Received: May 2021
DOI 10.17677/fn20714807.2021.03.01
Fluorine Notes, 2021, 136, 1-2
FLUORINATED ETHERS. COMMUNICATION 1. PREPARATION OF ETHERS BY WILLIAMSON REACTION AND THE ADDITION OF ALCOHOLS TO ALKENES AND ALKYNES
S.V. Vershilova, V.V. Kornilov, A. S. Tsyrulnikovaa,b, L. M. Popovaa,b, N. V. Lebedeva
a S.V. Lebedev Scientific Research Institute of Synthetic Rubber, Gapsalskaya str. 1, St. Petersburg, 198035, Russia
b Peter the Great St.Petersburg Polytechnic University, Novorossiyskaya str. 48, St. Petersburg, 194021, Russia
Abstract: This review considers the main synthesis methods of fluorinated ethers, namely, various variations of Williamson reaction and the addition of alcohols to alkenes and alkynes. In both methods, the initial fluorinated fragment can be present in initial alcohol (alcoholate) as well as in alkyl halide (or alkene), or in both initial components (depending on the desired structure of the target compound).
Key words: alkylation, alkyl halides, fluorinated alcohols, Williamson reaction, fluorinated alkenes.
Introduction
Fluorinated ethers have attracted the attention of researchers since the mid-20th century. It is known that the number, as well as the position of both individual (single) fluorine atoms and perfluorinated fragments, has a significant effect on chemical and physicochemical properties of organic molecules. In case of ethers, this approach often leads to products with required characteristics, but also requires the development of new synthesis methods. The combination of highly fluorinated (perfluorinated) and hydrocarbon fragments in the molecular structure, as well as varying the carbon chains length, make it possible to obtain materials that largely provide the development of new chemical technologies. Such technologies include “fluorine” biphasic catalysis, "fluorine" separation and the production of coolants, polymeric materials, self-organizing systems, biomedical designs and nanotechnologies in microelectronics, etc.
The introduction of a single fluorine atom into ether molecule is carried out by methods that are applicable to other classes of compounds. These methods include, for example, the replacement of another halogen with fluorine under the action of some metal fluorides (KF, HgF, HgF2), or with hydrogen fluoride.
Herewith, in most cases, the ether bond is resistant to the action of even strong fluorinating agents [1].
So, ethyl-β-fluoroethyl ether was obtained in 40% yield by heating (240-250°C) of the corresponding chlorine derivative with KF [1]:

The use of mercury monofluoride to replace bromine atom located at the secondary carbon in the 2,3-dibromopropylethyl ether molecule is described in the Swart’s papers (160-170°C, 1 day, 18.5%) [1].
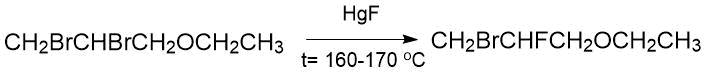
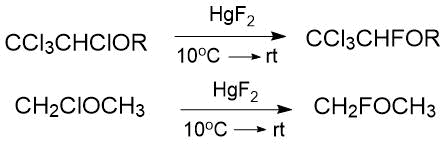
The use of mercury (II) fluoride makes it possible to substitute halogens in the α-position to the ether bond with fluorine under milder conditions (10–20 °C, 42%) [2].
There are two main ways to prepare polyfluorinated ethers:
1) reaction of alcohols (alcoholates) with alkyl halides, as well as tosylates, sulfates, sulfinates, etc.:
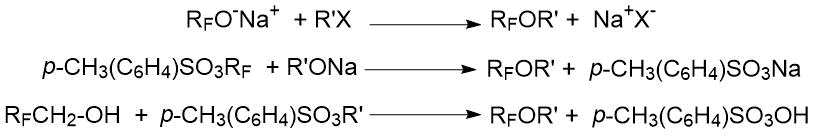
2) addition of alcohols to alkenes and alkynes:
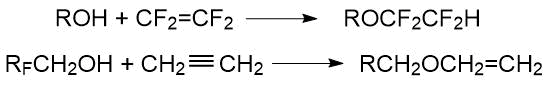
In both methods, the initial fluorinated fragment can be present in initial alcohol (alcoholate) as well as in alkyl halide (or alkene), or in both initial components (depending on the desired structure of the target compound).
In addition to main methods, there are several particular synthesis routes of fluorinated ethers with definite structure, which will be discussed in detail in the second part of this review. In particular, methyl fluoroalkyl ethers can be prepared by reacting fluorinated alcohols with diazomethane. In some cases, the addition reaction of polyfluoroalkyl iodides to alkenes (including fluorine-containing ones) is used. Such substances already have an ether bond in their structure. This method is convenient for introducing a perfluoroalkyl group into the existing structure.
In paper [3], A. Henne and M. Smook reported on unsuccessful attempt to prepare fluorinated ethers by one of classic methods - intermolecular dehydration of alcohols as an example of trifluoroethanol and 3,3,3-trifluoropropanol. Therefore, despite the fact that this reaction is theoretically possible for the synthesis of some specific ethers, it should not be considered as a universal method of preparation.
1. Alkylation of alcohols
1.1. Alkylation of fluorinated alcohols with alkyl halides
The classic method of preparing ethers is the Williamson reaction, which is reaction of alkali metal alcoholate with alkyl halide [4].
There are several options of this method with regard to fluorinated ethers. They differ in the realization conditions (catalyst, solvent, temperature, synthesis time), and in the generating alcoholates method.
One of the first examples of synthesis of fluorinated ethers by the reaction of polyfluoroalkanol alcoholates with alkyl halides is described in US Patent 2452944 (E.T. McBee et al.) [5].
