41, in C14 +C4H7 m/z 55, in C15 +C6H11 m/z 83, in C16-C20 +C4H7 m/z 55, in C24 +C3H7 m/z 43, in C28-C30 +C4H9 m/z 57. Along with the change in basic ions, the ratios of the intensities of the peaks completing fragmentation change. The base ion peak can be the result of preferential olefinic fragmentation, such as the base ion +C4H8 with m/z 56 100% in the spectrum of cyclohexane. The base ion of the lower homologues, while remaining in its series, can shift by 14‑28 a.m.u., depending on the molecular weight of the homologue, the even or odd number of carbon atoms.
These changes in the spectra are associated with the processes of fragmentation, stabilization, and structural factors that arise with an increase in molecular weight. However, the gradual increase in the intensities of all peaks of fragment ions, with successive abstractions of ethylene, in the spectra of cyclotriacontane C30H60, as well as hexacontane n-C60H122, confirms the existing relationship and dependence of the intensities of the ion peaks on their masses and energies.
Perfluoroeicosane and perfluorotributylamine ion series
In the spectra of n-perfluoroalkanes, in contrast to the spectra of n-alkanes, after the release of the primary radical, the last significant figure of the masses of all ions of a given series is the same.
The primary ion of the alkyl series of n-perfluoroalkanes corresponds to an intense peak [+.M- .F] + in the spectra of C1-C3 homologues, a noticeable peak in the C6-C16 spectra and a very weak, trace (Tr) peak in the spectra of higher homologues. Ejection of a fluorine atom opens the chain, which is fragmented by successive cleavages of CF2 [4].
Due to the very low intensity, intermediate fragment peaks of perfluoroeicosane n-C20F42 ions, which terminate the peak with m/z 769, are not visible. The ion with m/z 769 formally corresponds to the abstraction of the perfluoroalkyl radical .C5F11.
However, taking into account that the first fragment ion of all the lower homologues of n‑perfluoroalkanes C1-C14 is formed by the abstraction of a fluorine atom, it should be concluded that the ion with m/z 769 is formed as a result of the primary abstraction of a fluorine atom and five emissions of CF2.
Another process of fragmentation of n-perfluoroalkanes, leading to the formation of a series of peaks for alkenyl ions +CF2(CF2)nCF = CF2, is the abstraction of three M-57 fluorine atoms.
Series [M-3.F] begins with the abstraction of 2 fluorine atoms and the formation of a rearrangement radical cation with a terminal vinyl group protecting one of the “chain flanks” from fragmentation.
An excited rearrangement of the radical cation expels the third fluorine atom, which is detached from the opposite terminal CF3 group (5).
[M]+. -2 .F → CF3(CF2)n +CF-CF2. * -.F → .CF2 (CF2)n+CF-.CF2 (5)
An ion with two radical centers is capable of cyclization.
An M-57 liftoff is usually accompanied by a CF2 blowout, so instead of an M-57 it corresponds to an M-107 pulloff. It is in this way that the formation of the first fixed fragment ion with m/z 931 0.5% (M-107) perfluoroeicosane C20F43 MW 1038 NIST #: 239239 ID #: 36518 DB: mainlib occurs.
The next fixed alkenyl ion with m/z 731 is 0.1%. (Figure 2) formed after 4 consecutive detachments of CF2.
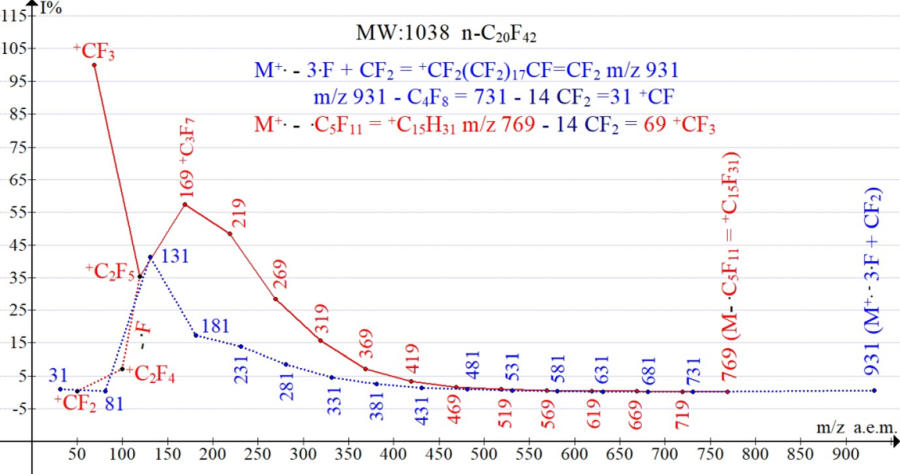
Figure 2. Two series of perfluoro-eicosane fragment ions NIST #: 239239 ID #: 36518 DB: mainlib.