Sodium trifluoroethylate, previously obtained by reacting trifluoroethanol with sodium in dioxane, was heated in a metal ampoule with ethyl bromide (130°C, 89 h). Fractional distillation gave 2,2,2-trifluoroethyl ethyl ether (CF3CH2OCH2СН3), with boiling point 50,3°С, density 1,065 g/cm3 and nD25 1,3065.
In the reaction with polyfluoroalkanols, the alkyl bromides were preferable to the corresponding chlorides or iodides because they provided a higher alcohols conversion and the yield of target ethers [3].
According to the standard procedure, A. Henne and M. Smook [3] treated fluorinated alcohols with metallic sodium, placed in autoclave, added alkyl halide and then kept at 200°C at a pressure of 36 atm for 15 h. The results are presented in Table. 1.1.
Table 1.1.
|
ROH |
RX |
Product *) |
Conversion/Yield, % |
Boiling point (°С) / density (g/cm3) |
|
CF3CH2OH |
CH3I |
CF3CH2OСН3 |
- |
31,2(746) / 1,1661(3°С) |
|
CF3CH2OH |
CH3Br |
43 / 61 |
||
|
CF3CH2OH |
C2H5Cl |
CF3CH2OС2Н5 |
Small amounts |
49,9(742) / 1,0910(20) |
|
CF3CH2OH |
C2H5Br |
-/ 60 |
||
|
CF3CH2OH |
CClF2CH3 |
CF3CH2OСF2CН5 |
28 / 24 |
37,8 /- (decomposes at storage) |
|
CF3C2H4OH |
CH3Br |
CF3C2H4OСН3 |
-/43 |
54,9(753) / 1,1129(20) |
|
CF3C2H4OH |
C2H5Br |
CF3C2H4OС2 Н5 |
-/30 |
72,3(746) / 1,0593(20) |
|
*) Na, 200 °С, 36 atm, 15 h, |
||||
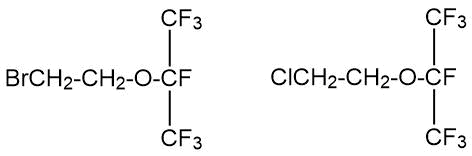
A.G. Pittman, El Cerrito and W.L. Wasley [6] synthesized a number of ethers by the reaction of potassium heptafluoro-2-propanolate (CF3)2FCOK and potassium 1,2-dichloropentafluoro-2-propanolate (CF2Cl)2FCOK with 1-bromo-2-chloroethane or 1,2-dibromoethane according to the following scheme:
(CF3)2FCOK + CH2BrCH2Х → (CF3)2FCOCH2CH2B(Х)
where X = Cl, Br.
The initial alcoholates were prepared from fully halogenated acetone derivatives (in particular, from hexafluoroacetone) by treatment with potassium fluoride in diglyme. In this way, in particular, 2-(2-bromoethoxy) heptafluoropropane and 2-(2-chloroethoxy) heptafluoropropane were obtained:
Due to relatively high acidity of polyfluoroalkanols with general formula RFCH2OH [1], alkoxide ions can be obtained not only by treating these alcohols with alkali metals, but also by reacting with alkali metal hydroxides in a suitable solvent or in a biphasic aqueous systems.
So, in paper by D.N. Bazhin et al. [7], the alkylation of telomere alcohols [H(CF2CF2)nCH2OH (n = 1-3)] by alkyl halides with different hydrocarbon chain lengths in the presence of potassium hydroxide in DMSO medium, as well as under the conditions of biphasic system “dichloromethane/aqueous potassium hydroxide” is described:

where X=Cl, Br, R=C4H9 , C6H13, C10H21.
It was shown that in DMSO medium, the yield of target ethers (n = 1) was 45-55%, and in the mixture of dichloromethane/aqueous potassium hydroxide using phase-transfer catalyst (PTC) it was significantly higher (87-94%) for products with n = 2 or 3. The examples are shown in Table 1.2.
Table 1.2. Yields and characteristics of polyfluorinated ethers [7].
|
No. |
Ether formula |
Method*) |
Yield, % |
Boiling point (°С) / pressure (mm Hg) |
|
I |
HCF2CF2CH2O(CH2)3СН3 |
1 |
45 |
140-141 |
|
II |
HCF2CF2CH2O(CH2)5CH3 |
1 |
49 |
170-171 |
|
III |
HCF2CF2CH2O(CH2)9CH3 |
1 |
55 |
105-107/6 |
|
IV |
H(CF2CF2)2CH2O(CH2)3CH3 |
2 |
87 |
179-180 |
|
V |
H(CF2CF2)2CH2O(CH2)5CH3 |
2 |
89 |
196-198 |
|
VI |
H(CF2CF2)2CH2O(CH2)9CH3 |
2 |
94 |
195-196/30 |
|
VII |
H(CF2CF2)3CH2O(CH2)3CH3 |
2 |
89 |
201-202 |
|
VIII |
H(CF2CF2)3CH2O(CH2)5CH3 |
2 |
88 |
160-161/30 |
|
IX |
H(CF2CF2)3CH2O(CH2)9CH3 |
2 |
96 |
217-220/30 |
*) Method 1: RX, КОН, DMSO, then alcohol-telomere at 100°С, 8 h.
Method 2: CH2Cl2, КОН (40% aq.sol.), H(CF2CF2)nCH2OH, TEBAC, 40°С, then RX, 40°С, 5 h.
The synthesis of a number of hydrocarbon-fluorocarbon (HF) surfactants with ether bond is described in the paper of W. Huang and co-authors [8].
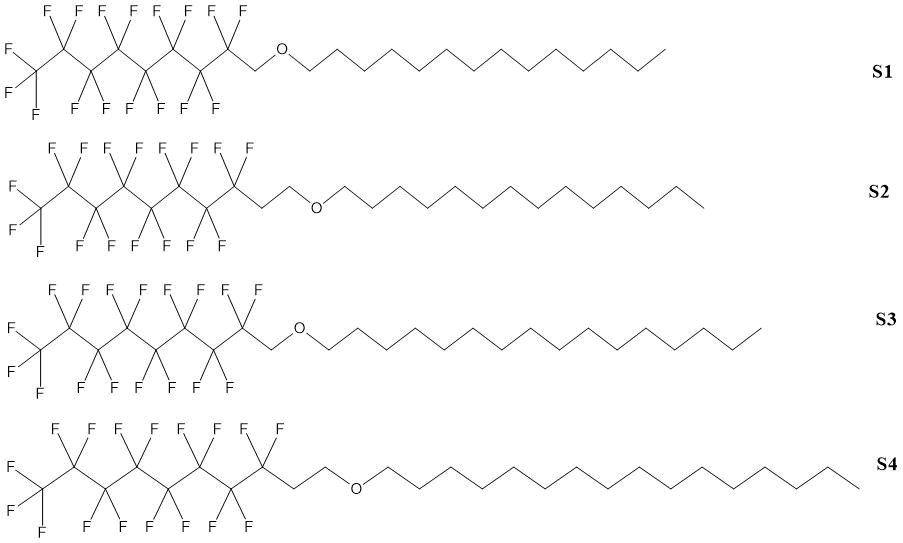
Alkyl ethers of 1H,1H-perfluorononan-1-ol and 1H, 1H, 2H, 2H-perfluorodecanol-1 (1H,1H,2H,2H-perfluorodecanol) were prepared in one stage by three variations of Williamson reaction (see Table 1.3).
Table 1.3. Yields and characteristics of 1H, 1H-perfluorononan-1-ol and 1H, 1H, 2H, 2H-perfluorodecan-1-ol alkyl ethers [8].
|
No. |
Ether |
Method*) |
Yield,% |
Boiling point (°С) |
|
1 |
S1 |
1 |
70 |
31 |
|
2 |
S2 |
2 |
70 |
35 |
|
3 |
S3 |
1 |
71 |
39 |
|
4 |
S4 |
3 |
52 |
42 |
*) Method 1: RF(CH2)nOH, THF, NaH, 40°С, 1 h, then RBr, boiling for 4 days.
Method 2: RF(CH2)nOH, С6Н6, THF, Bu4N+HSO4-, NaOH (50% aq. sol.), 10°С, 1.5 h, then RBr, 20°С, 4 days.
Method 3: RF(CH2)nOH, КОН, DMSO, RBr, 70°С, 2 days. Purification method - column flash- chromatography.
1.2. Alkylation of fluorinated alcohols with alkenyl halides
Ethers of polyfluoroalkanols containing an unsaturated hydrocarbon moiety are of increased interest, primarily as potential monomers.
So, vinyl ethers of various structures can be prepared by dehalogenation of 2-haloethoxy polyfluoroalkanes with potassium hydroxide in alcohol [6]:

The reaction of fluorinated alcohols with alkyl halide vinyl ether makes it possible to produce diethers with a perfluoroalkyl moiety and a terminal vinyl group [9]:
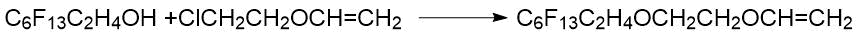
Allyl ethers of polyfluorinated alcohols in most cases also can be prepared by Williamson reaction through the reaction of polyfluoroalkanol alcoholates with allyl halides (chlorides, or bromides) under various conditions.
The preparation of such compounds by heating alkoxides of polyfluoroalkanols with allyl halides in an anhydrous solvent was described in [10]. The results are presented in Table 1.4.
The initial alcoholate was formed either preliminarily by treating the fluoroalkanol with sodium or in situ - using anhydrous potassium carbonate and acetone.
Table 1.4. Allyl polyfluoroalkyl ethers with the general formula ROCH2CH=CH2 [10].
|
ROH |
X-CH2CH=CH2 |
Process parameters |
Yield, % |
|
CF3CH2OH |
ClCH2CH=CH2 |
Na, diglyme, 80 °С, 20 h |
66 |
|
CF3(CF2)2CH2OH |
Br CH2CH=CH2 |
Na, (CH3CH2)2O, b.p., 16 h |
67 |
|
K2CO3, (CH3)2CO, b.p., 3 days |
52 |
||
|
CF3(CF2)6CH2OH |
ClCH2CH=CH2 |
K2CO3, (CH3)2CO, b.p., 3 days |
37 |
|
H(CF2)2CH2OH |
ClCH2CH=CH2 |
Na, diglyme, 80 °С, 20 h |
43 |
|
H(CF2)4CH2OH |
ClCH2CH=CH2 |
Na, diglyme, 80 °С, 20 h |
21 |
|
H(CF2)6CH2OH |
BrCH2CH=CH2 |
Na, (CH3CH2)2O, b.p., 16 h |
24 |
|
H(CF2)10CH2OH |
BrCH2CH=CH2 |
K2CO3, (CH3)2CO, b.p., 3 days |
25 |
|
K2CO3, (CH3)C(O)C2H5, b.p., 3 days |
35 |
The paper of Rakhimov and co-authors is devoted to the preparation of allyl ethers of trihydroperfluorinated alcohols with the general formula H(CF2CF2)nCH2OH (n=2,3) [11]. Ethers were prepared in 54–85% yield by reactions of trihydroperfluorinated alcohols with allyl iodide (or with allyl bromide) at temperatures up to 80°C in dioxane, which contained up to 1.1% water:

where X = I, Br; n = 2,3.