In the spectra of n-perfluoroalkanes during the abstraction of CF2 groups, as well as in the spectra of n-alkanes during the abstraction of C2H4, a sequential increase in the intensities of all peaks occurs (Figure 2). Some decrease in the intensity of the +C2F5 peak is a result of the parallel detachment of .F with the formation of +.C2F4. The separation of CF2 from the CF2=CF-CF2+ ion leads to a loss of mass, a decrease in the energy of the CF2=CF+ ion and to its partial destabilization.
Compared with the spectra of n-perfluoroalkanes, the spectrum of perfluorotributylamine (PFTBA) with a central nitrogen atom fixing the CF2 end groups of three perfluorobutyl substituents is interesting in terms of changes in fragmentation occurring in it [7].
The spectrum of PFTBA N(C4F9)3 MW671 (NIST #: 66003 ID #: 8854 DB: replib Contributor: GWA MILNE, NAT'L INSTITUTES OF HEALTH, USA) is the sum of three main ions series with radical cation centers on (C4F9)2N. +CF2-α and (C4F9)2NCF2+ .CF2-β σ-bonds of the central (“terminal”) group of atoms [M].+, as well as σ-bond F. +CF2(CF2)3N(C4F9)2 of one of the peripheral groups CF3. (Figure 3).
By analogy with n-perfluoroalkanes, during fragmentation of the perfluorobutyl ion PFTBA, as a result of the abstraction of two fluorine atoms, an additional alkenyl sub-ions series with m/z 181, 131, 81 is formed (6).
(C4F9)2N. +C4F9→ (C4F9)2N. + +C4F9 - 3CF2 → +CF3 m/z 69
+C4F9 – 2 .F → +CF2CF2CF=CF2 m/z 181 – CF2 → +CF2-CF=CF2 m/z 131 (6)
The abstraction of a fluorine radical from one of the CF3 groups (M-.F series) is accompanied by the abstraction of two more fluorine atoms from two other CF3 groups, so that the first peak of this series is the peak of the ion +.M-3.F m/z 614 4.1% .
Probably, the detachment of M-.F breaks the symmetry of the molecule and initiates symmetric detachment of two more fluorine atoms from the other two CF3 groups.
The peaks of ions with m/z 564, 514, 464, 414, 364, 314, 264, 214,164,114 corresponding to 10 detachments of CF2 from the M-3 .F ion with m/z 614 are completed by the [CF2NCF2]+ ion with m/z 114.
The +.M-3 .F m/z 614 series (by analogy with the series of n-perfluoroalkanes) has two additional alkenyl subseries formed by stabilizing terminal emissions of 2 more fluorine atoms from two CF2 groups adjacent to nitrogen of one of the C4F9 m/z substituents 576, as well as 4 fluorine atoms from two substituents C4F9 m/z 538.
[(C4F9)3N]+. –5.F →+CF2CF2CF=CFN(C4F9)2 –2.F → (+CF2CF2CF=CF)2N(C4F9) (7)
As a result of successive emissions of CF2, two subseries are formed:
- M-5 .F: 576, 526, 476, 426, 376, 326, 176, 126, 76 and respectively
- M-7 .F: 538, 488.438, 388, 338, 288, 238.188, 138.
All these peaks are clearly visible in the mass spectrum PFTBA ID #: 386460 DB: wiley_nist98.
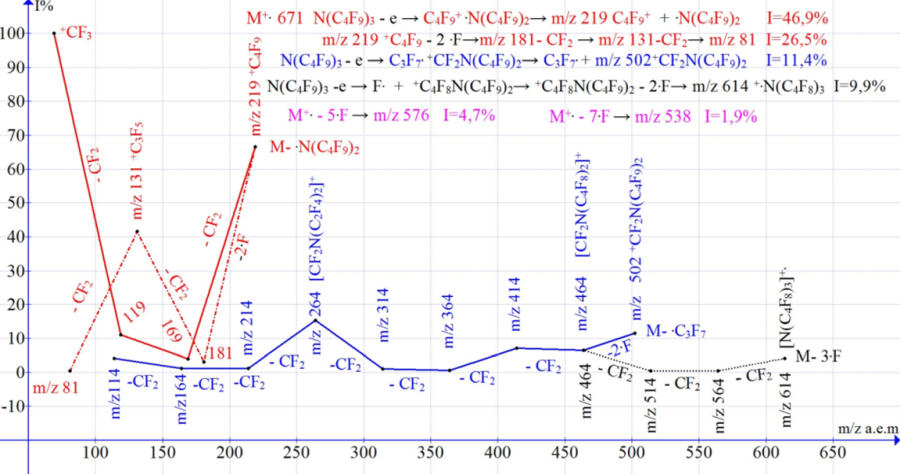
Figure 3. Four series of PFTBA fragment ions.
A sharp increase in the intensity of the +C4F9 ion peak m/z 219 is the result of a decrease in the excitation energy M+., which occurs when the .N(C4F9)2 radical is ejected with m/z 395. The decrease in the intensity of the +C4F9 ion peak m/z 219 is the result of 2 parallel paths of its fragmentation with the release of CF2 and the detachment of two fluorine atoms [4].
Three ions series in the spectra of PFTBA and n-perfluoroeicosane are similar to each other. Both spectra contain a series of alkyl fragment ions +CnF2n+1 and its alkenyl sub-series +CnF2n-1. In both spectra, after the detachment of fluorine atoms from the terminal CF3 groups, additional stabilizing detachment of two fluorine atoms from the terminal CF2-CF2 groups occurs.
Perfluorocyclohexane Ion Series
The mass spectrum of C6F12, as well as the spectra of cyclohexanes containing both fluorine atoms and hydrogen atoms, were first presented in [11]. Although the replacement of one fluorine atom with a hydrogen atom did not change the main peak of the spectrum, which still remained the +C3F5 m/z 131 ion, nevertheless, as a result of such a replacement, minor peaks disappeared: M+. and +C3F6 m/z 150, an intense peak +C5F9 m/z 231, as well as +C6F11 m/z 281. New hydrogen-containing peaks appeared +C6F10H m/z 263, +C5F8H m/z 213, +C4F6H m/z 163, +C3F4H m/z 113, +C2F2H m/z 63, fragmented with both HF detachment and CF2 detachment. The replacement of one or several fluorine atoms with hydrogen atoms did not allow clarifying the fragmentation sequence of perfluorocyclohexane.
Fragment ions C6F12 MW 300 (NIST #: 34431 ID #: 115484 DB: main) can be grouped into five distinct series (Table 3).
Table 3.Five series of perfluorocyclohexane fragment ions.
|
Series |
C6F12 |
M 300 0,7% |
-CF2 |
-CF2 |
-CF2 |
-CF2 |
-CF2 |
∑%= 308,8 |
Current I % |
|
1 |
M-.F |
281 3.7% |
231 20.9% |
181 21.4% |
131 100.0% |
81 2.1% |
31 21.8% |
170.6 |
55.2 |
|
2 |
M-2.F |
262 Tr |
212 0.1% |
162 3.9% |
112 2.5% |
62 1.2% |
7.7 |
2.5 |
|
|
3 |
M-3.F |
243 Tr |
193 2.7% |
143 2.0% |
93 15.0% |
43 0.3% |
20.0 |
6.5 |
|
|
4 |
M/2 |
150 0.4% |
100 29.2% |
50 5.1% |
34.7 |
11.3 |
|||
|
5 |
*M-.C3F5 |
169 Tr |
119 5.5% |
69 70.3% |
75.8 |
24.5 |
Tr - intensity of the peak in% at the trace level (trace)
Three series of perfluorocyclohexane ions (1-3) are formed after the primary abstraction of one, two, and also three fluorine atoms from one of the terminal CF2CF2CF2 groups of the chain of the broken ring.
The parent ions of these three series are alkenyl ions:
- Series 1 - ion with m/z 281 3.7% CF2=CF- (CF2)3-+CF2, or a cyclizable biradical cation .CF2-+CF-(CF2)3-.CF2 ;
- Series 2 - linear cation with m/z 262 Tr% CF2=C+-(CF2)3-.CF2 ;
- Series 3 - ion with m/z 243 Tr% CF2=C=CF-(CF2)2-+CF2 , or a cyclizable biradical cation .CF2-+C=CF-(CF2)2-.CF2 ;
As a result of successive abstractions of CF2 from alkenyl ions 281, 262, and 243, intermediate and final ions are formed with m/z 31 +CF 21.8%, 62 +C2F2 1.2%, and 43 +C2F 0.3% (Table 3).
Two more ions series in the spectrum of C6F12 arise as a result of the breaking of 2 C-C bonds of the cycle. This is a series of N 4 11.3%, - the result of symmetric decay M/2 with the formation of ion +C3F6 m/z 150 0.4%, fragmentation with the detachment of CF2 and the formation of ion +C2F4 29%. And series N 5 24.5%, - asymmetric rearrangement decay +.M-.C3F5 *+CF2CF2CF3 m/z 169 Tr.
It is from the rearrangement ion *+C3F7 during the abstraction of CF2 that ions are formed: +C2F5 5.5% and +CF3 70%. The asymmetric rearrangement decay of +.C6F12 with the detachment of .C3F5, in comparison with the detachment of M/2, is 2 times more intense.
When comparing the ion currents of series (Table 3), the most intense 55.2% series is series 1 (detachment of the first fluorine atom). It is followed by a series of 5-24.5% and 4-11.3% (detachments .C3F5 and C3F6). Series with detachments of three and two fluorine atoms have minimum intensities of 6.5 and 2.5%, respectively.
The sequences of formation and graphs of four series of fragmentation of perfluorocyclohexane with real intensities of the formed ions are shown in Figure 4.