The preparation of allyl 2,2,3,3,4,4,5,5-octafluoropentyl and allyl 1,1,2,2-tetrafluoropropyl ethers was described in the paper by Polish researchers (H. Maciejewski et al.) [12] Allyl chloride, sodium hydroxide, benzene and a small amount of dimethylaminopyridine were added to the initial fluorinated alcohol. The reaction mass was kept for 8 h at a temperature of 70-80°C and with constant stirring. At the end of this reaction, resulting products were filtered and subjected to fractional distillation to isolate the fraction with boiling point of 140 °C. The target ethers yield was at least 78%.
D. Lazzari and co-authors prepared allyl ethers of 1H,1H-perfluorobutan-1-ol, 1H,1H-perfluorohexan-1-ol and 1H,1H,8H,8H-perfluorooctane-1,8-diol by heating equivalent amounts of corresponding alcohols, allyl bromide and crushed sodium hydroxide in autoclave (80 °С, 7 h) in yields of 69, 67, and 62%, respectively [13].
Synthesis and characteristics of perfluoro-tert-butoxy allyl and propargyl ethers are presented in US Patent 0323672 (I. Horvath et al.) [14].
The paper of French researchers [15] describes the preparation of polyfluoroalkyl allyl ethers from the corresponding fluorinated alcohols and allyl chloride (or allyl bromide) using tetrabutylammonium bisulfate Bu4NHSO4 as a phase transfer catalyst:

where: X=Cl, Br ; R-OH = CF3CH2-OH, CF2HCF2CH2-OH, ClCF2-CF2CH2-OH, etc.
In [16] the authors found that under the conditions of heterophase process (aqueous alkali) a high conversion of lower polyfluoroalkanols (C≤5) was achieved by using a solubilizer, for example, 1,4-dioxane. In the case of fluorinated alkanols with longer chains (C> 5) the use of tetrabutylammonium bromide (TBAB) as a phase transfer catalyst (CTP) was required:

where: R=CF3CH2-; CF3(CF3)CH-; HCF2CF2CH2-; H(CF2CF2)2CH2; H(CF2CF2)3CH2-; (CF2CF2)4CH2-
1.3. Alkylation of alcohols with polyfluoroalkyl halides
Bening and co-authors [17] described the reaction of CF3CH2Cl with СН3СH2ONa (130°С, ≈ 55 h, steel bomb, EtOH abs.), the yield of the target ether was 25%. The molecular weight (130) determined by cryoscopy method was close to theoretical for CF3CH2OC2H5.
In the paper of Chinese researchers from Shanghai Institute of Organic Chemistry, the reaction of 2-chloro-1,1,1-trifluorethane with aliphatic alcohols in an aqueous medium was described. This reaction led to the formation of alkyl trifluoroethyl ethers with sufficient satisfactory yield. The reaction was carried out in autoclave at elevated temperature and pressure [18]:
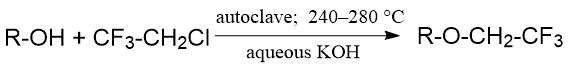
The ether yield varied within range of 42-70%. The increase in length of hydrocarbon chain (as well as in branching) required more severe conditions: for example, in the reaction with n-butanol, 2-methylpropanol, 1-methylpropanol, the conversion of CF3CH2Cl reached 60-70% (280°C, 12 h), and with tert-butanol was only 7% (283°C, 14 h) (see Table 1.5).
Table 1.5. Yields, characteristics and conditions for synthesis of trifluoroethyl ethers [18].
|
Ether formula ROCH2CF3, |
T, °С |
Pressure, atm. |
τ, h |
Conversion, % |
Boiling point (°С) |
Yield, % |
|
R = CH3 |
260 |
76 |
10 |
74 |
31-33 |
55 |
|
R = C2H5 |
240 |
60 |
11 |
87 |
48-50 |
67 |
|
R = n-C4H9 |
280 |
110 |
13 |
61 |
82-85 |
42 |
|
R = (CH3)2CHCH2 |
285 |
115 |
10 |
63 |
80-82 |
45 |
|
R=C2H5(CH3)CH |
280 |
115 |
11 |
70 |
81-83 |
64 |
|
R = (CH3)3C |
285 |
110 |
14 |
7 |
̶ |
̶ |
|
R = CF3CH2 |
250 |
102 |
13 |
77 |
60-62 |
70 |
J.A Yong and P. Tarrant showed [19] that heating of 1-chloro-1,1,2,2-tetrafluoroethane (CHF2CF2Cl) with sodium ethylate (CH3CH2ONa) the substitution proceeded along chlorine atom, and the target 1,1,2,2-tetrafluoroethoxyethane was formed in 66-72% yield.
It was described [20] the reaction of trifluoroethanol with chlorodifluoromethane (CHClF2) in the presence of KOH (solid), which led to the formation of difluoromethyl 2,2,2-trifluoroethyl ether (boiling point 29-30°C):

It was found that conversion of chlorodifluoromethane increased with a significant excess (2-4 times) of trifluoroethanol, with increasing pressure, as well as with addition of water in reaction mixture.
|
Parameters |
Conversion CHClF2, % |
|
|
1 |
70°С, р = 100 kPa |
21,4 |
|
2 |
60-90°С, 1.55 MPa, 4 h |
40,2 |
|
3 |
90-100°С, 1,59-1,17 MPa, 20 h |
32 |
|
* |
80-95°С, 0,79 MPa, 2 h |
53 |
* in the presence of water
In China patent (W. Xu and H. Li) [21], the reaction of 1,1,1,3,3,3-hexafluoroisopropanol with fluoromethyl halides (CH2FX, X = Cl, Br, I) was considered under various conditions:
(CF3)2CHOH + CHFX → (CF3)2CHOCH2F
The yield of the target ether, depending on halide used, increased in the series I > Br > Cl and reached 85%.