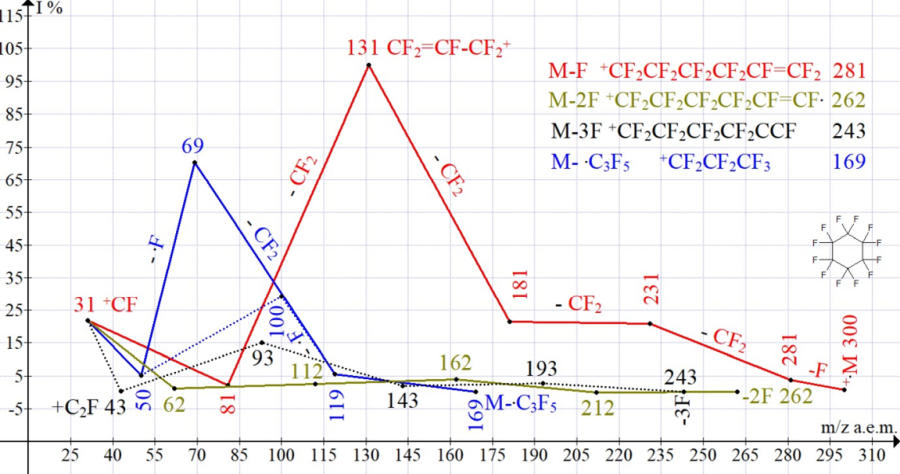
Figure 4. Four series of perfluorocyclohexane fragment ions.
Formation of three ions series with detachment of one, two and three fluorine atoms is a general process of fragmentation of perfluorocarbon compounds associated with the spread of kinetic energy of ionizing electrons, which corresponds to the quasi-equilibrium theory of monomolecular reactions [7].
Since ionizing electrons transfer different amounts of energy to the molecules of the compound under study, molecular ions have a set of internal energy values, therefore, for the same fragmentation path, there is a set of decay rates, which distinguishes molecular ions from systems where the reacting particles are in thermal equilibrium and where each given reaction has one given rate at a given temperature [1].
The ions series in the spectra of perfluorocyclohexane, perfluoroeicosane, PFTBA do not confirm that fragmentation and decay of M.+ occurs at different speeds along the same path. Molecular ions do have a set of internal energy values. This explains the primary detachment of one, two, and also three fluorine atoms in three different series of perfluorocyclohexane fragmentation. One and three fluorine atoms in the spectrum of perfluoroeicosane. Two, three, five, as well as seven fluorine atoms in four series of the PFTBA spectrum. Nevertheless, it is at the stage of primary abstraction of fluorine atoms that the excitation energies of ions of all series are equalized, further fragmented by the successive ejection of CF2. The stability of the base ion +CF3 m/z 69 * 70% (C3V group) is probably related to its symmetry and its mass, as well as to the lack of energy for CF2 detachment, since such detachment occurs only to an insignificant extent only in homologues of n-perfluoroalkanes with minimal masses C1 and C2.
Perfluorodecalin Ion Series
Depending on the mutual orientation of the two fluorine atoms in positions 4a and 8a, perfluorodecalin exists in cis and trans sterioisomeric forms.
The spectrum of perfluorodecalin (mixture of cis and trans isomers) C10F18 MW 462 (NIST #: 227559 ID #: 36292 DB: main) is the sum of five ions series:
- Series 1 - cycle break with the release of seven CF2 groups: +.M 462, 412 0.5%, 362 0.2%, 262 0.4%, 212 1.1%, 162 4.7 %, 112 2.2%, 62 .CF =+CF 0.2%. ∑9.3%.
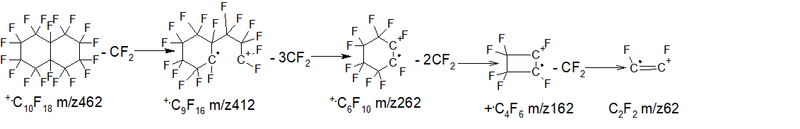
- Series 2 - ejection of a fluorine atom +.M- .F = 443, then 7 detachments of CF2: 443 1.7%, 393 6.3%, 343 4.8%, 293 28.3%, 243 22.5%, 193 7.0%, 143 6.1%, 93 10.7% (ions series +.M- .F + (CF2)7 ) ∑87.4%.
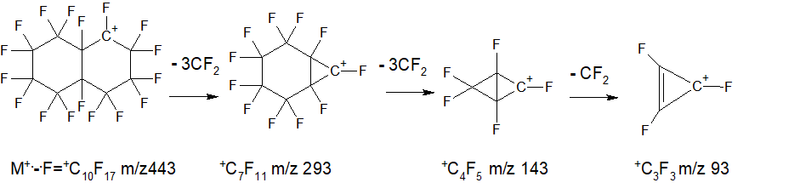
- Series 3 - burst cycle with ejection +.M- CF2= .CF 381, then six CF2 gaps: 381 0.2%, 331 0.8%, 281 0.7%, 231 2.1%, 181 9.2%, 131 77.4%, 81 0.7%, 31 4.9%. (series of alkenyl ions CF2=+CF) ∑96.0%.
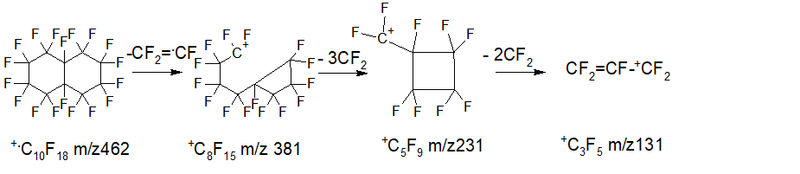
- Series 4 * (rearrangement) series - cycle rupture with the release of the radical .C6F9 +.M- .C6F9 (m/z 243) = +CF2CF2CF2CF3 m/z 219, then three CF2 detachment: 219 1.3%, 169 5.9%, 119 13.7%, 69 100% - (alkyl ions series) ∑ 120.9%.
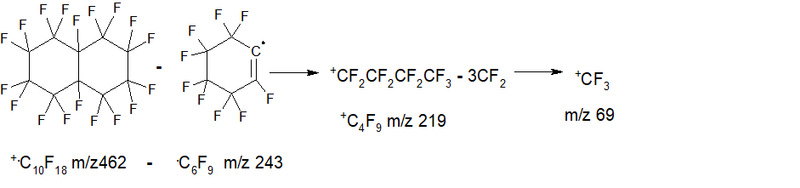
- Series 5 emission of 3 fluorine and CF2: +.M-107 (M- 3.F-CF2) = 355 1.7%, then six detachments of CF2: 305 1.3%, 255 2.4% , 205 4.0%, 155 4.4%, 105 0.9% +C4F3 (trifluorotetrahedran cation) ∑14.7%.
Table 4. Five series of fragmented perfordecalin ions.
|
Ion series |
M C10F18 462 0,2% |
|
-CF2 |
-CF2 |
-CF2 |
-CF2 |
-CF2 |
-CF2 |
-CF2 |
∑% 343,6% |
Current I 100% |
|
1 |
M- 7(CF2) |
462 0.2% |
412 0.5% |
362 0.2% |
262 0.4% |
212 1,1% |
162 4,7% |
112 2,2% |
62 0,2% |
9,5 |
2,7% |
|
2 |
M-F=443 |
443 1,7% |
393 6,3% |
343 4,8% |
293 28,3% |
243 22,5% |
193 7,0% |
143 6,1% |
93 10,7% |
87,4 |
25,4% |
|
3 |
M-CF2CF. |
381 0,2% |
331 0,8% |
281 0,8% |
231 2,1% |
181 9.2% |
131 77,4 |
81 0,7% |
31 4,9% |
96,1 |
28,0% |
|
M/2 =231 |
231 2.1% |
181 9.2% |
131 77,4 |
81 0,7% |
31 4,9% |
94,3 |
|||||
|
4* |
M-.C6F9 M-243 |
219 1,3% |
169 5.9% |
119 13,7% |
69 100% |
120,9 |
35,2% |
||||
|
4 |
100 14,0% |
50 1,0% |
15,0 |
4,4% |
|||||||
|
5 |
M-3F+CF2 |
355 1,7% |
305 1,3% |
255 2,4% |
205 4,0% |
155 4,4% |
105 0,9% |
14,7 |
4,3 % |
Ion peaks in bold have the highest intensity.
Of the five series of perfluorodecalin (Table 4), the most intense, with the maximum values of the total ionic current, are three series:
- N4* M- .C6F9, leading to the formation of a base peak +CF3 (35.2%);
- N3 M- .CFCF2, leading to peak CF2=CF-+CF2 (28.0%);
- N2 M- .F leading to a peak with m/z 293 +C7F11, (25.5%);
terminated with ion +C3F3 93 10.7%.
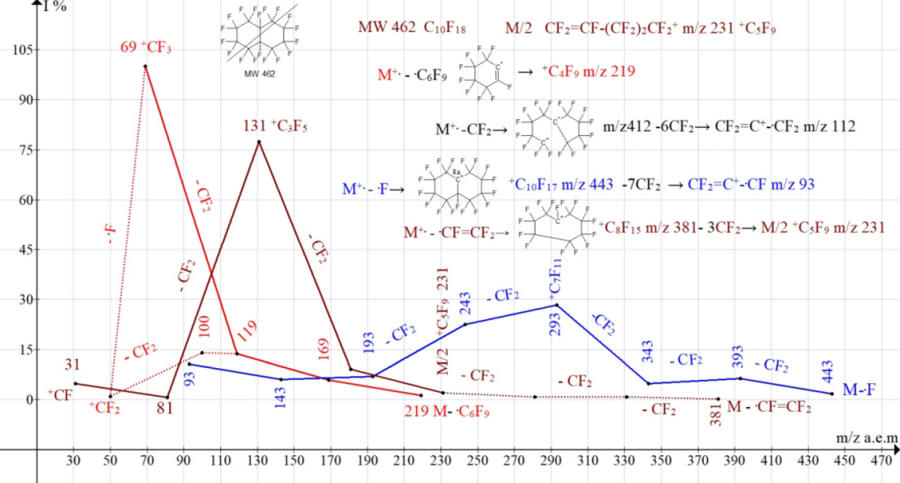
Figure 5. Three series of fragmented perfluorodecalin ions.