1.4. Reaction of polyfluoroalkanols with alkyl sulfates and sulfonates
Like non-fluorinated alcohols, polyfluoroalkanols form ethers when alkylated with dialkyl sulfates.
So, the synthesis of methyl and ethyl ethers by reaction of halogenated isopropanols (1,1,1,3,3,3-hexafluoro, 1-chloro-1,1,3,3,3-pentafluoro- and 1,3 -dichloro-1,1,3,3-tetrafluoro-) with dimethyl sulfate (or diethyl sulfate) in the presence of aqueous KOH was described (see Table 1.6) [22]:
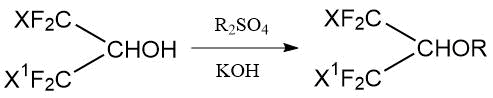
Table 1.6. Conditions for synthesis of methyl (ethyl) polyfluorochloroisopropyl ethers [23].
|
Ether |
Reaction conditions |
Conversion,% |
Boiling point (°С); nD20 |
|
(CF3)2CHOCH3 |
heating, 30 min |
55,4 |
50; 1.27505 |
|
(CF3)2CHOC2H5 |
boiling, 2 h |
36,9 |
64,2; 1,28981 |
|
(CF3)CClF2CHOCH3 |
10°С during addition of dimethyl sulfate, then heating at 45-50°С for 30 min |
67 |
80; 1,32029 |
|
(CClF2)2CHOCH3 |
10 °С during addition of dimethyl sulfate, then heating at 45-50°С for 30 min |
43,5 |
110; 1,36362 |
R.D. Bagnall with co-authors [23] treated 2,2,3,3-tetrafluoropropanol with dimethyl sulfate in the solution of KOH (at room temperature, 12 h), the yield of 2,2,3,3-tetrafluoropropyl methyl ether was 83%:
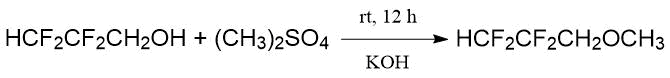
The same paper reported about synthesis of fluorohalogenated methylpropyl ethers for testing them as potential inhalation anesthetics.
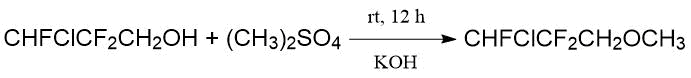
O. Scherer and H. Hahn [24] also used dimethyl sulfate to obtain 1,1,1-trifluoroethyl methyl ether (40°C, 1 h) (see Example 3 in Table 1.7). Symmetric hexafluorodiethyl ether was prepared by treating СF3CH2ONa alcoholate with toluenesulfonic acid trifluoroethyl ether (100°C, 10 h) (see Example 1 in Table 1.7). According to the one-stage procedure the mixture of alcoholate and p-toluenesulfonyl chloride was kept under similar conditions. It led to the target ether with a lower yield of 63% (see Example 2 in Table 1.7):
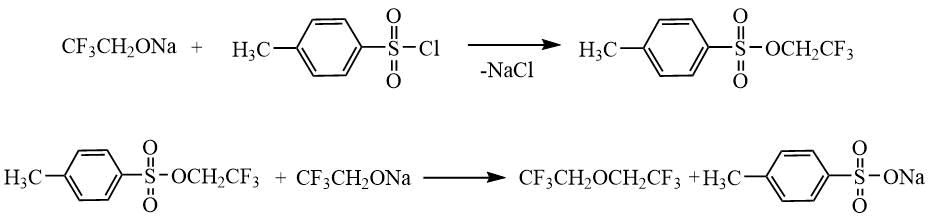
Table 1.7. Yields and characteristics of trifluoroethylalkyl ethers prepared by the reaction of alcohols with dimethyl sulfate [24].
|
No. |
Ether formula |
Yield,% |
Boiling point, °С |
|
1 |
CF3CH2OCH2CF3 |
85 |
63.8-64 |
|
2 |
CF3CH2OCH2CF3 |
63 |
64 |
|
3 |
CF3CH2OCH3 |
80-85 |
31-32 |
Synthesis of 2,2,2-trifluoroethyl methyl ether by methoxylation of tosylate CH3C6H4SO3CH2CF3 was described by B. Lee et al. [25]. The reaction of CH3C6H4SO3Cl with 2,2,2-trifluoroethanol in the presence of KOH (20% aq. sol.) gave the corresponding tosylate (0°С, 7 h, 96%). Subsequent treatment of CH3C6H4SO3CH2CF3 with sodium methoxide (20°С, 6 h) afforded to obtain the target ether (after rectification: 99.8% purity, 88.6% yield).
Methyl ether of p-toluenesulfonic acid was used by Zisman et al. to obtain methyl ether of 1H,1H,7H-trihydroperfluoroheptanol (CHF2(CF2)5CH2OH) [26] (3.5% aq. sol. NaOH, boiling, 16 h, yield 40-50%; boiling point 81-82°С at 2 mm Hg, d20 1.6323 g/cm3). The diether CHF2(CF2)5CH2O(CH2)6OCH2(CF2)CHF2 was prepared in a similar way. These ethers were considered as potential solvents, dielectrics, lubricating and cooling agents.