The intensities of the peaks of series 3 M- .CF =CF2 and linear series 4 * M - .C6 F9 increase to maximum values with a decrease in their masses to 69 and 131, respectively. But in the cyclic series 2 (+.M- .F), the most intense ion with m/z 293 28% arises not at the end, but in the middle of the series, which is possibly associated with an increase in the stability of the cation +C7F11 m/z 293, the further fragmentation of which leads to the formation of polycyclic ions +C4F5 m/z 143 and +C3F3 m/z 93, with high strength of small cycles [12] and low intensity of peaks.
Ions series 1,1,2,2,3,3,3а, 4,4,5,5,5а, 6,6,7,7,8,8,8a, 8b-icosafluoroacenaphthylene (acenaphthylene).
The spectrum of acenaphthylene C12F20 MW524 (NIST #: 394464 ID #: 115492 DB: mainlib) includes seven ions series. The graphs of the C12F20 ion series are shown in Figure 6.
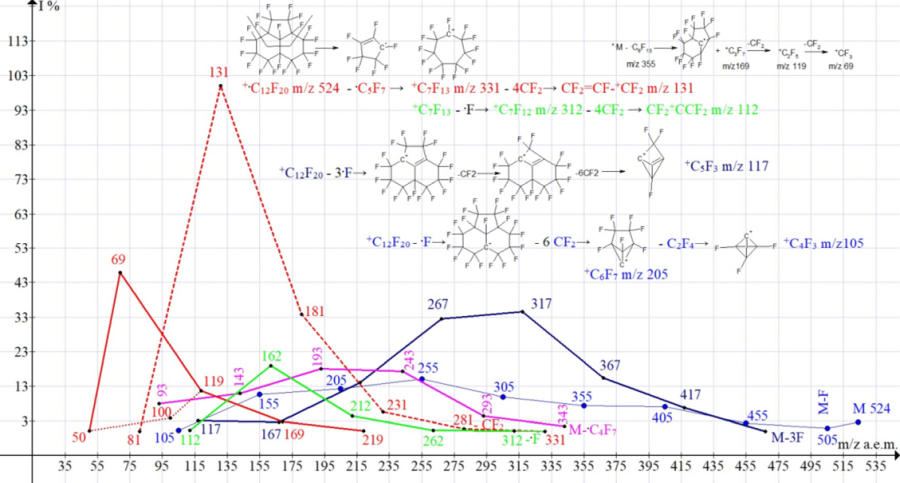
Figure 6. Graphs of the series of C12F20 ions of perfluoroacenaphthylene.
Seven series of fragmented perfluoroacenaphthylene ions:
- Series 1 +.M 524 2.7%, detachment of the atom .F +.M- .F = 505 0.9%, then eight detachments of CF2: 455 2.3%, 405 7.1%, 355 7 .4%, 305 9.9%, 255 15.1%, 205 12.3%, 155 10.7%, 105 0.3%; ∑% = 68.7%. (13.6%).
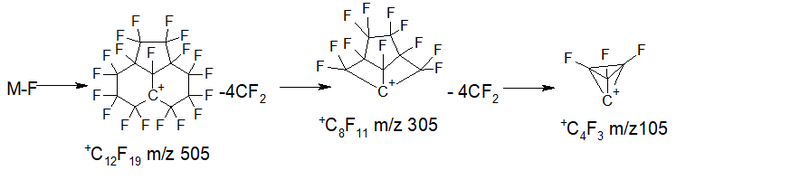
- Series 2 +.M 524 2.7%, detachment of two fluorine and CF2: M- 88 ( 2 .F + CF2) = 436 C11F16 0.1%, then 7 detachments of CF2: 386 2.7% , 336 2.8%, 286 6.3%, 236 7.9%, 186 5.3%, 136 0.7%, 86 0%; ∑% = 28.5%. (5.6%).
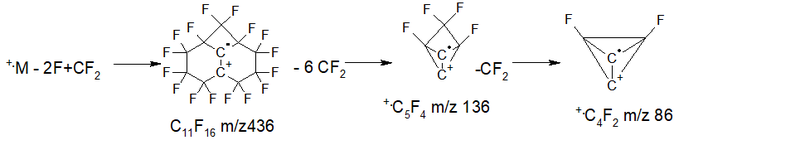
- Series 3 +.M 524 2.7%, detachment of 3 .F and CF2 = 417 +C11F15 6.8%, then 2nd detachment of CF2: 367 15.5%, 317 34.6%, then another four breakaway CF2: 267 32.5%, 217 14.1%, 167 2.6%, 117 3.1%; ∑% = 111.9%. (22.1%).