Alkylation of alcoholates of polyfluoroalkanols H(CF2CF2)nCH2OН by tosylates of non-fluorinated alcohols allowed Bazhin et al. [7] to perform the synthesis of a ethers series with the general formula H(CF2CF2)nCH2OR (KOH, THF, Н2О, catalyst, -60°C, time according to the results of two stages 8 h, yield 87-96%):
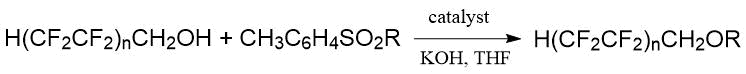
Japanese researchers synthesized F-tert-butyl methyl and ethyl ethers by two methods using the reaction of sodium and potassium perfluoro-tert-butylates with alkyl sulfate [27]. The first method consisted in the reaction of potassium perfluoro-tert-butylate prepared in situ with diethyl sulfate in ethanol:
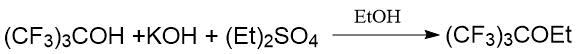
The second method involved the reaction of pre-isolated sodium perfluoro-tert-butylate with dialkyl sulfate in tetraglyme.
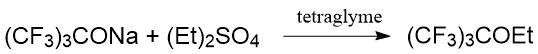
The preparation of di(polyfluoroalkyl) ethers by the reaction of polyfluoroalkylchlorosulfites with polyfluorinated alcohols was described in the papers of A.I. Rakhimov et al. [28, 29, 30]. The results are presented in Table 1.8:
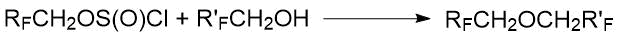
Table 1.8. Yields and characteristics of di(polyfluoroalkyl) ethers [28].
|
Ether formula |
Method |
Yield, % |
Boiling point, °С / pressure, mm Hg |
nD20 |
d204 |
|
H(CF2CF2)2CH2OCH2(CF2CF2)2H |
1 |
59 |
103/2 |
1.3385 |
1.7344 |
|
2 |
52 |
- |
|||
|
HCF2CF2CH2OCH2CF2CF2H |
1 |
98 |
65/1 |
1.3575 |
1.6251 |
|
H(CF2CF2)3CH2OCH2(CF2CF2)3H |
1 |
57 |
130/1 |
1.3370 |
1.8014 |
|
HCF2CF2CH2OCH2(CF2CF2)2H |
2 |
85 |
83/2 |
1.3500 |
1.6790 |
|
HCF2CF2CH2OCH2(CF2CF2)3H |
2 |
51 |
95/1 |
1.3450 |
1.7310 |
|
HCF2CF2CH2OCH2(CF2CF2)4H |
1 |
34 |
130/1 |
- |
- |
|
H(CF2CF2)2CH2OCH2(CF2CF2)3H |
1 |
62 |
110/1 |
1.3380 |
1.7647 |
*) Method 1: R´FCH2OH, СHCl3 (ether), DMF (catalyst), RFCH2OS(O)Cl, -10°С, then r.t., 1 day.
Method 2: R´FCH2OH, ТEА, pentane (hexane), RFCH2OS(O)Cl, -10°С, then r.t., 1 day.
It was noted that the use of DMF was economically more profitable and led to a cleaner target product, because triethylammonium salt formed by second method significantly contaminated ethers [30].
2. Addition of alcohols to alkenes and alkynes
2.1. Addition of alcohols to alkenes
The addition of alcohols to fluoroolefins and fluorochloroolefins proceeds quite easily in many cases.
The reactivity of polyfluoroalkenes is determined by stabilizing effect of substituents to carbanion and changes as follows in the series of perfluoroalkenes:
CF2=CF2 < CF3CF=CF2 << (CF3)2C=CF2 [31]
When one fluorine atom is replaced by a halogen, the relative reactivity increases:
CF2=CF2 < ClCF=CF2 < BrCF=CF2 [31]
For the first time, the addition of fluoroalkenes to alcohols was reported in a patent by DuPont Company [32]. Tetrafluoroethylene and chlorotrifluoroethylene were added to alcohols in autoclave at elevated temperatures (50-145°C) in the presence of sodium alcoholates as a catalyst:

The reaction of alcohols with chlorotrifluoroethylene was investigated in the paper of Park and Laecher [33]. The reaction was carried out by bubbling fluoroalkene through a saturated solution of potassium hydroxide in alcohol:

where R=CH3, C2H5, iso-C3H7, n-C4H9.
The ether yield reached 70-85%.
The paper [34] describes the reaction of alcohols (methanol, ethanol, n-propanol, n-butanol, and n-amyl alcohol) with TFE, in particular, a detailed description of preparation of n-butyl 1,1,2,2-tetrafluoroethyl ether is presented.
The reaction of TFE with n-butanol in the presence of base (10% solution of n- С4Н9ОNa) proceeded under relatively mild conditions (0 - 38 °С, pressure) in the yield of the target ether of 81%:
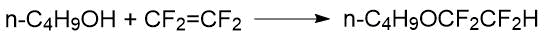
The reaction of perfluoroisobutylene with methanol and ethanol was considered in the Knunyants’s paper [35]. This reaction took place at room temperature under conditions of bubbling perfluoroisobutylene through alcohol with stirring. In addition to target product with a fairly high yield (64.7% for methyl 1,1,3,3,3-pentafluoro-2-(trifluoromethyl) propyl ether and 53% for ethyl 1,1,3,3,3-pentafluoro-2-(trifluoromethyl) propyl ether) alkene derivatives were present in the reaction products (8-10%):
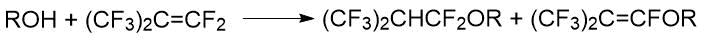
where R= CH3; C2H5
Other papers describe the reaction of perfluoroisobutylene with 2-chloroethanol [36], as well as with allyl ether [37]. In both cases, the ethers formation was accompanied by side processes.
Perfluoroisobutylene in the presence of cesium fluoride with chlorodimethyl and methyl-1-chloroethyl ethers formed methyl(perfluoro-tert-butyl) methyl (b.p. 65-67°С, yield 61.5%) and methyl-1-(perfluoro-tert -butyl) ethyl (b.p. 83-85°C, yield 67.9%) ethers, respectively [38]:

The severe reaction conditions were required in the case of the addition of TFE to alcohols containing a trifluoromethyl group (for example, 2,2,2‑trifluoropropanol). This process is described by Henne and Smuck in [3] (see Table 2.1). The reaction required the use of autoclave. The alcoholate from alcohol and metallic sodium were prepared directly during the reaction.
Table 2.1.
|
ROH |
Process conditions |
Conversion / Yield, % |
Boiling point, °С / pressure, mm Hg |
dt |
|
CF3CH2OH |
180 °С, 40 atm, 16 h |
- /78,5 |
56.7 / 760 |
1.4874 |
|
CF3CH2СН2 OH |
200 °С, 20 atm |
54 / 59 |
88.2 / 744 |
1.4087 |
Japanese patent describes the method for producing hexafluoropropyl 2,2,2-trifluoroethyl ether in yield of 89% by reacting 2,2,2-trifluoroethanol with hexafluoropropene in the presence of bases (H2O, 25°C, pressure, 24 h) [39]. Both inorganic (NaOH, KOH, NaH, Ca(OH)2 and CaH2) and organic (primary, secondary and tertiary amines) compounds were used as bases. It was noted that 1,2,3,3,3-pentafluoro-1-(2,2,2-trifluoroethoxy)-1-propene (~ 10%) was formed as a co-product.
An interesting feature of the reaction of perfluorinated alcoholates with allyl halides was noted in the paper of Canadian scientists M.E. Redwood and C.J. Willis [40]. For example, the reaction of allyl bromide with cesium heptafluoroisopropylate resulted in a halogen substitution product:
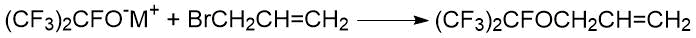
2.2 Addition of alcohols to alkynes
In general cases, polyfluoroalkanols in the presence of alcoholates react with acetylene to form vinyl ethers:
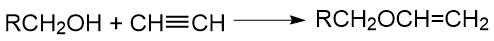
So, 1,1,1-trifluoroisopropyl vinyl ether was obtained by Lyon et al. [41] when acetylene reacts with trifluoroisopropanol (containing 12% potassium alcoholate) with heating in an ampoule (150 C, 260 psi, 4.5 h).
Addition of polyfluoroalkanols to acetylene in gas phase (porcelain tube, 18-20% zinc acetate on SKLTM coal, 190-200°C, excess pressure 12-15 mm Hg) allowed Rostovsky et al. [42] to obtain vinyl ethers H(CF2)nCH2OCH=CH2, (n=2, 3) in 80 and 92% yield.
References
- Milos Hudlicky, Organic Fluorine Chemistry, translation from Czech., Ed. A. N. Sergeeva, GNTI HL, M., 1961, 372 pp. (in Russian)
- Mason C.T., Allan C.C., Preparation and Properties of some α-Fluoroethers, J. Am. Chem. Soc., 1956, 78, 1682-1684.
- A.L. Henne, M.A. Smook, Fluorinated Ethers, J. Am. Chem. Soc, 1950, 72, 4378-4379.
- Williamson, A., On etherification, J. Chem. Soc., 1852, 4, 229-239.
- Patent US 2452944 (1948), Fluorinated ethers, E.T. McBee, Wm.E. Weesner.
- Patent US 3465045 (1969), Fluorinated vinyl ethers and use thereof, A.G.Pittman, L.W.Wasley; Patent US 3799992 (1974), Fluorinated vinyl ethers and use thereof, A.G.Pittman, L.W.Wasley.
- D.N. Bazhin, T.I. Gorbunova, A.Ya. Zapevalov, V.I. Saloutin, Synthesis of polyfluorinated ethers, Russ. J. Appl. Chem., 2005, 78(10), 1646-1650.
- W. Huang, C. Jin, D.K. Derzon, T.A. Huber, J.A. Last, P.P. Provencio, A.S. Gopalan, M. Dugger, D.Y. Sasaki, Synthesis of etherlinked fluorocarbon surfactants ant their aggregational properties in organic solvents, J. of Colloid and Interface Sci, 2004, 272, 457-464.
- Boutevin, B. Youssef, B., Synthese d’ethers vinyliques a chaine laterale fluoree, Fluorine Chem, 1989, 44(3), 395-412.
- O.W. Steward, O.R. Pierce, Fluoroalkyl-and 3-(fluoroalkoxy)propylpolysiloxanes, J. Org. Chem., 1961, 26(8), 2943-2947.
- A. I. Rakhimov, E. V. Shurubtsova, and N. A. Storozhakov, Synthesis of Allyl Ethers Derived from Polyfluorinated Alcohols, Russian Journal of General Chemistry, 2007, 77(2), 317-318.
- H. Maciejewski, J. Karasiewich, B. Marciniec, Efektywna synthesa fluorofunkcyjnych (poly‑)siloxanow, Polimery, 2012, 57(6), 449-455.