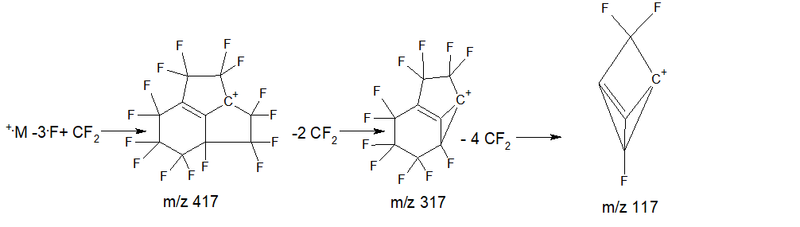
- Series 4* (rearrangement) series +.M 524 2.7%, abstraction of the radical .C4F7 m/z 181 = 343 +C8F13 1.5%, then five detachments of CF2: 293 4.4%, 243 17.4%, 193 18.1%, 143 11.0%, 93 8.0%; ∑% = 63.1% (12.5%).
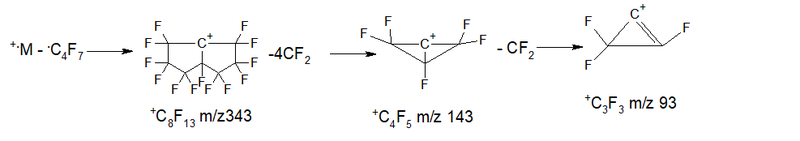
- Series 5 +.M 524 2.7%, abstraction of the radical .C5F7 m/z 193 (+.M-193 = 331 +C7F13 0%), then 4th abstraction of CF2: 281 0.8%, 231 5.7%, 181 33, 9%, 131 100%, 81 0%; ∑% = 143.1% (28.3%).
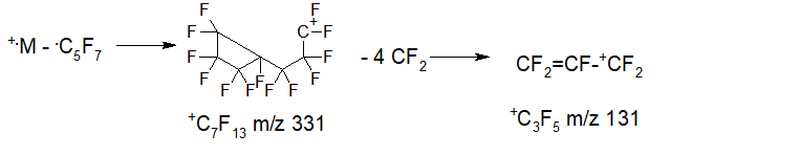
- Series 6* (rearrangement) series +.M 524 2.7%, separation of octafluorocyclopentene C5F8 m/z 212 (+.M – C5F8 = 312 +C7F12 0.1%), then four separation of CF2: 262 0.3%, 212 4.5%, 162 19.0%, 112 0.3%; ∑% = 26.9% (5.3%).
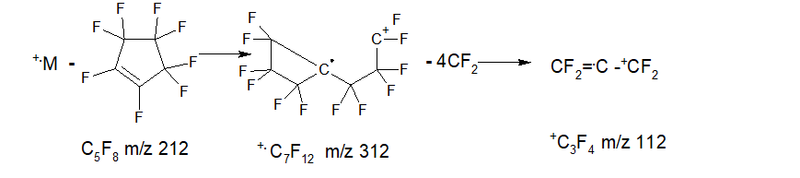
- Series 7* (rearrangement) series +.M 524 2.7%, abstraction of the radical .C9F13 m/z 355 (+.M - 355 = 169 +C3F7 2.8%), then two abstraction of CF2: 119 11.7%, 69 45, eight%; ∑% = 63.0% (12.5%).
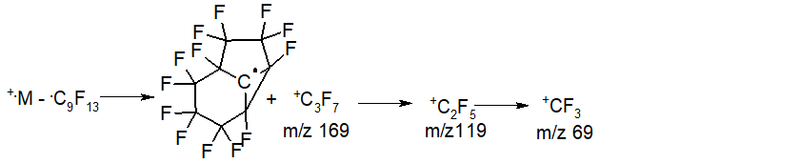
In the spectrum of C12F20, the series with the maximum values of the total ionic current are three series:
- N5 M- .C5F7, leading to the formation of a base peak CF2=CF-+CF2 (28.3%);
- N3 M-3 .F leading to a peak with m/z 317 +C9F11 (22.1%);
- N1 M-.F leading to a peak with m/z 255 +C7F9 (13.6%);
In contrast to series 5 (the maximum peak of the ion CF2=CF-+CF2 100%) and series 7 (the intense peak of the +CF3 ion 45.8%), in which the most intense ion peaks are formed at the end of fragmentation, in series 3 the most intense ion peak is formed in the middle of the series (ion +C9F11 m/z 317 35%), which may be the result of ion stabilization during fragmentation or rearrangement.