- D.Lazzari, M.C.Cassani, G.Solinas, M.Pretto Fluoroalkyl allyl ethers: Useful building blocks for the synthesis of environmentally safer fluorinated multiblock molecules, J. Fluor. Chem., 2013, 156, 34-37.
- Patent US 0323672 (2014), Perfluoro-t-buthoxy allyl and propargyl ethers, I.T. Horvath, K.C. Lau, M.Y. Lui, E. Law, K.C.P. Wong.
- Boutevin, B., Youssef, B., Boileau, S., Garnault, A.M. Synthese d’ethers et de thioethers allyliques fluores par catalyse par transfert de phase, J. Fluor. Chem, 1987, 35, 399-410.
- L. Popova , A. Tsyrulnikova, S. Vershilov, J. Bazarnova, E. Aronova, L. Osetrova, Allyl type monomers for hard surface coating protection, E3S. Web of Conferences, 2019, 140, 7-14.
- Patent US 2336921(1943), Fluorine compound, A.F. Benning, J.D. Park.
- K. Wu, Q.-Y. Chen, Synthesis of trifluoroethyl ethers from 2,2,2-trifluoroethyl chloride (HCFC-133a) in high temperature aqueous medium, J. Fluor. Chem., 2002, 113, 79-83.
- Yong J.A., Tarrant P., A New method of preparing of esters of difluoroacetic acid, JACS, 1950, 72, 1860-1861.
- Patent US 3637477 (1972), Method of preparing of CF3CHClOCHF2, L.S.Croix.
- Patent China CN101659603 (2010), Method of producting fluoromethyl 1,1,1,3,3,3-hexafluoroisopropyl ether, Weiguo Xu and Hua Li.
- Патент США 3911024 (1975), Fluorinated isopropyl derivatives, L.S.Croix.
- R.D. Bagnall, W. Bell, K. Pearson, New inhalation anaesthetics: II. Fluorinated methyl propyl ethers, J. Fluor. Chem., 1978, 11, 93-107.
- Patent DE 1076113 (1960), Verfahren zur herstellung von 1,1,1-trifluoraethylalkylaethern, O. Dr. Scherer, H. Dr.Hahn, Hoechst AG.
- Patent CN 109867612 (2019), Method for preparing hydrofluoroether through two-step process, W. Li, D. Zhang, D. Liu, Z. Guo, Zhejiang res inst chemical ind LTD.
- Patent US 2824141 (1958), Fluoroethers, W.A. Zisman, J.G. O’Rear.
- N. Takada, T. Abe, A. Sekiya, Preparation and physicochemical properties of F-tert-butyl alkyl ethers, J
- Rakhimov A.I., Nalesnaya A.V., Vostrikova O.V., Synthesis of di (polyfluoroalkyl) ethers, J. Applied Chemistry, 2004, 77(9), 1573-1574. (in Russian)
- A.I. Rakhimov, A.V. Nalesnaya, R.V. Fisechko, Features of catalysis in the reaction of polyfluoroalkyl chlorosulfites with saturated monohydric alcohols, Russ. J. Gen. Chem., 2008, 78(11), 2075-2081.
- Patent RU 2346926 (2009), Method of obtaining simple polyfluoroalkyl ethers, Rakhimov A.I., Nalesnaya A.V., Fisechko R.V., VolgSTU.
- Chambers R.D., Fluorine in Organic Chemistry, New York etc. John Wiley and Sons, 1973, 385 p.
- Patent US 2409274 (1946), Polyfluoro organic ethers and their preparation, W. E. Hanford, G. W. Rigby, DuPont.
- J. D. Park, D. K. Vail, K. R. Lea and J. R. Lacher, Polyfluoro Alkyl Ethers and their Preparation, J. Am. Chem. Soc., 1950, 72, 1550-1552.
- J.D. Park, M.L. Sharrer, W.H. Breen and J.R. Lacher, The Action of Alkanols on Tetrafluoroethylene, J. Am. Chem. Soc., 1951, 73, 1329-1330.
- I.L. Knunyants, L.S. German and B.L. Dyatkin, Reactions of fluoroolefins. 6. Reactions of perfluoroisobutene and perfluoropropene with nucleophilic reagents, Russian Chemical Bulletin, 1956, 5(11), 1387-1394.
- R.J. Koshar, Th.C. Simmons, F.W. Hoffman, The addition of alcohols to octafluoroisobutene, J. Am. Chem. Soc., 1957, 79(7), 1741-1744.
- Kurykin M.A., German L.S., Reactions of trans-perfluoropentene-2 with alcoholates, Izv. Academy of Sciences of the USSR. Ser. Chem., 1981, 11, 2647-2650. (in Russian)
- Delyagina N.I., Dyatkin B.L., Knunyants I.L., Reaction of perfluoro-tert-butyl anion with α-haloethers and imidiol halides / Zh. Org. Khim., 1974, 10(5), 935-937. (in Russian)
- Patent JP3482488 (2003), Method for producing fluorine-containing ether compound, J. Murata, A. Sekiya, M. Tamura.
- Redwood M.E., Willis C.J., Fully fluorinated alkoxides. Part II. Ethoxides, propoxides, and butoxides., Can. J. Chem., 1967, 45(4), 389-395.
- Patent US 2749369 (1956), Fluorinated ethers and method of producting, J.A. M.Lyon.
- Patent SU 784191 (1982). Process for producing fluorine-contaning vinyl ethers, E.N. Rostovskij, L.D. Budovskaya, V.N. Ivanova.
ARTICLE INFO
Received 27 May 2021
Accepted 04 June 2021
Available online June 2021
Recommended for publication by Prof. S.M. Igumnov
Fluorine Notes, 2021, 136, 1-2