Conclusion
A detailed study of the ionic series of the spectrum makes it possible to determine the main and minor fragmentation pathways, as well as their sequences and ratios.
In the spectra of perfluoroeicosane, perfluorotributylamine, perfluorocyclohexane, and perfluoropolycycloalkanes, as a result of the primary abstraction of one, two, three, or several fluorine atoms, the excitation energies of the parent ions formed are averaged, further fragmented by successive detachments of CF2.
In a series of homologues of n-alkanes and cycloalkanes, as a result of the abstraction of primary radicals or neutral molecules, the excitation energies of the former parent ions are also averaged, further fragmented by C2H4 emissions.
Thus, as a result of the detachment of primary radicals, the fragmentation processes acquire an equilibrium character.
After the abstraction of primary radicals in the spectra of n-alkanes and cycloalkanes, as well as n-perfluoroalkanes, a gradual increase in the intensities of all peaks of fragment ions occurs, which is difficult to explain by their synchronously increasing structural stabilization.
In four series of cyclotriacontane, the most intense fragment ions are formed during the abstraction of the same total mass of ethylene (C2H4)12, equal to 308 a.m.u. This fact confirms the consumption of the same energy spent on the abstraction of ethylene in different ions series.
In the spectra of homologues of n-alkanes and cycloalkanes, the sequential increase in the intensities of the peaks of fragment ions occurs as a result of a sequential decrease in their masses and sequential expenditure of the ion excitation energy for ethylene emissions. An increase in the intensities of all peaks of ions with a decrease in their mass as a result of successive detachments of the constant group is a general tendency of all fragmentation processes associated with the redistribution of the excitation energy. The base peaks in the spectra of C6-C30 cycloalkanes, as well as C1-C11 n-alkanes, change depending on the molecular weight of the homologue, as well as the even or odd number of carbon atoms.
In the ions series of the spectra of higher homologues of cycloalkanes, as well as n-alkanes, the intensities of peaks with m/z 97-56 reach their maximum values in the interval when the ratio of the mass of the formed ion to the mass of ethylene is not more than 3.5: 1 and not less than 2:1.
Fragmentation of PFTBA, in particular the detachment of three fluorine atoms from three terminal CF3groups, confirms the influence of the symmetry of the molecule on its fragmentation upon ionization by electrons.
The search for variants of primary detachments and the resulting ions series can also be used in the analysis of the spectra of more complex molecules.
Even in the absence of regular fragment groups in the compound under study, the consideration of all variants of abstraction of primary radicals and the establishment of several corresponding ionic series of the spectrum is more productive than the description of the interpreted spectrum as a whole.
Acknowledgments
This work was supported by Ministry of Science and Higher Education of Russian Federation using scientific equipment of Molecules Structure Study Center of INEOS RAS.
References
- Takhistov V.V., Practical mass spectrometry of organic compounds, Ed. Leningrad. University, 1977, 197-198, 268 pp. (in Russian)
- Speck D. D., Venkataraghavan R., McLafferty F.W., Org. Mass Spectrom., 1978, 13, 209-214.
- Zenkevich I.G., Ioffe B.V., Interpretation of mass spectra of organic compounds, Leningrad, Khimia, 1986, 175 pp. (in Russian)
- Kagramanov N.D., Algorithms for fragmentation of n-alkanes and n-perfluoroalkanes, Fluorine notes, 2020, 1(128), 3-4.
- Kagramanov N.D., A new perspective on the mclafferty rearrangement in the spectra of n-carboxylic acids and their methyl and 2,2,2-trifluoroethyl esters. Fluorine notes, 2020, 5(132), 3-4
- Takhistov V.V., Practical mass spectrometry of organic compounds, Ed. Leningrad. University, 1977, 74, 268 pp. (in Russian)
- Kagramanov N.D., Three series ions of perfluorotributylamine mass-spectrum (PFTBA). Fluorine notes, 2020, 3(130), 1-2.
- Rosenshtock H.M., Wallenstein H.B., Warhafig A., Eyring H., Proc. Natl. Acad. Sci USA, 1952, 38, 667.
- Landsberg G.S., Elementary physics textbook, vol. 1, Mechanics. Heat. Molecular Physics, Nauka, Moscow, 1971, 122-127, 656pp.
- Landau L.D., Lifshitz Е.M., Theoretical physics, 4th ed, Nauka, Moscow, 1988, Т.1., Mechanics, 26, 215pp. (in Russian)
- J.R.Majer, Mass spectrometry of fluorine compounds, Advances in fluorine chemistry, vol. 2, London Butterworths, 1961, 75-80.
- Zaikin V.G., Mikaya А.I., Vdovin V.М., Mass-spectrometry of small cycle, Nauka, Moscow, 1983. (in Russian)
ARTICLE INFO
Received 26 April 2021
Accepted 12 May 2021
Available online June 2021
Recommended for publication by PhD M. A. Manaenkova
Fluorine Notes, 2021, 136, 3-4
